Abstract
Objective/Background:
Poor quality and inadequate sleep are associated with impaired cognitive, motor, and behavioral components of sport performance and increased injury risk. While prior work identifies sports-related concussions as predisposing factors for poor sleep, the role of sleep as a sports-related concussion risk factor is unknown. The purpose of this study was to quantify the effect of poor sleep quality and insomnia symptoms on future sports-related concussion risk.
Patients/Methods:
In this study, 190 NCAA Division-1 athletes completed a survey battery, including the Insomnia Severity Index (ISI) and National Health and Nutrition Examination Survey (NHANES) Sleep module. Univariate risk ratios for future sports-related concussions were computed with ISI and NHANES sleepiness scores as independent predictors. An additional multiple logistic regression model including sport, sports-related concussion history, and significant univariate predictors jointly assessed the odds of sustaining a concussion.
Results:
Clinically moderate-to-severe insomnia severity (RR = 3.13, 95% CI: 1.320–7.424, p = 0.015) and excessive daytime sleepiness two or more times per month (RR = 2.856, 95% CI: 0.681–11.977, p = 0.037) increased concussion risk. These variables remained significant and comparable in magnitude in a multivariate model adjusted for sport participation.
Conclusion:
Insomnia and daytime sleepiness are independently associated with increased sports-related concussion risk. More completely identifying bidirectional relationships between concussions and sleep requires further research. Clinicians and athletes should be cognizant of this relationship and take proactive measures – including assessing and treating sleep-disordered breathing, limiting insomnia risk factors, improving sleep hygiene, and developing daytime sleepiness management strategies – to reduce sports-related concussion risk and support overall athletic performance.
Keywords: Sports-related concussion, Sleep quality, Insomnia severity index, Relative risk, Daytime sleepiness, College athletes
1. Introduction
An estimated 3.3 million mild traumatic head injuries, including those from motor vehicle accidents and sports-related concussions, are reported by emergency departments in the United States each year [1–3]. Many more sports-related concussions are managed by sports medicine teams and an estimated 50% of sports-related concussions go unreported by those who sustain them [4–6]. Consequently, reducing sports-related concussion risk is a significant public health focus and identifying predisposing concussion risk factors is of paramount importance.
Prior research indicates that the risk of sustaining a sports-related concussion is anywhere from 1.75 to 11 times greater for those with a history of one or more prior concussions [7–11]. In one of the largest retrospective studies to date (n = 12,320 participants), the odds of sustaining a sports-related concussion was 10.65 times greater than for those with a history than those without [8]. Therefore, while the specific magnitude of this effect is not necessarily clear, it is apparent that prior sports-related concussion is a predisposing factor.
Other sports-related concussion risk factors include sex [12–19], age [16,17,20–22], and attention-deficit hyperactive disorder (ADHD) or other learning disorders (LD) [23,24]. However, the findings are less clear, and often conflicting, for these risk factors. For example, some reports indicate that females are at greater risk for sports-related concussions [12,15,18], whereas others indicate that males are [13,17] and yet others indicate no differences in risk [16,19]. Many of these risk factors likely interact with each other. One recent study utilized multiple risk factors to create a composite weighted risk score based on univariate odds ratios. This study identified sports-related concussion history as the most indicative risk factor, with level of play, history of headache treatment, contact sport participation, and ADHD/LD as additional risk factors [8].
