Abstract
End stage kidney disease increase the risk of COVID-19 related death but how the kidney replacement strategy should be adapted during the pandemic is unknown. Chronic hemodialysis makes social distancing difficult to achieve. Alternatively, kidney transplantation could increase the severity of COVID-19 due to therapeutic immunosuppression and contribute to saturation of intensive care units. For these reasons, kidney transplantation was suspended in France during the first epidemic wave. Here, we retrospectively evaluated this strategy by comparing the overall and COVID-19 related mortality in kidney transplant recipients and candidates over the last three years. Cross-interrogation of two national registries for the period 1 March and 1 June 2020, identified 275 deaths among the 42812 kidney transplant recipients and 144 deaths among the 16210 candidates. This represents an excess of deaths for both populations, as compared with the same period the two previous years (mean of two previous years: 253 in recipients and 112 in candidates). This difference was integrally explained by COVID-19, which accounted for 44% (122) and 42% (60) of the deaths in recipients and candidates, respectively. Taking into account the size of the two populations and the geographical heterogeneity of virus circulation, we found that the excess of risk of death due to COVID-19 was similar for recipients and candidates in high viral risk area but four-fold higher for candidates in the low viral risk area. Thus, in case of a second epidemic wave, kidney transplantation should be suspended in high viral risk areas but maintained outside those areas, both to reduce the excess of deaths of candidates and avoid wasting precious resources.
Keywords: COVID-19, ESRD, mortality, renal transplantation, SARS-CoV-2
Graphical abstract
Editor’s Note.
This is one of several articles we think you will find of interest that are part of our special issue of Kidney International addressing the challenges of dialysis and transplantation during the COVID-19 pandemic. Please also find additional material in our commentaries and letters to the editor sections. We hope these insights will help you in the daily care of your own patients.
In December 2019, an outbreak of apparently viral pneumonia of unknown etiology emerged in the city of Wuhan, in the Chinese province of Hubei.1 On January 9, 2020, the World Health Organization announced the discovery of a novel coronavirus officially named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),2 which is the pathogen responsible for this infectious respiratory disease called coronavirus disease 2019 (COVID-19). The disease quickly spread from Wuhan, and as of July 17, 2020, more than 13 million cases have been confirmed in 209 countries,3 leading the World Health Organization to consider COVID-19 the first pandemic to be triggered by a coronavirus. Strengthened surveillance was implemented in France on January 10, 2020, resulting in the identification of the first 3 confirmed European cases on January 24, 2020.4 COVID-19 then progressed very fast, and France went into lockdown on March 17, 2020.
Among the various alarms raised by the pandemic was its impact on the population of patients with end-stage renal disease (ESRD). ESRD patients need renal replacement therapy, which consists of either dialysis or kidney transplantation. Because the latter provides both reduced risk of mortality and better quality of life for a lower cost than chronic hemodialysis, it is largely considered to be the preferred modality of treatment for ESRD.5 Yet the fact that renal transplant recipients require therapeutic immunosuppression raised concern that they might have greater susceptibility to severe infection and increased viral burden.6, 7, 8 A second concern was that the hospitals facing the massive increase of admissions into intensive care units, due to severe forms of COVID-19, might not have the resources in terms of staff and equipment to care for donors and recipients. In light of these possibilities, and in the presence of a therapeutic alternative (i.e., hemodialysis), the national regulatory agency,9 in conjunction with the scholarly societies involved,10 , 11 decided to suspend the activity of renal transplantation on March 20, 2020.
National lockdown proved to be effective in decreasing the contact rate and therefore the number of infectious cases, resulting in a drastic drop of the basic reproductive number (R0, i.e., the expected number of new cases generated by a single infectious case) from 3.3 at the beginning of the epidemic to 0.47.12 As expected from this value of <1, the epidemic died out, and the restriction policies were progressively eased from May 11 onward, allowing resumption of renal transplantation activity.
Taking advantage of the national registry, which prospectively collects the follow-up data from renal transplant recipients and candidates (CRISTAL), as well as the French nationwide Registry of Solid Organ Transplant Recipients with COVID-19 (French SOT COVID, clinicaltrials.gov #NCT04360707), we continued to compare the impact of the pandemic on the mortality of renal transplant recipients and candidates. Our goal was to determine which of the 2 populations was at higher risk of death due to COVID-19 and thereby provide a rationale to decide whether renal transplantation should be maintained or suspended in case of a second wave of the disease.
