Abstract
Background
Thymosin α1 therapy was commonly used in patients with coronavirus disease 2019 (COVID-19), while its impact on outcomes and which patients could benefit from thymosin α1 therapy were uncertain.
Study design and methods
Patients with COVID-19 from 19 designated hospitals between January 1 to February 29, 2020 were included, and the main exposure of interest was administration of thymosin α1. The primary outcome was 28-day mortality. Propensity score matching (PSM) was used to account for baseline confounders, cluster analysis and Cox proportional hazard model was used to account for subgroup analysis.
Results
A total of 771 patients were included, and 327/771 (42.4%) patients received thymosin α1 therapy. The 28-day mortality in thymosin group was significantly lower than that in control group (41.3% vs. 60.6%, p < 0.001). After PSM 522 patients were included in analysis and the 28-day mortality in thymosin α1 group and control group were 51.0% and 52.9% respectively, with no significant difference. In subgroup analyses, the association between thymosin α1 therapy and 28-day mortality appeared to be stronger among male patients (HR 0.673, 95% CI 0.454–0.998; p = 0.049). There were no benefits of thymosin α1 in 28-day mortality in other subgroups. There were two phenotypes after cluster analysis, but no benefits of thymosin α1 were shown in phenotype 1 (HR 0.823 95% CI 0.581–1.166; p = 0.273) and phenotype 2 (HR 1.148 95% CI 0.710–1.895; p = 0.442).
Conclusion
There was no association between use of thymosin α1 and decreased mortality in critically ill COVID-19 patients. Subgroups analysis and phenotype analysis also showed no differences on mortality after thymosin α1 therapy.
Keywords: COVID-19, Thymosin α1, Mortality
Abbreviation
- COVID-19
coronavirus disease 2019
- PSM
propensity score matching
- NCP
novel coronavirus pneumonia
- LCA
latent class analysis
- ARDS
acute respiratory distress syndrome
- ICU
intensive care unit
- HFNO
high flow nasal oxygen
- NIV
noninvasive mechanical ventilation
- IMV
invasive mechanical ventilation
- SOFA
Sequential Organ Failure Assessment
- PaO2/FiO2
ratio of arterial oxygen partial pressure to inhaled oxygen concentration
- IQR
interquartile range
- RR
respiratory rate
- LDH
lactate dehydrogenase
- CRP
C-reactive protein
- IL-6
interleukin-6
- INR
international normalized ratio
- PT
prothrombin time
- APTT
activated partial thromboplastin time
- SMD
standardized mean difference
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CRRT
continuous renal replacement therapy
- ECMO
Extracorporeal Membrane Oxygenation
- HR
heart rate
1. Background
Since December 2019 a cluster of patients of pneumonia caused by COVID-19 have been identified in Wuhan and soon this virus spread at a tremendous rate which swept through the whole world [1], [2], [3]. Many studies have showed significant decrease of lymphocyte counts, which demonstrated probable immunosuppression in critically ill COVID-19 patients [1], [2], [3]. Recent studies have also reported that T lymphocyte were correlated with the severity of COVID-19 and had a good predictive value for NCP [4], [5]. Therefore immunotherapy focusing on T cells might have potential benefits for NCP patients.
During the therapy of COVID-19 patients, many immunomodulatory drugs were used guided by clinicians’ experience and thymosin α1 was one of them. Thymosin α1 is an acidic peptide purified from calf thymus tissue and has an acetylated aminoterminus, which is a potent inducer of T cells [6]. In previous study, thymosin α1 could acted as an immune modulator, exerted great biological influence in activating and regulating the function of immune system. When pathogens invaded, innate immunity was the first defense mechanism to protect our body from infection and cellular injury [7]. Notably, a multicenter RCT shows thymosin α1 therapy could lower short-term, all-cause mortality and promote the percentage of HLA-DR antigen expressed on lymphocytes in patients with severe sepsis [8]. Many COVID-19 patients received thymosin α1 therapy guided by clinicians’ experience, however, there was no study exploring the effects of thymosin α1 on COVID-19 patients’ outcome.