One previously uninvestigated, or unreported, potential risk factor is the role of sleep. Currently the National Sleep Foundation recommends that teenagers (14–17 years old) and young adults (18–25 years), the populations in whom most sports-related concussion research is currently conducted, obtain 8–10 or 7–9 h of sleep per night, respectively [25]. However, recent estimates suggest that many individuals in these age categories, including athletes, do not obtain sufficient nightly sleep [26–28]. With respect to athletics, this is especially problematic. Chronic under-sleeping, sleep restriction, and sleep deprivation are associated with, and can result in, mental fatigue [29,30]; attentional lapses [30,31]; increased reaction time [29,32]; impaired predictive visual tracking [31,32]; reduced postural and dynamic motor control [29,33]; degraded intra- and inter-personal emotion regulation, perception, and responsiveness [34]; and increased impulsivity and risk-taking behavior [35–37]. Furthermore, sport performance indices (including cardiovascular [38,39], endurance [38], strength [40,41], and accuracy [31,32] outcomes) are reduced or impaired under inadequate or low quality sleeping conditions and daytime sleepiness or fatigue. Thus, individuals with habitually poor sleep may be more likely to have degraded overall sport performance, take more on-field risks, make poorer in-the-moment decisions, have less body control to appropriately act and react to cues, as well as experience brief lapses in attention to, and poor visual tracking of, in-game activities. These effects may reduce athletes’ capacity to avoid or minimize injury, including sports-related concussions.
In addition to these adverse performance outcomes, less than optimal amounts of sleep (≤7–8 h of sleep per night) is associated with increased injury risk [42–44]. However, prior investigations into injury risk have not specifically identified the risk for head injury or sports-related concussion. The purpose of this study was, therefore, to identify the extent to which self-reported, sleep-related outcomes affect the risk of sustaining a sports-related concussion in a sample of college athletes. We hypothesized that, in addition to concussion history, poor self-reported sleep would be associated with increased concussion risk.
2. Methods
2.1. Participants
Data were collected from surveys administered to NCAA Division-1 athletes (n = 190) over the summer and during the first two weeks of the Fall 2016 semester. In order to be eligible for the survey, students had to be at least 18 years of age. Selection favored returning students. Students were recruited through flyers, in-person solicitations at training facilities, and word of mouth among students and athletics staff. All surveys were administered online, using the student’s phone, tablet, computer, or a study-provided tablet. Participants were paid for completing surveys. The Institutional Review Board of the University of Arizona approved this study.
2.2. Questionnaires
Individuals participating in the study completed a comprehensive battery of questionnaires. This battery included demographic information (ie, age, sex, sport, self-reported sports-related concussion history) and validated self-report measures related to sleep including the Insomnia Severity Index (ISI) [45], Pittsburgh Sleep Quality Index (PSQI) [46], and Fatigue Severity Scale (FSS) [47]. These are all standard screening measures used across a wide variety of populations. Of note, the PSQI was scored using two different cutoff scores to identify poor quality sleep, ≥5 and ≥8. This is because even though ≥5 is the standard cutoff for the PSQI [46], more recent work has shown that ≥8 may be more appropriate for individuals following a traumatic brain injury [48].
The National Health and Nutrition Examination Survey’s (NHANES) Sleep module was used to assess daytime sleepiness with the item, “In the past month, how often did you feel excessively or overly sleepy during the day?” Response options included Never, Rarely (one time per month), Sometimes (2–4 times per month), Often (5–15 times per month), or Almost Always (16–30 times per month) [49].
Injury data were extracted from the student’s athletic medical record at least one year after completing the survey (with consent) and linked to individual survey responses. All medical visits during the student’s time at the university were examined and coded. All instances of a sports-related concussion (sports-related concussion) were noted and the date of that injury was also recorded so that time from survey could be computed.
