Abstract
The early postpartum period is crucial for mothers who have a complicated delivery due to preeclampsia. In mothers with symptoms of COVID-19, there may be severe and sometimes fatal consequences. We report the first maternal death in Balouchestan (Iran) due to complicated delivery with preeclampsia concomitant with COVID-19 postpartum. The patient was asymptomatic for COVID-19 during the delivery and rapidly progressed to severe respiratory distress and coagulopathy in the early postpartum period. Mothers with preeclampsia features may be at risk for severe COVID-19, and detailed assessments are essential for these patients during the COVID-19 pandemic.
Keywords: COVID-19, Preeclampsia, Pregnancy, Hypertension
1. Introduction
Due to the physiological changes in the immune and cardiopulmonary systems, pregnant women are more likely to develop severe illness after infection with respiratory viruses such as COVID-19 [1]. Women infected by COVID-19 had higher rates of preeclampsia [2]. Features of Preeclampsia could be present in some pregnancies with a severe course of COVID-19, that might be distinguished from actual preeclampsia by a more detailed investigation [3]. Herein we present a case report of a complicated postpartum preeclampsia concomitant with COVID-19 that developed rapidly with severe symptoms a few days after vaginal delivery.
Fig. 1.

Patient′s CT Scan.
2. Methods
2.1. Case presentation
A 19-year-old primigravida mother with the gestational age of 38 weeks (GA = 38 W), was presented to the referral center in Iranshahr (IRAN) with a referral letter from primary health care (PHC) due to her high blood pressure (BP = 140/90 mm Hg.min) on May 16th, 2020, at 01:10 pm. At the first assessment, the vital signs were normal BP = 140/90 mm Hg. In the vaginal examination, the cervix closed, and there were no uterine contractions. She was admitted to the maternity ward for vaginal delivery. Since the hospital protocol dictated testing at risk patients such as pregnant women for the COVID-19, she was tested for symptoms of COVID-19, which proved to be insignificant. Also, her physical examination was normal, and the patient's history was unremarkable. She had no complaints of headache, blurring of vision, diplopia, or epigastric pain and Laboratory investigation were as: Urine protein = 3+, hemoglobin (Hb = 9/5), platelet blood count (PLT:319), lactate dehydrogenase (LDH:687), blood urea nitrogen (BUN = 10), creatinine (Cr:0.7), prothrombin time (PT = 12), partial thromboplastin time (PTT:40), international normalized ratio (INR:1), aspartate aminotransferase test (AST:28), alanine transaminase (ALT:25) . Her treatment started by Sulfate therapy, so a Foley catheter was fixed, and a double-sided bed guard was installed on her bed. Blood pressure control was done by Amp labetalol 20 mg, and to terminate the pregnancy, 50 µg misoprostol administered sublingually.
After 8 h, at 09:30 pm, labor induction started with oxytocin while the fetal heart was being monitored. Vaginal delivery took place at 5 am, on May 17th; the placenta was expelled, and a healthy baby was born. The vaginal bleeding was normal, the uterus was well contracted, and vital signs were within the normal range. On May 19th, laboratory investigation was normal, BP was 130/80 mmHg, and she had no symptoms of COVID-19. Therefore, after receiving a prescription for antihypertensive medication to control her BP she was discharged from the hospital in a well, stable condition.
She came back to the same center Four days after delivery on May 23rd, at 11:00 am with chief complaints of shortness of breath, chest pain, headache, and fatigue in the past two days. In the beginning, vital signs were: BP: 170/80 mmHg, PR: 104 bpm, RR:20 per/min, T:37/6℃, and Spo2 84%. After counseling with an infectious disease specialist, a gynecologist, and an anesthesiologist, the infectious specialist suggested the risk of COVID-19. She was admitted to the isolation ward of the coronavirus at 11:30 am. Samples were collected from the nasopharyngeal swab to test for COVID-19, and laboratory reports indicated the ESR level of 66. Therefore, O2 therapy was started using a mask at the range of 6–8 L per min, and to control her BP, amlodipine 5 mg twice a day was prescribed. The chest CT revealed ground-glass opacity of both lungs and bilateral pleural effusion. The treatment started immediately with Cap Kaletra 200 mg for five days-BD, Cap azithromycin (500 mg on day 1, then 250 mg day 2–5), Tab hydroxychloroquine 600 mg/day for ten days, Amp apotel 150 mg/ml, 6/7 ml TDS, Amp pantoprazole 40 mg IV, and O2 therapy with a mask (6–8 L per min). Blood pressure control proved to be effective .
Table 1.