Different combinations of clinical and biological parameters may cluster into novel phenotypes that may respond differently to treatments [9], [10]. Unsupervised machine learning approaches such as k-means and latent class analysis (LCA) have been applied to identify distinct phenotypes in sepsis, acute respiratory distress syndrome (ARDS) and other critical illnesses [11], [12], [13]. Similarly, it is reasonable to explore the therapy response of thymosin α1 to patients with different clinical phenotypes of COVID-19.
In this multicenter retrospective study, we aimed to elucidate the association between thymosin α1 therapy and 28-day mortality in patients with COVID-19. We hypothesized that specific clinical phenotypes of COVID-19 might benefit from thymosin α1 therapy.
2. Methods
2.1. Study design and participants
We performed a retrospective cohort study which was conducted in 19 designated hospitals for COVID-19 in Wuhan (Hubei Province), Huangshi (Hubei Province), Shenzhen (Guangdong Province), and Jiangsu Province. The study was approved by the ethics committee of Jin Yin-tan Hospital (KY-2020-10.02). Written informed consent was waived by the Ethics Commission because of the outbreak of COVID-19. Patient-level informed consent was not required.
All adult patients with COVID-19 who were admitted to intensive care units (ICUs) of the participating hospitals between January 1 to February 29, 2020 were screened. inclusion criteria were: (1) > 18 years of age; (2) laboratory-confirmed diagnosis of COVID-19 [14]; (3) severe respiratory failure requiring advanced respiratory support (i.e. high flow nasal oxygen [HFNO], noninvasive mechanical ventilation [NIV], and invasive mechanical ventilation [IMV]), circulatory shock, or multiorgan failure.
2.2. Data collection and outcome
Demographic data, chronic comorbidities, vital signs and laboratory results within the first 24 h after hospital admission were extracted from electronic medical records. Treatment and outcome data were also recorded. Sequential Organ Failure Assessment (SOFA) scores were calculated to assess the severity of illness by using data from the first 24 h after admission. In cases when arterial blood gas analysis was not available, PaO2/FiO2 ratio was calculated based on Rice equation [15]. The main exposure of interest was the administration of thymosin therapy. All data were collected by using a case record form modified from the standardized International Severe Acute Respiratory and Emerging Infection Consort. The primary outcome in the present study was 28-day mortality.
2.3. Statistical analysis
Values were presented as the mean (standard deviation) or median (interquartile range (IQR)) for continuous variables as appropriate and as the total number (percentage) for categorical variables. Comparisons between groups were made using the chi-square test or Fisher’s exact test for categorical variables and Student’s t test or Mann-Whitney U test for continuous variables as appropriate.
PSM was used in our study to decrease the effects of data bias and confounding variables. A Cox regression model was also used to characterize the relationship between thymosin therapy and 28-day mortality. Given the previous studies and the number of events, baseline variables that were considered clinically relevant or that showed a univariate relationship with the outcome (p < 0.20) were entered into the multivariate Cox proportional-hazards regression model as baseline confounders.
2.4. Cluster analysis
To derive the phenotypes, we selected 8 markers including Lymphocyte, CRP, IL-6, PT, APTT, D-Dimer, RR, PaO2/FiO2 based on previous research and their association with outcomes. The missing data were summarized in the supplement (e-Table 1), and the missing values were obtained with the multiple imputation method. Standardized transformation was used for the dataset, additionally, non-normally distributed variables were log-transformed prior to standardized transformation. Gap Statistics, calinsky criterion and average silhouette method were used to determine the optimal number of phenotypes. Once the optimal number was determined, we applied consensus k means to identify phenotypically-distinct categories in patients with COVID-19. The standardized mean differences (SMDs) and p values were calculated to evaluate the differences between groups or phenotypes, and p < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 software (IBM, Somers, NY) or RStudio (version 1.2.5019).