2.3. Statistical analyses
2.3.1. Primary analyses
Individuals were grouped by sport-related concussion occurrence following the survey (0 = no future sports-related concussion; 1 = one or more future sports-related concussions). Demographic and self-reported sleep-related outcome scores (including total scores and individual item scores) were analyzed using two sample Manne–Whitney U tests and Fisher’s exact tests as appropriate. All p-values for the ISI, PSQI, and FSS individual item scores were Holm-adjusted within-questionnaire to control for multiple comparisons. We computed univariate risk ratios for future incidence of a sports-related concussion to test our primary hypothesis. Individual binary predictors included:
Subclinical to more severe insomnia (1 = ISI score ≥ 8)
Moderate-to-severe clinical insomnia (1 = ISI score ≥ 15)
Clinically significant sleep disruption for healthy individuals (1 = PSQI total score ≥ 5)
Clinically significant sleep disruption for post-TBI individuals (1 = PSQI total score ≥ 8)
Clinically significant fatigue (1 = FSS ≥ 36)
Excessive daytime sleepiness occurring at least twice per month (1 = NHANES Sleepiness response 2, 3, or 4)
Additional univariate risk ratios were computed for prior history of sports-related concussion (1 = any history of sports-related concussion), sex (1 = Male), and higher concussion prevalence sport participation in our sample [50,51] (1 = football, soccer, women’s basketball; 0 = any other sport). In order to identify independent sports-related concussion risk factors, a multiple logistic regression model was fit using all statistically significant (p < 0.05) univariate predictors. An automated forward-and-backward stepwise variable selection method simplified this model to limit multi-collinearity between predictors.
2.3.2. Secondary analyses
To examine further the association between self-reported sleep outcomes and sports-related concussion risk, two additional logistic multiple logistic regression models were fit. First, total scores on the questionnaires exhibiting statistically significant between-group differences were entered as continuous variables, with sport participation and sports-related concussion history as additional covariates. The purpose of this model was to determine the association between increasing total scores and sports-related concussion risk, rather than classification based on dichotomized values. Second, to examine how individual elements of the questionnaires (eg, nighttime insomnia items versus daytime impairment items on the ISI) may increase sports-related concussion risk, individual sleep questionnaire item scores were entered into a logistic regression model, along with NHANES sleepiness scores, sport participation, and sports-related concussion history. To limit the number of individual items entered into the initial model, candidate questionnaire items included only those that were statistically significant (adjusted p < 0.05) based on the two sample Manne–Whitney U tests. Both additional models utilized the automated forward stepwise variable selection as before.
All statistical analyses were conducted in R (v. 3.5.0) [52]. Data wrangling and cleaning was performed using functions from the dplyr [53], tidyr [54], stringr [55], and lubridate [56] packages. Stepwise variable selection was accomplished using the stepAIC function in the MASS package [57]. Plots were created using the ggplot2 package [58]. Univariate relative risk and odds ratios were computed using the epitab function in the epitools package [59] with small sample adjustment.
3. Results
3.1. Demographics
Descriptive characteristics for this sample, stratified by sports-related concussion occurrence after the survey, are contained in Tables 1 and 2 as well as Fig. 1. The two subsamples were well-matched in terms of age, sex, race/ethnicity, academic year, and relationship status. While no association between individual sport participation and sports-related concussion occurrence was observed, an association was present when dichotomizing sports by relative concussion prevalence [50,51]. Individuals sustaining a concussion exhibited statistically significantly greater ISI and FSS total scores at the time of the survey. The average time from the survey until a sports-related concussion was 100.2 ± 62.7 days and only one individual sustained two or more concussions within the follow-up time-frame.
Table 1.
Continuous sample demographic and self-reported characteristics.