Results
Temporospatial distribution of the COVID-19 cases among renal transplant recipients and candidates
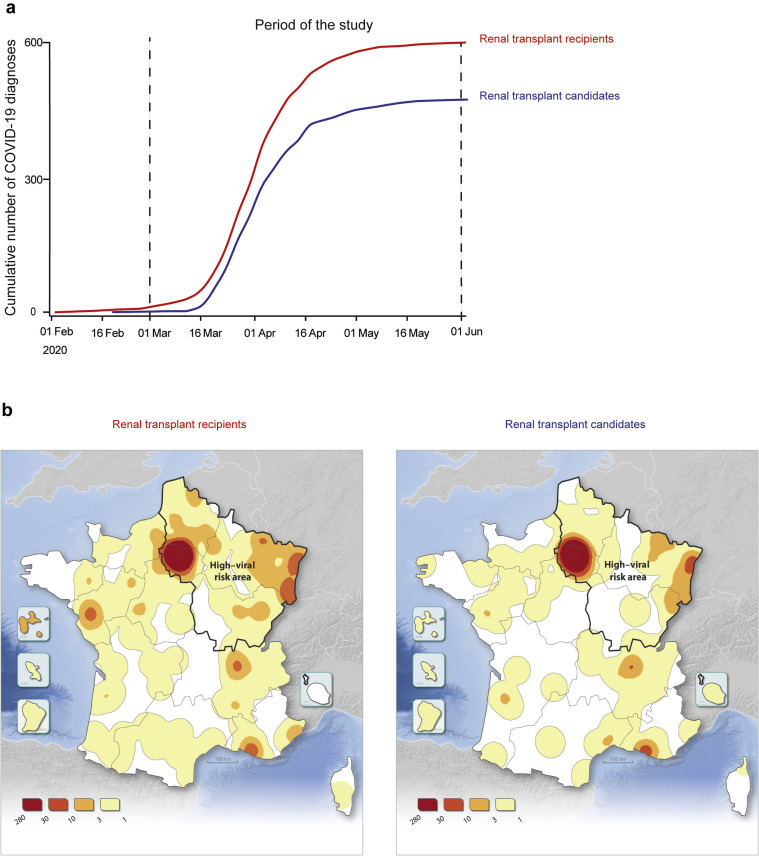
Among a total of 42,812 kidney transplant recipients and 16,210 candidates (active and inactive) on the national waiting list for a kidney graft on February 1, 2020 (Figure 1 ), 606 (1.42%) recipients and 478 (2.95%) candidates were diagnosed with confirmed COVID-19 infection before June 1, 2020 (Figure 2 a). The fact that the proportion of renal transplant candidates infected with SARS-CoV-2 is twice that of recipients is consistent with the difficulties in achieving social distancing in patients relying on chronic hemodialysis as renal replacement therapy.
Figure 1.
Flowchart of the IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) study. COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease.
Figure 2.
Spatio-temporal characteristics of the epidemic of coronavirus disease 2019 (COVID-19) in France. (a) Cumulative incidence of the cases of COVID-19 diagnosed in France from February 1 to June 1, 2020 for renal transplant recipients (red curve) and candidates (blue curve). This study focuses on COVID-19–related mortality. Taking into account that (i) all cases were diagnosed between March 1 and April 1 and (ii) the 99% confidence interval for the delay between COVID-19 diagnosis and death is 30 days, the period of the study was set from March 1 to June 1, 2020. (b) Heat maps showing the geographic distribution of the cases of COVID-19 for renal transplant recipients (left panel) and candidates (right panel). The limit of the area in which the circulation of the virus was more intense is shown (high–viral risk area defined by the French government on May 11, 2020).
The curves of cumulative incidence (Figure 2a) indicate that the COVID-19 cases were diagnosed between March 1 and May 1, 2020, with a similar kinetic in the 2 populations. Given that the present study focuses on death due to COVID-19 and because the 99th percentile of the delay between diagnosis and death due to COVID-19 is 30 days (data not shown), we set the period of analysis for this study to be between March 1 and June 1, 2020.
The intensity of SARS-CoV-2 circulation was extremely heterogeneous in France. The regions that had both a high prevalence of COVID-19 (more than 10% of emergency service admission for a suspicion of COVID-19) and/or a saturation of intensive care units (more than 80% of COVID-19 patients in intensive care units) on May 11, 2020 were classified as a high viral–risk area by the government.13 On May 20, 2020, the incidences of COVID-19 were, respectively, 11.9 per 105 inhabitants in the high viral–risk area versus 5.8 in the rest of France.
The spatial distributions of the cases of COVID-19 for renal transplant recipients (Figure 2b) and candidates (Figure 2c) were similar, with, as expected, a higher density of cases in the Northeast quarter (which corresponds to the high viral–risk area).
Mortality of renal transplant recipients and candidates over the study period
In France, the health status of both renal transplant recipients and candidates is prospectively monitored, and the data are recorded in the CRISTAL national registry. In addition, from the outset of the global pandemic, the Société Francophone de Transplantation set up a specific registry in which all the cases of COVID-19 diagnosed in solid organ recipients were prospectively collected (French SOT COVID Registry; clinicaltrials.gov #NCT04360707).14 Cross-interrogation of these 2 databases for the period March 1 to June 1, 2020 identified 275 deaths among the 42,812 renal transplants, 122 (44%) of which were due to COVID-19. Of the 16,210 candidates on the national waiting list, 144 died over the same period, 60 (42%) owing to COVID-19 (Figure 1). The main clinical characteristics of these patients are presented Table 1 . Regardless of the reason, ESRD patients (recipients and candidates) who died during the study period were older, more frequently diabetic, and had more often cardiovascular comorbidities. This was expected since these characteristics are associated not only with a higher risk for COVID-19–related death,8 but also with the risk of death from other causes in these populations. In contrast, higher body mass index, a known risk for COVID-19–related death in the general population,8 was associated with death from COVID-19 but not from other causes in both recipients and candidates (Table 1). Another interesting finding is the fact that renal transplant recipients who died from COVID-19 were more frequently within their first year of transplantation (Table 1). The first-year post-transplantation is the period during which the depth of therapeutic immunosuppression is at a maximum,15 especially for the patients that received a depleting induction.16 We therefore went on testing whether depleting induction was associated with a higher risk of death due to COVID-19 in recipients transplanted within the last 12 months. Among the 3313 renal recipients transplanted after February 1, 2019, a total of 1901 (57.4%) received thymoglobulin or alemtuzumab. The distribution of the latter into 3 categories—(i) alive on June 1, 2020 (n = 1890; 99.4%); (ii) death from other (than COVID-19) cause (n = 3; 0.15%); and (iii) death from COVID-19 (n = 8; 0.42%)—was not different than that of the 1412 (42.6%) recipients that did not receive depleting induction (no induction or induction with anti-R-IL2): n = 1398 (99%), n = 6 (0.42%), and n = 8 (0.56%) in the 3 previous categories, respectively (χ2; P = 0.55).