3. Results
3.1. Baseline characteristics
During the study period, a total of 771 critically ill patients with COVID-19 were included in the final analysis. Of the study cohort, 327 (42.4%) patients received thymosin α1 therapy while hospitalized and the rest 444 (57.6%) patients were in control group. The characteristics of the thymosin group and control group were presented in Table 1 . In general, patients in thymosin group were younger (64 (IQR 55–71) vs. 66 (IQR 57–74); p = 0.011), had a significantly lower SOFA score (3 (IQR 1–4) vs. 4 (IQR 2–5); p < 0.001), APAPCHE II score (9 (IQR 6–13) vs. 11 (IQR 8–15); p < 0.001), and were characterized by higher levels of PaO2/FiO2 ratio, lymphocytes, albumin and lower levels of RR, bilirubin, LDH, CRP, PCT, IL-6, D-dimer and troponin when compared with control group (Table 1). During the stay in ICU, more patients in thymosin group received antivirus agent therapy (87.5% vs. 71.6%, p < 0.001), and the percentages of patients receiving NIV, CRRT or ECMO were higher than those in control group (Table 2 ).
Table 1.
Clinical characteristics of patients in Thymosin group and control group.
| Variables | Total (n = 771) | Thymosin group (n = 327) |
Control group (n = 444) |
p-value |
|---|---|---|---|---|
| DEMOGRAPHIC | ||||
| Age, median (IQR), year | 65 (56–72) | 64 (55–71) | 66 (57–74) | 0.011 |
| Male, n (%) | 486 (63.0) | 202 (61.8) | 284 (64.0) | 0.534 |
| COMORBIDITIES | ||||
| Hypertension, n (%) | 328 (42.5) | 140 (42.8) | 188 (42.3) | 0.896 |
| Diabetes, n (%) | 141 (18.3) | 61 (18.7) | 80 (18.0) | 0.821 |
| Coronary heart disease, n (%) | 99 (12.8) | 44 (13.5) | 55 (12.4) | 0.661 |
| COPD, n (%) | 39 (5.1) | 14 (4.3) | 25 (5.6) | 0.398 |
| Heart failure, n (%) | 35 (4.5) | 10 (3.1) | 25 (5.6) | 0.090 |
| Chronic kidney disease, n (%) | 17 (2.2) | 4 (1.2) | 13 (2.9) | 0.111 |
| CLINICAL FEATURES AND CLINICAL FINDINGS | ||||
| Fever, n (%) | 663 (86.0) | 276 (84.4) | 387 (87.2) | 0.275 |
| Cough, n (%) | 560 (72.6) | 215 (65.7) | 345 (77.7) | < 0.001 |
| Dyspnea, n (%) | 461 (59.8) | 153 (46.8) | 308 (69.4) | < 0.001 |
| Diarrhea, n (%) | 90 (11.7) | 27 (8.3) | 63 (14.2) | 0.011 |
| Fatigue, n (%) | 408 (52.9) | 148 (45.3) | 260 (58.6) | < 0.001 |
| RR, median (IQR) | 23 (20–29) | 22 (20–26) | 24 (20–30) | < 0.001 |
| PaO2/FiO2 ratio, median (IQR), mmHg | 94.4 (41.6–205.5) | 118.3 (44.2–295.0) | 76.9 (38.1–166.4) | < 0.001 |
| MAP, median (IQR), mmHg | 94.7 (86.7–102.8) | 95.3 (88.3–102.7) | 94.0 (85.7–103.0) | 0.293 |
| Lymphocytes, median (IQR), ×109/L | 0.6 (0.4–0.9) | 0.7 (0.5–1.0) | 0.6 (0.4–0.9) | < 0.001 |
| Bilirubin, median (IQR), umol/L | 12.9 (9.3–19.6) | 11.9 (8.7–17.9) | 13.7 (9.6–20.8) | 0.002 |
| Albumin, median (IQR), g/L | 30.3 (27.0–35.0) | 32.2 (28.0–38.2) | 29.4 (26.6–32.2) | < 0.001 |
| LDH, median (IQR), U/L | 469.5 (329.3–622.8) | 438.0 (299.0–596.0) | 491.0 (351.0–647.0) | 0.016 |
| CRP, median (IQR), mg/L | 80.5 (32.2–141.2) | 60.1 (24.5–116.5) | 92.0 (45.9–151.3) | < 0.001 |
| PCT, median (IQR), ng/ml | 0.2 (0.1–0.5) | 0.1 (0.1–0.4) | 0.2 (0.1–0.6) | < 0.001 |