| SRC after surveya |
|||
|---|---|---|---|
| No | Yes | p-valueb | |
| N | 171 | 19 | |
| Age | 20.59 [19.59–21.31] | 19.98 [19.44–20.93] | 0.217 |
| CES-D Total score | 9 [5–15] | 13 [7.5–17] | 0.143 |
| PSQI Total score | 8 [6–10] | 8 [6–10.50] | 0.558 |
| Item 2: Sleep Latency | 15 [10–30] | 12 [5–25] | 1.000c |
| Item 4: Total sleep time | 7 [6–8] | 7 [6.35–8.00] | 1.000c |
| Item 5a: Difficulty falling asleep | 1 [1–2] | 2 [1–2] | 1.000c |
| Item 5b: Difficulty maintaining sleep | 1 [0–2] | 1 [1–2] | 1.000c |
| Item 5c: Bathroom use | 1 [0–2] | 1 [0–1] | 1.000c |
| Item 5d: Cannot breathe comfortably | 0 [0–0] | 0 [0–1] | 1.000c |
| Item 5e: Loud snoring or coughing | 0 [0–0] | 0 [0–0] | 1.000c |
| Item 5f: Feel too cold | 0 [0–0.5] | 0 [0–1] | 0.987c |
| Item 5g: Too hot | 1 [0–2] | 2 [1–2] | 0.076c |
| Item 5h: Nightmares | 0 [0–1] | 0 [0–1] | 1.000c |
| Item 5i: Pain | 0 [0–0] | 1 [0–1] | 0.142c |
| Item 6: Overall sleep quality | 1 [1–1] | 1 [0.5–1.5] | 1.000c |
| Item 7: Medication use to help sleep | 0 [0–0] | 0 [0–0.5] | 1.000c |
| Item 8: Trouble staying awake | 0 [0–1] | 1 [0.5–1.5] | 0.03c |
| Item 9: Daytime motivation | 1 [0–1] | 1 [1–2] | 0.008c |
| ISI Total score | 6 [4–10] | 10 [5.5–15] | 0.024 |
| Item 1: Difficulty falling asleep | 1 [0–2] | 1 [0.5–2] | 0.518c |
| Item 2: Difficulty staying asleep | 1 [0–1] | 1 [0–1.5] | 0.254c |
| Item 3: Waking up too early | 1 [0–2] | 1 [1–2] | 0.033c |
| Item 4: Sleep pattern satisfaction | 2 [1–3] | 3 [2–3] | 0.053c |
| Item 5: How noticeable are your sleeping problems to others | 0 [0–1] | 1 [0.5–2] | 0.010c |
| Item 6: How worried are you about your sleep problems | 0 [0–1] | 1 [0–2] | 0.055c |
| Item 7: How much do your sleep problems interfere with daily function | 1 [0–2] | 2 [1–3] | 0.008c |
| FSS Total score | 28 [21–35] | 33 [30–38.5] | 0.023 |
| Item 1: Lower motivation when fatigued | 5 [3–6] | 6 [5–7] | 0.193c |
| Item 2: Exercise brings on fatigue | 4 [3–5] | 4 [3.5–5] | 0.931c |
| Item 3: I am easily fatigued | 2 [1–4] | 3 [2–4] | 0.314c |
| Item 4: Fatigue interferes with physical functioning | 3 [2–5] | 3 [3–5.5] | 0.429c |
| Item 5: Fatigue causes frequent problems | 2 [2–3] | 3 [2–4] | 0.429c |
| Item 6: Fatigue prevents sustained physical functioning | 3 [2–4] | 3 [2–4] | 0.429c |
| Item 7: Fatigue interferes with duties/responsibilities | 3 [2–4] | 3 [2–4] | 0.429c |
| Item 8: Fatigue is among my most disabling symptoms | 2 [1–4] | 4 [3–5] | 0.017c |
| Item 9: Fatigue interferes with work/family/social life | 2 [1–4] | 4 [2.5–4] | 0.072c |
Notes:
Individual questionnaire items are described in italics.
Statistically significant p-values are highlighted in bold for visibility.
Values are median [inter-quartile range].
p-values are for Wilcoxon Rank Sum tests.
Holm-adjusted p-value to control for multiple comparisons within questionnaire.
Abbreviations: SRC: sports-related concussion; CES-D: Center for Epidemiological Studies Depression Scale; PSQI: Pittsburgh Sleep Quality Index; ISI: Insomnia Severity Scale; FSS: Fatigue Severity Scale.
Table 2.