Table 1.
Characteristics of patients deceased during COVID-19 epidemic
| Recipients |
Pa | Pb | Candidates |
Pc | Pd | Pe | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alive on June 1 | Death from other causes | Death due to COVID-19 | Alive on June 1 | Death from other causes | Death due to COVID-19 | ||||||
| N | 42469 | 153 | 122 | 16,042 | 84 | 60 | 0.60 | ||||
| Male | 26,255 (61.8) | 105 (68.6) | 71 (58.2) | 0.08 | 0.41 | 9993 (62.3) | 59 (70.2) | 43 (71.7) | 0.13 | 0.13 | 0.08 |
| Age, yr | 56.9 ± 15 | 70 ± 10.5 | 66.5 ± 12.1 | <0.01 | <0.01 | 57.5 ± 13.9 | 63 ± 11.4 | 62.7 ± 10.5 | <0.01 | <0.01 | 0.04 |
| Cardiovascular disease | 3563 (13.4) | 26 (29.9) | 35 (33) | <0.01 | <0.01 | 2832 (18.7) | 25 (32.9) | 10 (17.5) | <0.01 | 0.82 | 0.04 |
| Diabetes | 5352 (18.9) | 26 (26) | 51 (46.8) | 0.07 | <0.01 | 4674 (30.1) | 42 (53.2) | 29 (49.2) | <0.01 | <0.01 | 0.77 |
| BMI, kg/m2 | 25.8 ± 5.2 | 25.5 ± 5.2 | 28 ± 5.2 | 0.5 | <0.01 | 26.2 ± 5.3 | 25.7 ± 5.8 | 28.4 ± 4.8 | 0.41 | <0.01 | 0.63 |
| Blood group | 0.16 | 0.24 | 0.39 | 0.5 | 0.27 | ||||||
| A | 18,423 (43.4) | 69 (45.1) | 47 (38.5) | 5308 (33.1) | 21 (25) | 15 (25) | |||||
| B | 1874 (4.4) | 10 (6.5) | 6 (4.9) | 537 (3.3) | 2 (2.4) | 2 (3.3) | |||||
| AB | 4599 (10.8) | 9 (5.9) | 20 (16.4) | 2369 (14.8) | 15 (17.9) | 12 (20) | |||||
| O | 17,555 (41.4) | 65 (42.5) | 49 (40.2) | 7828 (48.8) | 46 (54.8) | 31 (51.7) | |||||
| Cause of ESRD | 0.02 | <0.01 | <0.01 | <0.01 | 0.23 | ||||||
| Diabetes | 3403 (8) | 16 (10.5) | 27 (22.1) | 2641 (16.5) | 28 (33.3) | 21 (35) | |||||
| Glomerulonephritis | 10,738 (25.3) | 36 (23.5) | 17 (13.9) | 3151 (19.6) | 12 (14.3) | 9 (15) | |||||
| Nephroangiosclerosis | 2654 (6.2) | 19 (12.4) | 15 (12.3) | 1917 (12) | 9 (10.7) | 10 (16.7) | |||||
| Other | 11,201 (26.4) | 38 (24.8) | 37 (30.3) | 4207 (26.2) | 24 (28.6) | 12 (20) | |||||
| Polycystic kidney disease | 6860 (16.2) | 26 (17) | 18 (14.8) | 1952 (12.2) | 3 (3.6) | 4 (6.7) | |||||
| Tubulointerstitial nephritis | 7610 (17.9) | 18 (11.8) | 8 (6.6) | 2172 (13.5) | 8 (9.5) | 4 (6.7) | |||||
| Previous transplantation | 5538 (13) | 15 (9.8) | 14 (11.5) | 0.24 | 0.61 | 3423 (21.3) | 25 (29.8) | 14 (23.3) | 0.06 | 0.71 | 0.04 |
| Time since transplantation | |||||||||||
| Duration, mo | 114.2 ± 95.7 | 121.8 ± 94 | 99.5 ± 83.9 | 0.32 | 0.09 | ||||||
| ≤1 yr | 3404 (8) | 9 (5.9) | 16 (13.1) | 0.33 | 0.04 | ||||||
| Last eGFR MDRD, ml/min | 52.5 ± 22.7 | 39.8 ± 21 | 36.1 ± 19.9 | <0.01 | <0.01 | ||||||
| Time on dialysis, mo | 34.8 ± 46.7 | 39.8 ± 52.7 | 53 ± 43.6 | 0.25 | <0.01 | 40.4 ± 54.2 | 63.3 ± 60.1 | 42.2 ± 45.9 | <0.01 | 0.8 | 0.13 |
BMI, body mass index; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MDRD, Modification of Diet in Renal Disease.
The P values are for the following comparisons: arecipients alive vs. recipients deceased from other cause; brecipients alive vs. recipients deceased from COVID-19; ccandidates alive vs. candidates deceased from other causes; dcandidates alive vs. candidates deceased from COVID-19; edeaths from COVID-19 recipients vs. candidates.