| IL-6, median (IQR), U/ml | 13.1 (8.3–29.5) | 14.8 (8.4–25.9) | 12.4 (8.3–35.1) | < 0.001 |
| INR, median (IQR) | 1.1 (1.0–1.2) | 1.1 (1.0–1.2) | 1.1 (1.0–1.3) | 0.235 |
| PT, median (IQR), s | 13.6 (12.0–15.3) | 13.1 (11.9–14.8) | 14.0 (12.0–15.5) | 0.001 |
| APTT, median (IQR), s | 33.7 (27.5–40.2) | 33.6 (28.0–37.9) | 33.8 (26.6–41.3) | 0.814 |
| D-dimer, median (IQR), ug/L | 2.9 (0.7–9.6) | 1.4 (0.5–7.5) | 3.8 (1.0–14.5) | < 0.001 |
| Serum creatine, median (IQR), μmol/L | 71.5 (57.0–94.0) | 69.0 (56.0–88.8) | 74.0 (59.4–98.0) | 0.027 |
| Troponin, Median (IQR), pg/ml | 26.0 (10.0–98.8) | 15.5 (9.0–41.0) | 30.0 (10.1–134.8) | < 0.001 |
| SEVERITY and OUTCOME | ||||
| SOFA score, median (IQR) | 3 (2–5) | 3 (1–4) | 4 (2–5) | < 0.001 |
| APACHE II score, median (IQR) | 10 (7–14) | 9 (6–13) | 11 (8–15) | < 0.001 |
| 28-day mortality | 404 (52.4) | 135 (41.3) | 269 (60.6) | < 0.001 |
COPD = chronic obstructive pulmonary diseases; RR = respiratory rate; PaO2/FiO2 ratio = Ratio of arterial oxygen partial pressure to inhaled oxygen concentration; MAP = mean arterial pressure; LDH = lactate dehydrogenase; CRP = C-reactive protein; PCT = procalcitonin; IL-6 = interleukin-6; INR = international normalized ratio; PT = prothrombin time; APTT = activated partial thromboplastin time; SOFA = Sequential Organ Failure Assessment; APACHE II = Acute Physiology and Chronic Health Evaluation II.
Table 2.
Treatments during ICU stay in Thymosin group and control group.
| Variables | Total (n = 771) | Thymosin group (n = 327) |
Control group(n = 444) | p-value |
|---|---|---|---|---|
| Any antivirus agent, n (%) | 604 (78.3) | 286 (87.5) | 318 (71.6) | < 0.001 |
| NIV, n (%) | 392 (50.8) | 185 (56.6) | 207 (46.6) | 0.006 |
| IMV, n (%) | 328 (42.5) | 145 (44.3) | 183 (41.2) | 0.386 |
| Vasoactive drugs, n (%) | 325 (42.2) | 129 (39.4) | 196 (44.1) | 0.192 |
| CRRT, n (%) | 106 (13.7) | 60 (18.3) | 46 (10.4) | 0.002 |
| ECMO, n (%) | 43 (5.6) | 27 (8.3) | 16 (3.6) | 0.005 |
NIV = Noninvasive mechanical ventilation; IMV = Invasive mechanical ventilation; CRRT = continuous renal replacement therapy; ECMO = Extracorporeal Membrane Oxygenation.
3.2. Outcome
The overall 28-day mortality of all patients was 52.4%, while in thymosin group the mortality was 41.3%, which was significantly lower than that in control group (41.3% vs. 60.6%, p < 0.001) (Table 1). After adjustment for baseline confounders (age, sex, hypertension, diabetes, SOFA score, PaO2/FiO2, RR, lymphocyte, LDH, hs-TnI, D-dimer, antiviral agent use, gamma-globulin use, glucocorticoid use), the Cox proportional hazard model showed that thymosin α1 therapy wasn’t associated with decreased 28-day mortality in the overall population (HR 0.885, 95% CI 0.647–1.209; p = 0.150) (Table 3 ). In subgroup analyses, the association between thymosin therapy and 28-day mortality appeared to be stronger among male patients (HR 0.673, 95% CI 0.454–0.998; p = 0.049). There were no benefits of thymosin α1 in 28-day mortality in other subgroups (Table 3).