Categorical sample demographic and self-reported characteristics.
| mTBI after surveya |
|||
|---|---|---|---|
| No | Yes | pb | |
| N | 171 | 19 | |
| Sex n (%) | 0.560 | ||
| Female | 80 (46.8%) | 7 (36.8%) | |
| Male | 91 (53.2%) | 12 (63.2%) | |
| Prior mTBI history n (%) | 0.044 | ||
| No | 114 (66.7%) | 8 (42.1%) | |
| Yes | 57 (33.3%) | 11 (57.9%) | |
| Sport type | 0.038 | ||
| Low SRC prevalence | 118 (69.1%) | 8 (42.1%) | |
| High SRC prevalencec | 53 (30.1%) | 11 (57.9%) | |
| NHANES Sleepiness Questionnaire n (%) | 0.025 | ||
| Never | 20 (11.7%) | 0 (0%) | |
| Rarely (1 time per month) | 41 (24%) | 2 (10.5%) | |
| Sometimes (2–4 times per month) | 62 (36.3%) | 6 (31.6%) | |
| Often (5–15 times per month) | 40 (23.4%) | 7 (36.8%) | |
| Almost always (16–30 times per month) | 8 (4.7%) | 4 (21.1%) | |
Notes:
Values are n (%).
p-values are for Fisher’s exact tests.
Statistically significant p-values are highlighted in bold for visibility.
Abbreviations: SRC: sports-related concussion; NHANES: National Health and Nutrition Examination Survey.
Fig. 1.
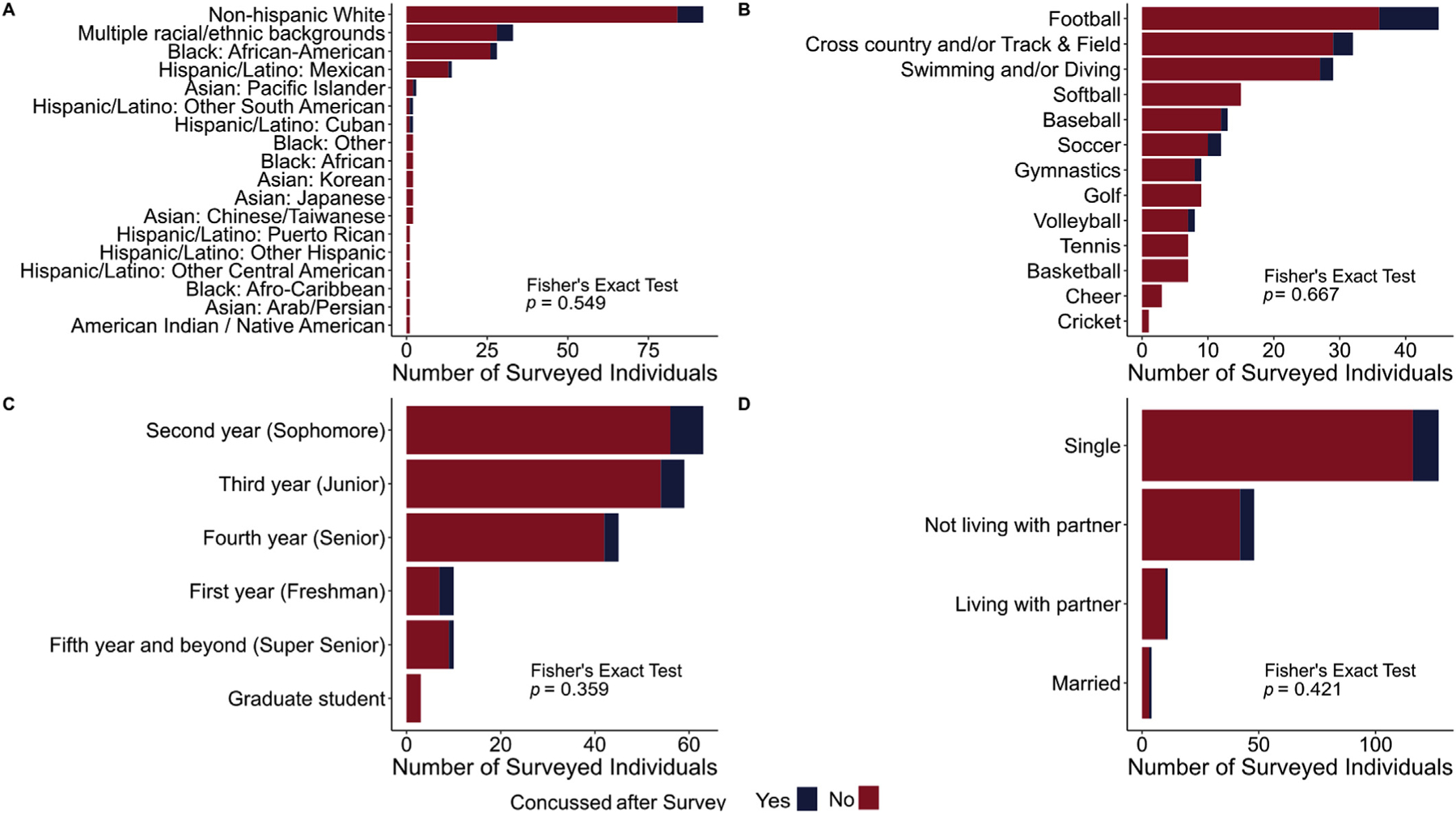
Proportions of individuals sustaining (blue) or not sustaining (red) a sports-related concussion (blue) broken down by (A) racial/ethnic groups, (B) individual sports, (C) academic years, and (D) relationship statuses. Fisher’s exact tests were used to assess associations between these demographics and sports-related concussion occurrence after the survey date. No statistically significant associations were observed.
3.2. Risk ratios