Values are n (%) or mean (± SD), unless otherwise indicated.
The COVID-19 pandemic induced an excess of mortality in both renal transplant recipients and candidates
In order to better assess the impact of the pandemic on the mortality of renal transplant recipients and candidates, we took advantage of the fact that these 2 populations retained similar characteristics over the last 3 years (Table 2 ). We therefore went on comparing the daily incidence of deaths observed between March 1 and June 1, 2020 to the number recorded over the same period the 2 previous years. The daily incidence of deaths in 2018 and 2019 was stable over the period and higher for renal transplant recipients (2.76 ± 0.20 deaths/d; Figure 3 a) than for candidates (1.21 ± 0.08 deaths/d; Figure 3b). The latter difference (ratio = 2.28) is largely explained by the fact that the renal transplant recipient population is larger than that of candidates (n = 42,812 vs. n = 16,210, respectively, ratio = 2.64).
Table 2.
Evolution of the characteristics of renal transplant recipients and candidates over the past 3 years
| Recipients |
P | Candidates |
P | Pa | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | ||||
| N | 39,865 | 41,210 | 42,812 | 14,418 | 15,289 | 16,210 | |||
| Male | 24,694 (61.9) | 25,463 (61.8) | 26,474 (61.8) | 0.90 | 8856 (61.4) | 9468 (61.9) | 10,112 (62.4) | 0.23 | 0.23 |
| Age, yr | 56.1 ± 14.9 | 56.5 ± 15 | 57.0 ± 15 | <0.001 | 55.9 ± 13.9 | 56.8 ± 13.8 | 57.5 ± 13.9 | <0.001 | <0.01 |
| Cardiovascular disease | 3131 (14.1) | 3355 (13.7) | 3635 (13.6) | 0.21 | 2480 (18.4) | 2698 (18.7) | 2876 (18.8) | 0.74 | <0.01 |
| Diabetes | 4687 (19.5) | 5033 (19.2) | 5437 (19.1) | 0.47 | 3906 (28.3) | 4300 (29.1) | 4756 (30.3) | <0.001 | <0.01 |
| BMI, kg/m2 | 25.7 ± 5.2 | 25.7 ± 5.2 | 25.8 ± 5.2 | 0.09 | 26.1 ± 5.4 | 26.2 ± 5.3 | 26.2 ± 5.3 | 0.11 | <0.01 |
| Blood group | 0.93 | 0.68 | <0.01 | ||||||
| A | 17,430 (43.7) | 17,941 (43.6) | 18,559 (43.4) | 4714 (32.7) | 4997 (32.7) | 5354 (33) | |||
| B | 1715 (4.3) | 1791 (4.3) | 1898 (4.4) | 490 (3.4) | 539 (3.5) | 541 (3.3) | |||
| AB | 4284 (10.8) | 4419 (10.7) | 4639 (10.8) | 2064 (14.3) | 2270 (14.8) | 2399 (14.8) | |||
| O | 16,418 (41.2) | 17,041 (41.4) | 17,698 (41.4) | 7150 (49.6) | 7483 (48.9) | 7916 (48.8) | |||
| Cause of ESRD | 0.23 | 0.08 | <0.01 | ||||||
| Diabetes | 3102 (7.8) | 3247 (7.9) | 3452 (8.1) | 2357 (16.4) | 2534 (16.6) | 2697 (16.6) | |||
| Glomerulonephritis | 10,252 (25.7) | 10,508 (25.5) | 10,801 (25.2) | 2918 (20.2) | 3031 (19.8) | 3176 (19.6) | |||
| Nephroangiosclerosis | 2351 (5.9) | 2505 (6.1) | 2690 (6.3) | 1538 (10.7) | 1696 (11.1) | 1940 (12) | |||
| Other | 10,574 (26.5) | 10,885 (26.4) | 11,302 (26.4) | 3791 (26.3) | 4054 (26.5) | 4248 (26.2) | |||
| Polycystic kidney disease | 6295 (15.8) | 6598 (16) | 6910 (16.1) | 1830 (12.7) | 1896 (12.4) | 1962 (12.1) | |||
| Tubulointerstitial nephritis | 7291 (18.3) | 7464 (18.1) | 7654 (17.9) | 1981 (13.7) | 2076 (13.6) | 2185 (13.5) | |||
| Previous transplantation | 5270 (13.2) | 5438 (13.2) | 5576 (13) | 0.67 | 3269 (22.7) | 3311 (21.7) | 3467 (21.4) | 0.02 | <0.01 |
| Time since transplantation, mo | 110.4 ± 92.9 | 112.1 ± 94.2 | 114.2 ± 95.7 | <0.001 | |||||
| Last eGFR MDRD, ml/min | 52.8 ± 22.1 | 53 ± 22.4 | 52.3 ± 22.7 | <0.001 | |||||
| Time on dialysis, mo | 35.4 ± 48.4 | 35.1 ± 47.6 | 34.9 ± 46.7 | 0.25 | 40.5 ± 54.7 | 40.3 ± 53.5 | 40.6 ± 54.2 | 0.90 | <0.01 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MDRD, Modification of Diet in Renal Disease.
Values are n (%) or mean (± SD), unless otherwise indicated.
The P values for the comparison of the clinical characteristics of recipients and candidates in 2020.
Figure 3.