Table 3.
Subgroup analyses of the association between Thymosin therapy and 28-day mortality.
| Variable | No.of patients | Adjusted HR (95%CI) | lower | upper | p value |
|---|---|---|---|---|---|
| All | 771 | 0.885 | 0.647 | 1.209 | 0.150 |
| Sex | |||||
| Male | 486 | 0.673 | 0.454 | 0.998 | 0.049 |
| Female | 285 | 1.145 | 0.650 | 2.019 | 0.355 |
| Age | |||||
| ≤65 | 401 | 0.802 | 0.491 | 1.309 | 0.377 |
| >65 | 370 | 1.087 | 0.706 | 1.674 | 0.704 |
| Comorbidity | |||||
| Hypertension | 328 | 1.129 | 0.721 | 1.768 | 0.595 |
| Diabetes | 141 | 1.312 | 0.608 | 2.831 | 0.489 |
| SOFA | |||||
| ≤4 | 504 | 1.070 | 0.702 | 1.631 | 0.753 |
| >4 | 219 | 0.748 | 0.447 | 1.253 | 0.270 |
| SPO2/FiO2 | |||||
| >150 mmHg | 370 | 0.885 | 0.615 | 1.272 | 0.509 |
| ≤150 mmHg | 207 | 0.982 | 0.458 | 2.104 | 0.963 |
| Lymphocyte | |||||
| >0.6 × 109/L | 348 | 0.765 | 0.469 | 1.249 | 0.285 |
| ≤0.6 × 109/L | 390 | 0.921 | 0.573 | 1.480 | 0.735 |
| IMV | |||||
| Yes | 442 | 0.817 | 0.438 | 1.522 | 0.524 |
| No | 329 | 0.942 | 0.640 | 1.385 | 0.761 |
| Vasoactive drug | |||||
| Yes | 446 | 0.778 | 0.420 | 1.440 | 0.424 |
| No | 325 | 0.923 | 0.628 | 1.357 | 0.683 |
| Thymosin use days | |||||
| <7 | 59 | 1.163 | 0.765 | 1.729 | 0.767 |
| ≥7 | 216 | 0.697 | 0.471 | 1.025 | 0.089 |
| Phenotypes | |||||
| Phenotype 1 | 432 | 0.823 | 0.581 | 1.166 | 0.273 |
| Phenotype 2 | 339 | 1.148 | 0.710 | 1.895 | 0.442 |
SOFA = Sequential Organ Failure Assessment; PaO2/FiO2 ratio = Ratio of arterial oxygen partial pressure to inhaled oxygen concentration; IMV = Invasive mechanical ventilation.
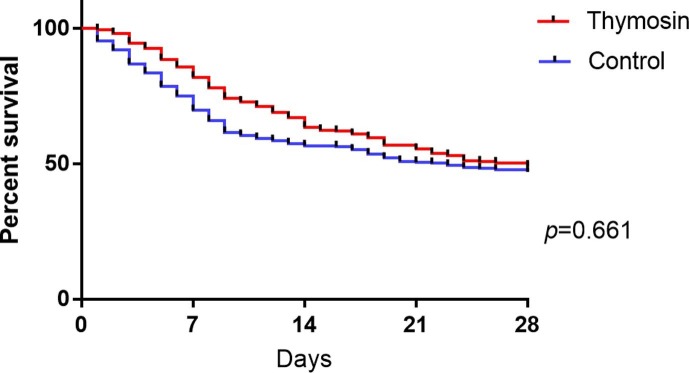
Considering the data bias and confounding variables, PSM was used to adjust baseline characteristics including age, sex, comorbidities, SOFA, HR, RR, PaO2/FiO2, lymphocyte, LDH, bilirubin, troponin and D-dimer. After PSM, there were 261 patients in each group and there were no significant differences of baseline characteristics in two groups (eTable 1). However, after PSM the 28-day mortality in thymosin group and control group were 51.0% and 52.9% respectively, without significant difference (Fig. 1 ).
Fig. 1.
Probability of Survival and Subgroup Analyses of the Risk of Death at 28 Days after PSM.