Details of the univariate relative risk ratios are contained in Table 3. Any prior history of concussion, participating in a sport with a traditionally high sports-related concussion prevalence, self-reported moderate-to-severe insomnia via the ISI (scores ≥ 15), and experiencing excessive daytime sleepiness on two or more days per month via the NHANES were individually associated with sustaining a future sports-related concussion. Notably, dichotomized fatigue severity scores were not associated with an increased risk of future concussion despite the total scores being significantly greater in the concussed group (Table 1).
Table 3.
Univariate relative risk ratios for future sports related concussion
| Measure | SRC after survey |
95% CI for RR |
||||
|---|---|---|---|---|---|---|
| No | Yes | RR | Lower | Upper | p-value | |
| Prior SRC History | ||||||
| No | 114 | 8 | ||||
| Yes | 57 | 11 | 2.211 | 0.935 | 5.23 | 0.044 |
| Sporta | ||||||
| Low prevalence | 118 | 8 | ||||
| High prevalence | 53 | 11 | 2.425 | 1.027 | 5.730 | 0.038 |
| Sex | ||||||
| Female | 80 | 7 | ||||
| Male | 91 | 12 | 1.282 | 0.528 | 3.113 | 0.473 |
| NHANES Sleepiness | ||||||
| 0–1 times per month | 61 | 2 | ||||
| 2 + times per month | 110 | 17 | 2.856 | 0.681 | 11.977 | 0.037 |
| ISI | ||||||
| ISI <8 | 99 | 7 | ||||
| ISI ≥8 | 72 | 12 | 1.911 | 0.787 | 4.639 | 0.092 |
| ISI <15 | 154 | 13 | ||||
| ISI ≥15 | 17 | 6 | 3.13 | 1.32 | 7.424 | 0.015 |
| PSQI | ||||||
| PSQI <5 | 19 | 1 | ||||
| PSQI ≥5 | 152 | 18 | 1.112 | 0.157 | 7.89 | 0.699 |
| PSQI <8 | 76 | 6 | ||||
| PSQI ≥8 | 95 | 13 | 1.427 | 0.567 | 3.595 | 0.335 |
| Total sleep ≥8hrs | 44 | 7 | ||||
| Total sleep < 8hrs | 127 | 12 | 0.516 | 0.234 | 1.346 | 0.289 |
| FSS | ||||||
| FSS <36 | 129 | 14 | ||||
| FSS ≥36 | 42 | 5 | 1.021 | 0.389 | 2.685 | 1 |
Abbreviations: SRC: Sports-related concussion; RR: Relative risk ratio; mTBI: Mild traumatic brain injury; PSQI: Pittsburgh Sleep Quality Index; ISI: Insomnia Severity Scale; FSS: Fatigue Severity Scale; NHANES: National Health and Nutrition Examination Survey.