Comparison of the daily incidence of deaths over the past 3 years. (a,b) The moving average (10 days) method was used to plot the daily incidence of deaths observed from March 1 to June 1 over the past 3 years for, respectively, (a) renal transplant recipients and (b) candidates. (c,d) Stacked histograms showing, for each day of the period March 1 to June 1, 2020, the distribution between the number of deaths (moving average 10 days) due to coronavirus disease 2019 (COVID-19; black bar) versus cause (white bar) for, respectively, (c) renal transplant recipients and (d) candidates. (e) Heat maps showing the geographic distribution of the renal transplant candidates deceased due to COVID-19 during the first peak (left panel: March 1–April 15, 2020) and the second peak (right panel: April 16, June 1).
The curve of the daily incidence of deaths for 2020 increased over the curves of the 2 previous years, thus demonstrating an excess of mortality for both renal transplant recipients (Figure 3a) and candidates (Figure 3b). This excess of mortality was integrally explained by COVID-19, as shown in Figures 3c and d.
Of note, the incidence of COVID-19–related deaths followed a different kinetics in the 2 populations. Instead of a single peak pattern observed for renal transplant recipients, the daily incidence of deaths due to COVID-19 for renal transplant candidates followed a double-peak pattern. Except the younger age for patients of the second peak, comparing the candidates of the 2 peaks did not reveal meaningful differences regarding their clinical characteristics (Table 3 ) or geographic localization (Figure 3e).
Table 3.
Comparison of the clinical characteristics of candidates deceased due to COVID-19 in the first versus the second peak of the epidemic
| Candidates deceased due to COVID-19 (n = 60) |
|||
|---|---|---|---|
| First peak (March 1–April 15, 2020) | Second peak (April 16–June 1, 2020) | P | |
| N | 37 (62) | 23 (38) | |
| Male | 25 (67.6) | 18 (78.3) | 0.37 |
| Age, yr | 65.4 ± 8.7 | 58.3 ± 11.8 | 0.04 |
| Cardiovascular disease | 5 (14.7) | 5 (21.7) | 0.5 |
| Diabetes | 16 (44.4) | 13 (56.5) | 0.37 |
| BMI, kg/m2 | 29.1 ± 4.9 | 27.2 ± 4.6 | 0.1 |
| Blood group | 0.82 | ||
| A | 10 (27) | 5 (21.7) | |
| B | 2 (5.4) | 0 (0) | |
| AB | 7 (18.9) | 5 (21.7) | |
| O | 18 (48.6) | 13 (56.5) | |
| Cause of ESRD | 0.4 | ||
| Diabetes | 10 (27) | 11 (47.8) | |
| Glomerulonephritis | 7 (18.9) | 2 (8.7) | |
| Nephroangiosclerosis | 6 (16.2) | 4 (17.4) | |
| Other | 7 (18.9) | 5 (21.7) | |
| Polycystic kidney disease | 4 (10.8) | 0 (0) | |
| Tubulointerstitial nephritis | 3 (8.1) | 1 (4.3) | |
| Previous transplantation | 8 (21.6) | 6 (26.1) | 0.63 |
| Time on dialysis, mo | 47.9 ± 52.6 | 33.3 ± 31.8 | 0.19 |
BMI, body mass index; COVID-19, coronavirus disease 2019; ESRD, end-stage renal disease.
Values are n (%) or mean (± SD), unless otherwise indicated.
Excess mortality due to COVID-19 is higher for candidates than for recipients of a renal transplant and is influenced by virus circulation
A direct comparison of COVID-19–related deaths in renal transplant recipients and candidates is difficult for several reasons. First, the 2 populations largely differ in size. To overcome this problem, the daily incidence of deaths during the first wave of the COVID-19 epidemic was normalized over the size of the population (Figure 4 a). Comparing the 2 curves revealed an excess of mortality in candidates estimated at +73%.
Figure 4.
Comparison of the daily incidence of deaths during the epidemic between recipients and candidates. (a) The daily incidence of deaths (moving average 10 days), observed between March 1 and June 1, 2020 in renal transplant recipients (red curve) and candidates (blue curve), was normalized over the size of the population. (b–d) The daily incidence of deaths (moving average 10 days) observed between March 1 and June 1, 2020 was normalized over the mean number of deaths observed the same day for the 2 previous years. This mathematical transformation allows for direct comparison of the excess of risk of death due to the coronavirus disease 2019 epidemic for renal transplant recipients (red curve) and candidates (blue curve), despite the difference in size of the 2 populations. Comparisons were made at (a,b) the level of whole national territory, (c) inside the high–viral risk area, and (d) outside high–viral risk area.
This simple method has, however, several limitations, including the fact that it does not take into account the differences between candidates and recipients. Candidates are older, have a higher body mass index, and are more frequently diabetic with cardiovascular comorbidities (Table 2), which are all well identified risk factors for death (related to COVID-198 or not). To improve the comparability between the 2 populations, we took advantage of the fact that recipients and candidate populations were relatively stable in their characteristics over the past 3 years (Table 2), offering the possibility of determining for each population the excess of risk of death during the epidemic. We did that by dividing the daily incidence of deaths observed between March 1 and June 1, 2020 over the “expected” number of deaths: that is, the mean number of deaths observed the same day for the 2 previous years (Figure 4b). Comparing the area under the curve above the reference line (dashed black line) revealed that the COVID-19 pandemic was responsible for an excess of death that was 50% higher for patients on the national waiting list than for the recipients of a renal transplantation.