3.3. Cluster analysis
The consensus k means clustering models based on lymphocyte, CRP, IL-6, PT, APTT, D-dimer, RR, PaO2/FiO2 found that a 2-class model was the optimal fit with the 2 distinct phenotypes of those COVID-19 patients (e-Figure 1). Ultimately, 432 patients were classified as phenotype 1 with more severity and hyperinflammatory and other 339 patients were classified as phenotype 2 with less severity and hypoinflammatory. The differences of clinical characteristics between two phenotypes were in eTable 2. Patients in phenotype 1 was tend to higher SOFA score and APACHE II score. Lymphocyte count, PaO2/FiO2 and albumin were significantly lower while LDH, CRP, IL-6 and DDimer were significantly higher in phenotype 1 when compared with phenotype 2. Unfortunately, after adjusted by baseline confounders (age, sex, hypertension, diabetes, SOFA score, PaO2/FiO2, RR, lymphocyte, LDH, hs-TnI, D-dimer, antiviral agent use, gamma-globulin use, glucocorticoid use), no benefits of thymosin therapy in 28-day mortality were found in each phenotype (phenotype 1, HR 0.823 (95% CI: 0.581–1.166), phenotype 2, HR 1.148 (95% CI: 0.710–1.895); Table 3).
4. Discussion
This study mainly focused on the effects of thymosin α1 therapy on 28-day mortality in critically ill COVID-19 patients. The major findings of our study can be summarized as follows:1) thymosin α1 therapy was not associated with a difference in 28-day mortality in patients with COVID-19 after adjustment for baseline confounders; 2) subgroup analysis and phenotype analysis didn’t show benefits in 28-day mortality with thymosin α1 therapy.
Without substantial evidence, nearly half patients were given thymosin α1 therapy due to decreased lymphocyte and possible immunosuppression in critically ill COVID-19 patients [16]. Previous studies have proved thymosin α1′s effects in sepsis patients [8], [17] and some researches have showed thymosin α1′s efficacy as immunomodulatory treatment by regulating T cell subsets and inflammatory mediators [18]. All these findings indicated potential treatment effects of thymosin in COVID-19 patients. A recent published research showed that thymosin α1 reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells [19]. However, our retrospective study didn’t prove thymosin α1′s benefits of mortality in critically ill COVID-19 patients. This may be resulted from critical condition and complex pathophysiological changes in those patients. In our study, the overall mortality was 52.4% while it was only 21.1% in Yu’s study. Nearly half patients needed invasive mechanical ventilation in our study, which was much higher than that in Yu’s study. Lymphocytes of patients in our study was also much lower, which might indicated more severe immunosuppression. Considering populations’ differences, Yu’s results might proved that thymosin α1 only play roles in less severe subgroup among critically ill COVID-19 patients.
Yao’s study [20] included critically ill COVID-19 patients also showed that treatment with thymosin α1 could markedly decrease 28-day mortality and attenuate acute lung injury in critical type COVID-19 patients. However, after analyzing this research, three points may explain these different results. (1) The patients we included were more severe than Yao’s study (with lower PaO2/FiO2, higher SOFA score and higher mortality). (2) We have included more patients from more designated hospitals which could lead to a larger sample size and better external validation, however heterogeneity of treatments and managements among different hospitals might also lead to bias. (3) All patients in our study received treatments in ICU and nearly half of them needed invasive mechanical ventilation, but in Yao’s study, patients were from 4 ICUs and 4 general wards. Different respiratory support methods might also lead to different therapeutic responses. Since our study was a retrospective study, much heterogeneity of the population existed, we used two methods to adjusted these biases. After PSM or using Cox regression model, results were consistent in our study, which proved the reliability of our conclusion.
The insignificant association between thymosin α1 therapy and mortality cannot exclude the beneficial effect of thymosin α1 therapy among specific patients with COVID-19. However in subgroup analyses no positive findings were shown in our study. We used eight indicators to indentify two phenotypes with different characteristics. However, there was no differences of 28-day mortality between phenotype 1 and phenotype 2. It's worth mentioning that thymosin α-1 was a kind of immunomodulatory treatment, but there weren’t enough immune-related indicators, which made it difficult to figure out a specific group of COVID-19 patients who could benefit from thymosin α1 therapy.