Laboratory reports of the patient according to the days of hospitalization.
| Measure | US Units | SI Units | Reference Range | 16th May | 17thMay | 18thMay | 19th May | 23rd May | 24th May | 25th May | 26th May | 27th May | 28th May | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Virology | Covid19 | positive | ||||||||||||
| CBC | WBC | ×103/mm3 | ×109/liter | 4–10 | 9.8 | 7 | 7.5 | 9 | 11.5 | 7.7 | 11.8 | 9 | 11.5 | 12.5 |
| RBC | ×106/mm3 | ×1012/liter | 4.2–5.4 | 4.2 | 3.9 | 4.5 | 4.9 | ‘4,32 | 4.7 | 3.15 | 4.15 | 3.4 | 3.8 | |
| Hemoglobin | g/dl | ×0.6206 mmol/liter |
10–16 | 9.5 | 8.6 | 8.6 | 8.9 | 9.1 | 8.6 | 8.6 | 8.1 | 7.4 | 7.0 | |
| Hematocrit | % | × 0.01 | 35–40 | 28.2 | 25.8 | 24.3 | 27.2 | 28.2 | 26.3 | 27 | 24.5 | 22.2 | 22.3 | |
| Platelets | µl | ×106 L | 150–450 | 319 | 309 | 304 | 336 | 332 | 220 | 243 | 225 | 213 | 206 | |
| WBC Differential | Neut | % | × 0.01 | 45–75 | – | – | – | – | 79.0 | 77.1 | 87.5 | 70 | 80.7 | 90.3 |
| Lymph | % | × 0.01 | 16–46 | – | – | – | – | 10.4 | 15.6 | 11 | 13 | 15 | 8.4 | |
| Biochemistry | B.U.N | mg/dl | × 0.357 mmol/liter | 10–20 | 10 | 12 | 10 | 14 | 12 | 12 | 13.5 | 14 | 17 | 18 |
| Creatinine | mg/dl | × 88.42 µmol/liter | 0.7–1.4 | 0.7 | 0.8 | 0.8 | 0.7 | 0.5 | 0.7 | 0.9 | 0.6 | 0.6 | 0.5 | |
| Na-serum | mmol/liter | mmol/liter | 135–145 | – | – | – | – | 139 | 135 | 135 | 132 | 134 | 139 | |
| K-serum | mmol/liter | mmol/liter | 3.8–5 | … | – | – | – | 3.4 | 3.9 | 3.4 | 3.4 | 3.8 | 4.6 | |
| Blood Sugar | mg /dl | ×0.0555 mmol/liter |
70–130 | 95 | 105 | 108 | 88 | 100 | – | – | 105 | – | – | |
| S.G.OT (AST) | U/l | × 0.016675 µkat/liter |
<31 | 28 | 26 | 30 | 27 | – | – | – | 44 | 43 | 45 | |
| S.G.P.T (ALT) | U/l | × 0.016675 µkat/liter |
<31 | 25 | 25 | 27 | 29 | – | – | – | 104 | 98 | 95 | |
| Bilirubin total | mg/dl | × 17.1 µmol/liter |
0.3–1 | 0.6 | 0.6 | 0.6 | 0.5 | – | – | – | – | – | – | |
| Bilirubin Direct | mg/dl | × 17.1 µmol/liter |
0.1–0.3 | 0.2 | 0.2 | 0.1 | 0.2 | – | – | – | – | – | – | |
| Coagulation Test | PT | second | Second | 13–15 | 12 | 12.6 | 13 | 13.5 | 12 | 13 | 13.3 | 15 | 16.7 | 15 |
| PTT | second | Second | 25–42 | 40 | 29 | 33 | 26 | 37 | 28 | 37 | 46 | 61 | 33 | |
| I.N.R | Ratio | Ratio | 1.0–1.4 | 1 | 1 | 1.2 | 1 | 1 | 1.2 | 1.2 | 1.2 | 1.3 | 1.4 | |
| Immunology & Serology | ESR | mm/hr | mm/hr | 15–20 | _ | _ | _ | _ | – | – | – | – | – | – |
| C-Reaction Protein | mg/dl | × 95.238 nmol/liter |
<10 | – | – | – | – | 3+ | – | _ | 3+ | _ | _ |
On May 24th, vital signs were as BP:130/80, PR:82, RR:18 per/min, T: 36.8℃. The O2 saturation was 83% without O2 therapy. She was able to communicate and had a complaint of fatigue, while the result of RT-PCR was positive for COVID-19. On May 25th, the patient was having a fever (T: 39.8 ℃), which was controlled with Amp apotel 150 mg/ml, and the other vital signs were as BP:110/70 mmHg, PR: 88 bpm, RR: 25 per/min. On May 26th, at 5 pm, the patient developed severe respiratory distress, and SPO2 was 63%. Subsequently, the patient was transferred to ICU, and her vital signs were as: BP: 90/60 mmHg, PR: 87 bpm, RR: 30 per/min, and T: 37/2 ℃. While she was under cardiopulmonary monitoring, she was conscious, and the repeated ABG revealed PH:7/414, PCO2: 32.5 mmHg, PO2: 37 mmHg. Amp Potassium chloride 20 mEq, Salbutamol spray 200 metered doses, Amp hydrocortisone, and serum therapy was performed
Table 2.