This difference was, however, highly influenced by the intensity of virus circulation, as demonstrated by the analyses conducted separately for patients residing inside (Figure 4c) and outside (Figure 4d) the high–viral risk area. The excess of mortality due to COVID-19 inside the high–viral risk area was much higher than that observed outside this zone, but it was also very similar between the 2 populations (Figure 4c). In fact, the difference between the 2 populations was estimated to be <3%. In contrast, outside the high–viral risk area, the excess of risk of death due to COVID-19, which was low, was still much higher for renal transplant candidates than it was for renal transplant recipients (+432%; Figure 4d).
Discussion
Our study confirmed that the epidemic of SARS-CoV-2 infection, which affected France early in 2020, did impact the mortality of both renal transplant recipients and candidates. In both populations, an excess of deaths, integrally explained by COVID-19, was observed. Our findings, made in France only, are perfectly in line with the conclusions of recent reports from the US, UK, and other international registries.14 , 17 , 18
To make meaningful a direct comparison between these 2 populations, which differ by not only their size but also their clinical characteristics, we did not only rely on adjusting the daily incidence of deaths over the size of the populations but also calculated a relative daily incidence of deaths normalized over the “expected” number (ie, the mean number of deaths observed the same day over the last 2 years in the same patient population). These analyses revealed that the excess of mortality in 2020 due to the COVID-19 epidemic was globally higher (∼50%) for the candidates than for recipients of a renal transplant. This difference might be explained by the fact that most candidates are on chronic hemodialysis in France, which requires that they go 3 times a week to the dialysis center and makes it difficult to achieve efficient social distancing during the lockdown. This hypothesis is supported by both the results of the COWAIT prospective cohort study (ClinicalTrials.gov Identifier: NCT04376775) and the fact that the incidence of COVID-19 in candidates was twice that of recipients (2.9% vs. 1.4%). A difference was also observed in the UK.18 Another possible explanation is the fact that the level of kidney function, which has been shown to be a major predictor for death in COVID-19–infected patients,8 is lower in candidates than in renal transplant recipients. In line with this, we observed that recipients who died from COVID-19 had a lower estimated glomerular filtration rate at last follow-up than those who survived or died from another cause.
Another important finding of our study is that the difference between the 2 populations regarding the excess of mortality due to COVID-19 is highly influenced by the intensity of virus circulation. In the high–viral risk area, where most of the deaths due to COVID-19 were observed, the excess of risk was very similar for candidates and recipients. In contrast, in the geographic area where the circulation of the virus was lower, the excess risk of death due to COVID-19 was more than 4-fold higher for candidates than for recipients (for whom there was in fact no additional risk). This finding is important because it provides a rationale to modify our strategy in case of a second wave of COVID-19.19 Since the risk is similar in both populations, and the procedure is difficult to organize in the geographical areas where the circulation of the virus is intense, it seems reasonable to suspend again renal transplantation in these regions in case of a new epidemic. In contrast, in the rest of country, where circulation of the virus is low, our data indicate that the excess risk related to COVID-19 is major for candidates only. Given that hospital resources are less strained in these areas, it seems logical to maintain transplantation as long as possible in these territories.
It shall, however, be kept in mind that among the limitations of the present study is the fact that transplantation was totally suspended in France during the first wave of the pandemic. We therefore lack information regarding the real risk of death due to COVID-19 for patients undergoing renal transplantation during the peak of the crisis. The higher frequency of follow-up visits in the hospital (which makes social distancing more difficult to achieve), as well as the use of high-dose immunosuppression, including depleting induction (which induces profound lymphopenia for several weeks) likely contribute to increasing the risk of these patients as compared with recipients transplanted several years before.16 In agreement with this hypothesis, we observed that recipients who died from COVID-19 were more often within their first year of transplantation compared with those who survived the epidemic or died from another cause during the period. This problem could be mitigated efficiently by (i) selection of donors and recipients, (ii) implementation of telemedicine,20 and (iii) adaptation of an immunosuppressive regimen,21 since it was reported neither in the other (“vital”) organ (including heart, lung, and liver) transplantation programs that were not interrupted in France, nor in the countries in which the renal transplantation program continued during the epidemic.21
In conclusion, this French nationwide study demonstrates that the first wave of COVID-19 pandemic induced an excess of deaths in both renal transplant recipients and candidates, which predominated in the latter. In case of a second wave, our results suggest again suspending renal transplantation activity in the geographic areas in which the circulation of the virus is intense. However, renal transplantation should be maintained in the rest of the jurisdictions to both reduce the excess deaths in candidates and avoid wasting precious resources.22 This could be optimally achieved by following a phased approach depending on risk tolerance, hospital capacity, and degree of virus activity.21
Methods
Study population
All renal transplant recipients with a functional graft, and candidates registered on the national waiting list (active or inactive) for a kidney graft, on February 1, 2018, 2019, and 2020 were enrolled.
Cardiovascular comorbidities were measured at the time of wait-listing and included heart failure, ischemic heart disease (including history of myocardial infarction, coronary vascular disease, or unstable angina), and dysrhythmia but not hypertension.
The diagnostic criteria for COVID-19 were as follows: (i) evidence of SARS-CoV-2 infection on reverse transcriptase-polymerase chain reaction testing performed on nasopharyngeal swab specimens or (ii) presence of typical respiratory symptoms accompanied by evocative pulmonary lesions on low-dose chest computed tomography (CT) even when reverse transcriptase polymerase chain reaction yielded negative results.
Data sources
Data were extracted from 2 national registries: CRISTAL and French SOT COVID.