Our study had some limitations. First, the design of our study was a retrospective observational study and we considered only segmental measured confounders. Second, due to some missing data, we couldn’t definite thymosin α1′s effects in immunoregulation clearly. Third, cluster analysis required complete data but we imputed the missing data using statistical methods, which could lead to some bias. Finally, the lack of medical resources was obvious, especially in the early stage of the outbreak, and the management protocol of COVID-19 was changing and treatments in different hospitals varied. Therefore, the effect of thymosin α1 on COVID-19 needs to be explored in future studies.
5. Conclusion
Thymosin α1 therapy was widely used in patients with COVID-19, especially in patients with decreased lymphocyte. After adjustment for baseline confounders, there was no association between use of thymosin α1 and decreased 28 day mortality in critically ill COVID-19 patients, and subgroups analysis also showed no difference on mortality after thymosin α1 therapy.
Declarations
Ethical Approval and Consent to participate
Not available.
Consent for publication
All authors have confirmed the manuscript and approved the publication of the manuscript. The corresponding author has completed the “Consent to publish”.
Availability of data and material
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by grants 2020YFC0841300 and 2020YFC0843700 from the Ministry of Science and Technology of the People’s Republic of China, Key Research and Development Plan of Jiangsu Province (BE2018743 and BE2019749) and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) from Chinese Academy of Medical Sciences (2020-I2M-2-005 and 2019-I2M-1-001).
Authors' contributions
QS, conception and design, data analysis and manuscript writing. JFX, RQZ, XYL and HC, collection and assembly of data and data analysis. BD, HBQ and ZHT, manuscript revision. YY, conception and design, manuscript writing and final approval. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.107143.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of covid-19 in New York City. N Engl J Med. 2020;17 doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Li S.M., Liu J., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;18(55) doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang M., Guo Y., Luo Q., et al. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J Infect Dis. 2020;7 doi: 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClure J.E., Lameris N., Wara D.W., et al. Immunochemical studies on thymosin: radioimmunoassay of thymosin alpha 1. J Immunol. 1982;128(1):368–375. [PubMed] [Google Scholar]
- 7.Pei F., Guan X., Wu J. Thymosin alpha 1 treatment for patients with sepsis. Expert Opin Biol Ther. 2018;18(sup1):71–76. doi: 10.1080/14712598.2018.1484104. [DOI] [PubMed] [Google Scholar]
- 8.Wu J., Zhou L., Liu J., et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care. 2013;17(1):R8. doi: 10.1186/cc11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkerson M.D., Hayes D.N. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics (Oxford, England). 2010;26(12):1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rindskopf D., Rindskopf W. The value of latent class analysis in medical diagnosis. Stat. Med. 1986;5(1):21–27. doi: 10.1002/sim.4780050105. [DOI] [PubMed] [Google Scholar]
- 11.Seymour C.W., Kennedy J.N., Wang S., et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee C.S., Delucchi K., Parsons P.E., et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lanc. Respirat. Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Famous K.R., Delucchi K., Ware L.B., et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 2017;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diagnosis and management protocol of COVID-19 in China. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 15.Rice T.W., Wheeler A.P., Bernard G.R., et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 16.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 2020 03 27;5(1). [DOI] [PMC free article] [PubMed]
- 17.Huanling S, Rongju L, Min J. Effect and analysis of ulinastatin combined with thymosin on cardiopulmonary function and delirium in sepsis patients. Pak J Pharm Sci. 2019 May;32(3 Special):1281-1284. [PubMed]
- 18.Liu F., Wang H.M., Wang T., et al. The efficacy of thymosin α1 as immunomodulatory treatment for sepsis: a systematic review of randomized controlled trials. BMC Infect. Dis. 2016;09(15):16. doi: 10.1186/s12879-016-1823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y.P., Yue P., Zhen H.H., et al. Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M., Ji J.J., Zhong L., et al. Thymosin α1 therapy in critically ill patients with COVID-19: a multicenter retrospective cohort study. Int Immunopharmacol. 2020;6(88) doi: 10.1016/j.intimp.2020.106873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.