Statistically significant p-values are highlighted in bold for visibility.
3.3. Multiple logistic regression
The initial multiple logistic regression model included all statistically significant binary predictors indicated by the univariate relative risk models (prior sports-related concussion history, high sports-related concussion prevalence sport participation, ISI scores ≥ 15, and NHANES-rated excessive daytime sleepiness on two or more days per month). After variable selection, high concussion prevalence sport participation, insomnia severity, and daytime sleepiness were independently associated with increased odds of sustaining a sports-related concussion (Table 4 Primary model).
Table 4.
Independent association of predictors to sports-related concussion occurrence in multivariable models.
| Predictor | Odds ratio | Standard error | 95% CI | p-value |
|---|---|---|---|---|
| Primary model | ||||
| High SRC prevalence sport participationa | 3.240 | 1.671 | 1.193, 9.156 | 0.022 |
| Insomnia Severity Index score ≥15 | 3.047 | 1.782 | 0.929, 9.222 | 0.054 |
| Daytime sleepiness 2 + times per month | 4.789 | 2.190 | 1.251, 31.623 | 0.046 |
| Secondary model 1 (Total score model) | ||||
| High SRC prevalence sport participationa | 3.291 | 1.667 | 1.218, 9.266 | 0.020 |
| Insomnia Severity Index total score | 1.083 | 1.048 | 0.988, 1.189 | 0.088 |
| Daytime sleepiness 2 + times per month | 4.357 | 2.200 | 1.122, 28.923 | 0.062 |
| Secondary model 2 (Individual item model) | ||||
| High SRC prevalence sport participationa | 3.012 | 1.691 | 1.081, 8.696 | 0.036 |
| Daytime sleepiness 2 + times per month | 3.541 | 2.234 | 0.873, 23.956 | 0.116 |
| PSQI Item 9: Daytime motivation | 2.409 | 1.381 | 1.305, 4.696 | 0.007 |
3.4. Secondary analyses of risk factors
Initial secondary models were fit using high concussion prevalence sport participation, sports-related concussion history, NHANES sleepiness scores, and total scores on the ISI and the FSS or individual items scores that were statistically significantly different between groups (see Table 1 for statistically significant individual items). After stepwise variable selection, sport participation and experiencing excessive daytime sleepiness on two or more days per month were retained for both models and independently associated with increased odds of future sports related concussion (Table 4). For secondary model 2, daytime motivation on the PSQI was also independently associated with increased sports-related concussion odds (Table 4).
Supplemental Tables 1–4 explore further the relationship between sub-optimal sleep outcomes, high versus low concussion prevalence sport participation, and sports-related concussion occurrence. These tables highlight the fact that, while there were no differences in questionnaire outcomes between low- and high prevalence sports, the relative frequency of sports-related concussions in this sample was higher for those with greater insomnia severity and/or more frequent daytime sleepiness than for those without, regardless of the overall prevalence for sports-related concussion.
4. Discussion
The purpose of this study was to test the hypothesis that poorer self-reported sleep-related outcomes are associated with an increased risk of future sports-related concussion in a sample of Division 1 college athletes. We hypothesized that self-reported indicators of sub-optimal sleep would be associated with an increased risk for sports-related concussion. These hypotheses were confirmed.