CRISTAL was initiated in 1996 and is administered by the national regulatory agency (Agence de la Biomedicine). This registry prospectively collects data about all organ transplant recipients and candidates in France. Candidate and recipient demographic, clinical, and laboratory data are collected by transplantation teams at wait-listing, transplantation, and annually thereafter. Graft failures and recipient deaths are reported prospectively. Data accuracy is verified by CRISTAL research assistants.
French SOT COVID was initiated by the Société Francophone de Transplantation10 at the beginning of the COVID-19 epidemic in France to prospectively collect the data from all French renal transplant patients with COVID-19.
Ethics
The present study was conducted in accordance with French law. Because French legislation defines research studies based on the CRISTAL registry as part of the transplant outcome assessment, they do not require specific ethics committee review or approval.
The French SOT COVID Registry was approved by the Institutional Review Board of Strasbourg University (approval number 02.26) and registered at clinicaltrials.gov (NCT04360707). The need for informed consent was waived. However, all patients were informed about their inclusion in the registry.
Statistical analysis
Continuous variables are reported as means ± SD, and categorical variables are reported as frequencies (percentages). Differences between groups were assessed with the χ2 test or 2-sided Fisher’s exact test for categorical variables and analysis of variance test or Student’s t test or Wilcoxon’s rank-sum test for continuous variables.
All COVID-19 cases for candidates and recipients were geocoded at the home address. Smoothed maps were processed with a 30-km and exponential Stewart model.
Number of deaths per day among candidates and recipients between March 1st and June 1st, in 2018, 2019, and 2020, were assessed with 10-day moving averages.
All tests were 2-sided, and P < 0.05 was considered statistically significant. Analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute, Inc, Cary, NC).
Footnotes
see commentary on page 1404
Contributor Information
French nationwide Registry of Solid Organ Transplant Recipients with COVID-19:
Olivier Thaunat, Emmanuel Morelon, Charlene Levi, Fanny Buron, Alice Koenig, Thomas Barba, Sophie Caillard, Bruno Moulin, Samira Fafi-Kremer, Marc Hazzan, Anglicheau Dany, Alexandre Hertig, Jérôme Tourret, Benoit Barrou, Lionel Couzi, Pierre Merville, Anna Kaminski, Valérie Moal, Tristan Legris, Pierre-François Westeel, Maïté Jaureguy, Luc Frimat, Didier Ducloux, Jamal Bamoulid, Dominique Bertrand, Michel Tsimaratos, Florentine Garaix-Gilardo, Jérôme Dumortier, Sacha Mussot, Antoine Roux, Laurent Sebbag, Yannick Le Meur, Gilles Blancho, Christophe Masset, Nassim Kamar, Hélène Francois, Eric Rondeau, Nicolas Bouvier, Christiane Mousson, Matthias Buchler, Philippe Gatault, Jean-François Augusto, Agnès Duveau, Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Philippe Grimbert, Marie Matignon, Antoine Durrbach, Clarisse Greze, Renaud Snanoudj, Charlotte Colosio, Betoul Schvartz, Paolo Malvezzi, Christophe Mariat, Antoine Thierry, Moglie Le Quintrec, Antoine Sicard, Jean Philippe Rerolle, Anne-Élisabeth Heng, Cyril Garrouste, Henri Vacher Coponat, Éric Epailly, Olivier Brugiere, Sébastien Dharancy, Éphrem Salame, and Faouzi Saliba
Appendix
French nationwide Registry of Solid Organ Transplant Recipients with COVID-19
The authors wish to acknowledge the contribution of the members: Olivier Thaunat, Emmanuel Morelon, Charlene Levi, Fanny Buron, Alice Koenig, Service de Néphrologie, Hôpital Edouard Herriot, Lyon; Thomas Barba, Service de Médecine Interne, Hôpital Edouard Herriot, Lyon; Sophie Caillard, Bruno Moulin, Service de Néphrologie et Transplantation, Hôpitaux Universitaires de Strasbourg, Strasbourg; Samira Fafi-Kremer, Laboratoire de Virologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Marc Hazzan, Service de Néphrologie, Hôpital Huriez, Lille; Anglicheau Dany, Service de Néphrologie et Transplantation Adultes, AP-HP, Hôpital Necker, Paris; Alexandre Hertig, Jérôme Tourret, Benoit Barrou, Service de Néphrologie, AP-HP, Hôpital La Pitié Salpétrière, Paris; Lionel Couzi, Pierre Merville, Anna Kaminski, Service de Néphrologie—Transplantation—Dialyse, Hôpital Pellegrin, Bordeaux; Valérie Moal, Tristan Legris, Service de Néphrologie et Transplantation, AP-HM, Hôpital de la Conception, Marseille; Pierre-François Westeel, Maïté Jaureguy, Service de Néphrologie, CHU Amiens Picardie, Amiens; Luc Frimat, Service de Néphrologie, CHRU Nancy, Vandoeuvre; Didier Ducloux, Jamal Bamoulid, Service de Néphrologie, Hôpital Jean-Minjoz, Besancon; Dominique Bertrand, Service de Néphrologie, CHU de Rouen, Rouen; Michel Tsimaratos, Florentine Garaix-Gilardo, Service de Pédiatrie Multidisciplinaire, Hôpital La Timone, Marseille; Jérôme Dumortier, Service d’Hépato-Gastroentérologie, Hôpital Edouard Herriot, Lyon; Sacha Mussot, Antoine Roux, Centre chirurgical Marie Lannelongue, Le Plessis Robinson; Laurent Sebbag, Service d’insuffisance cardiaque, Hôpital Louis Pradel, Bron; Yannick Le Meur, Service de Néphrologie, Hôpital de la cavale blanche, Brest; Gilles Blancho, Christophe Masset, Service de Néphrologie—Transplantation, Hôtel Dieu, Nantes; Nassim Kamar, Service de Néphrologie et