Patient′s symptoms according to the date of hospitalization.
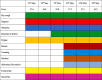 |
On the 27th, despite treatments, uncompensated metabolic acidosis led to a downturn in the patient's condition. The heart rhythm was sinus tachycardia, and the liver investigation was impaired, while PTT level was elevated. On May 28th, at 1:40 am, O2 saturation progressively decreased, and the patient needed intubation. ABG revealed PH = 7.419, PCO2 = 35.7 mmHg, PO2: 17 mmHg. At 2 am, the patient became intubated, put on a ventilator, and oxygen was given with Bag-Valve-Mask. At 6 am, the patient developed cardio-respiratory arrest and asystole. Unfortunately, after 45 min of CPR, and despite all efforts, the patient died.
3. Discussion
Pregnant women with severe COVID-19 can develop preeclampsia features that might be distinguished from actual preeclampsia by a more detailed assessment. It might not be a placental complication and could resolve spontaneously after recovery from severe pneumonia [3]. Severe COVID-19 could induce an unstable hemostatic leading to a hypercoagulable state as in other sepsis [4]. Sine COVID-19 and preeclampsia have overlapping clinical features; the differential diagnosis demands careful assessment in pregnant women presenting with hypertension, thrombocytopenia, or elevated liver enzymes [3]. Laboratory findings can be reminiscent of HELLP syndrome. Accordingly, measurements of prothrombin time, platelet count, and D-dimer in all patients presenting with COVID-19 may help risk stratification [5]. This knowledge could improve management and reduce misdiagnosis in pregnancies concomitant with COVID-19. Healthcare providers are warning to monitor pregnancies with suspected preeclampsia with more caution [3].
4. Conclusion
Early postpartum is crucial for mothers who have complicated delivery with preeclampsia, especially in those with symptoms of COVID-19 or even probably in asymptomatic COVID-19 carriers. It should be noted that there is a risk associated with COVID- 19 infection and maternal deterioration to severe respiratory distress and coagulopathy state in the postpartum period, especially early postpartum complicated with Preeclampsia features and high blood pressure. Therefore, a detailed investigation and multi-specialty team are required for all mothers with such features for comprehensive care during the COVID-19 pandemic.
Acknowledgments
Acknowledgments
We would like to thank Dr. Mohammad Mehran Aminifard, vice-chancellor and Dr. Asiye Dejkam, deputy of education of Iranshahr Medical University, for their kind support. We would also like to thank Rashideh Ghaderi and Asiye Damani personnel of the nursing and midwifery office of Iran and Khatam Hospitals for their kind assistance with this project.
Authorship contributions
All authors agreed to be accountable for all aspects of this report. Dr. B.S, Dr. B.P, and Dr. Z.M who were involved in patient management during her hospitalization. Also read and approved the report. Other authors involved in patient management and witting the first draft. All authors made critical revisions and approved the final manuscript.
Disclosure statement
None of the authors has a conflicting financial or propriety interest to disclose.
Funding sources
The authors received no specific grant from funding agencies in public, commercial or not-for-profit sectors for authorship and publication.
Ethical approval
This report study was approved by the Regional Ethics Committee at Iranshahr University of Medical Sciences. The Registration Code is IR.IRSHUMS.REC.1399.017. The written consent was obtained from the patient’s guardian.
References
- 1.Poon L.C., Yang H., Lee J.C.S., Copel J.A. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy, and puerperium: information for healthcare professionals. Ultrasound in Obstetrics Gynecol. 2020 doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di M.D., Khalili A., Saccone G., Rizzo G. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. American Journal of Obstetrics & Gynecology MFM. 2020;2(2) doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOGAn International Journal of Obstetrics & Gynaecology. 2020 doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pranata R., Huang I., Lim A.M., Wahjoepramono E.J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19 systematic review, meta-analysis, and meta-regression. Journal of Stroke and Cerebrovascular Diseases. 2020;29(8):104949. doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koumoutsea E.V., Vivanti A.J., Shehata N., Benachi A. COVID19, and acute coagulopathy in pregnancy. J. Thromb. Haemost. 2020;18(7):1648–1652. doi: 10.1111/JTH.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]