Transplantation, Hôpital Rangueil, Toulouse; Hélène Francois, Eric Rondeau, Service de Néphrologie, Dialyse et Transplantation, AP-HP, Hôpital Tenon, Paris; Nicolas Bouvier, Service de Néphrologie, Dialyse, Transplantation Rénale, CHU, Caen; Christiane Mousson, Service de Néphrologie, Dijon; Matthias Buchler, Philippe Gatault, Service de Néphrologie, Tours; Jean-François Augusto, Agnès Duveau, Service de Néphrologie, Dialyse, Transplantation, CHU Angers, Angers; Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Service de Néphrologie, CHU de Rennes, Rennes; Philippe Grimbert, Marie Matignon, Antoine Durrbach, Service de Néphrologie, Hôpital Henri-Mondor, Creteil; Clarisse Greze, Service de Néphrologie, AP-HP, Hôpital Bichat Claude Bernard, Paris; Renaud Snanoudj, Service de Néphrologie, Hôpital Foch, Service de Néphrologie et Transplantation Hôpital du Kremlin Bicêtre, Le Kremlin Bicetre; Charlotte Colosio, Betoul Schvartz, Service de Néphrologie, Hôpital Maison Blanche, Reims; Paolo Malvezzi, Service de Néphrologie, Hémodialyse, Transplantation rénale, Hôpital La Tronche, Grenoble; Christophe Mariat, Service de Néphrologie, CHU de Saint Etienne, Saint Etienne; Antoine Thierry, Service de Néphrologie, Hémodialyse et Transplantation rénale, Hôpital Jean Bernard, Poitiers; Moglie Le Quintrec, Service de Néphrologie-Transplantation-Dialyse, CHU Lapeyronie, Montpellier; Antoine Sicard, Service de Néphrologie, Hôpital Pasteur, Nice; Jean Philippe Rerolle, Service de Néphrologie, CHU Dupuytren, Limoges; Anne-Élisabeth Heng, Cyril Garrouste, Service de Néphrologie, CHU Gabriel Montpied, Clermont-Ferrand; Henri Vacher Coponat, Service de Néphrologie, CHU de La Réunion, Saint Denis; Éric Epailly, Service de Cardiologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Olivier Brugiere, Service d’hépatologie, Hôpital Foch, Suresnes; Sébastien Dharancy, Service d’hépatologie, Hôpital Huriez, Lille; Éphrem Salame, Service de Chirurgie Hépatique, Hôpital Universitaire de Tours, Tours; and Faouzi Saliba, Service d’Hépatologie, Centre Hépato-Biliaire Paul Brousse, Villejuif.
Disclosure
All the authors declared no competing interests.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization https://www.who.int Available at: Accessed January 10, 2020.
- 3.European Centre for Disease Prevention and Control. COVID-19 situation update worldwide https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases Available at: Accessed July 17, 2020.
- 4.Bernard Stoecklin S., Rolland P., Silue Y. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25(6):2000094. doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonelli M., Wiebe N., Knoll G. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 6.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382:2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson E.J., Walker A.J., Bhaskaran K. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020:584:430–436 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agence de la biomédecine [national regulatory agency] https://www.agence-biomedecine.fr Available at: Accessed March 20, 2020.
- 10.Société francophone de transplantation [The Francophone Society of Transplantation] https://www.transplantation-francophone.org Available at: Accessed March 20, 2020.
- 11.Société francophone de néphrologie dialyse et transplantation. https://www.sfndt.org Available at: Accessed March 20, 2020. [DOI] [PubMed]
- 12.Roques L., Klein E.K., Papaix J., Sar A., Soubeyrand S. Impact of lockdown on the epidemic dynamics of COVID-19 in France. Front Med (Lausanne) 2020;7:274. doi: 10.3389/fmed.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French government https://www.gouvernement.fr/info-coronavirus/carte-et-donnees Available at: Accessed May 20, 2020.
- 14.Caillard S., Anglicheau D., Matignon M. An initial report from the French SOT COVID Registry suggests high mortality due to Covid-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaunat O. Finding the safe place between the hammer and the anvil: sounding the depth of therapeutic immunosuppression. Kidney Int. 2015;88:1226–1228. doi: 10.1038/ki.2015.268. [DOI] [PubMed] [Google Scholar]
- 16.Chen C.C., Koenig A., Saison C. CD4+ T cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Front Immunol. 2018;9:275. doi: 10.3389/fimmu.2018.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cravedi P., Suraj S.M., Azzi Y. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20:3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravanan R., Callaghan C.J., Mumford L. SARS-CoV-2 infection and early mortality of wait-listed and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395:1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav A, Caldararo K, Singh P. Optimising the use of telemedicine in a kidney transplant programme during the coronavirus disease 2019 pandemic [e-pub ahead of print]. J Telemed Telecare.https://doi.org/10.1177/1357633X20942632. Accessed October 10, 2020. [DOI] [PMC free article] [PubMed]
- 21.Kumar D., Manuel O., Natori Y. COVID-19: A global transplant perspective on successfully navigating a pandemic. Am J Transplant. 2020;20:1773–1779. doi: 10.1111/ajt.15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loupy A., Aubert O., Reese P.P. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395:e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]