The relative risk of sustaining a sports-related concussion was 2–3x higher for individuals participating in a sport with a high prevalence of sports-related concussions, reporting any history of sports-related concussion prior to the survey date, self-reporting daytime sleepiness on two or more days in a month (via the NHANES Sleepiness questionnaire) or reporting moderate-to-severe levels of insomnia (via the ISI). Furthermore, after controlling for sport participation, the odds of sustaining a future sports-related concussion were 3.66–5.58x greater for individuals reporting moderate-to-severe insomnia and/or daytime sleepiness on two or more days per month. Collectively, the odds of sustaining a sports-related concussion for individuals who reported both moderate-to-severe insomnia severity and two or more days of excessive daytime sleepiness (n = 20) was 14.6x higher than for those who reported neither.
4.1. Sports-related concussion risk factors
Reducing the incidence of sports-related concussion is a public health concern. Consequently, identifying individual risk factors for sustaining a sports-related concussion has been the focus of considerable effort. Prior work has consistently demonstrated that individuals with previous concussions are at increased risk to sustain a future concussion [7–11]. Our present findings are consistent in magnitude with the majority of these findings (~2–3 times higher risk of future sports-related concussion with any prior history of concussion). However, in contrast to prior work, history of concussion was not retained in multivariate models in this sample. Rather, after controlling for participating in a sport with a traditionally higher sports-related concussion prevalence, self-report insomnia and daytime sleepiness were strong indicators of sustaining a concussion.
Additional studies have indicated that sex [12–19], age [16,17,20–22], and ADHD/LD [23,24], among others, may all increase the risk of sustaining a sports-related concussion. Yet, these findings are inconsistent. Relative to our findings, males were more likely than females to sustain a sports-related concussion, though this finding was not statistically significant. However, diagnoses of ADHD/LD were not available for analysis. Furthermore, age in our sample was not associated with an increased likelihood of sustaining a sports-related concussion, though this is not surprising given the narrow age range considered here.
4.2. Bidirectional relationships between sports-related concussion and sleep
There is evidence indicating that self-reported sleep disruption, daytime sleepiness, and fatigue are consequences of sports-related concussions. An estimated 30–80% of individuals with mixed severity traumatic brain injuries, of which sports-related concussions are a subset, report insomnia symptoms lasting well beyond the generally accepted clinical time course of recovery [60–62]. Recent evidence suggests that there may additionally be objective indicators of altered sleep quantity and quality following sports-related concussion [63–67]. However, the natural evolution of sleep disruption and recovery post-injury has not been clearly identified to date.
To our knowledge, this is the first study that has examined self-report sleep-related outcomes as a risk factor, rather than necessarily an outcome, for sports-related concussion. We observed a three-to five-fold increased likelihood of sustaining a sports-related concussion with either moderate-to-severe insomnia severity or daytime sleepiness, even after controlling for participating in a higher concussion prevalence sport. While likely not directly causative, there are several plausible explanations as to why individuals with these self-reported outcomes may be at increased sports-related concussion risk.
First, sub-optimal sleep is associated with increased reaction times [30,32,39]; attentional lapses [29,31]; degraded visual tracking [31,32]; reduced postural control and strength [29,33,40,41]; impaired emotional recognition, responsiveness, and control [34]; and increased impulsivity and risky behavior [35–37]. Second, the cardiovascular [38,39], endurance [38], strength [40,41], and accuracy [31,32] components of sport performance are reduced or impaired under inadequate or low quality sleeping conditions as well as daytime sleepiness. Consequently, individuals who experience poor sleep may be more likely to have degraded overall physical sport performance as well as brief lapses in attention to and poor visual tracking of in-game activities. Simultaneously, these individuals may make unusually poor or risky sport decisions (eg, take on-field risks), be unable to manage negative emotional responses to in-game situations, and have poorer body control during sport maneuvers at critical moments. Collectively, these effects may reduce an individual’s capacity to avoid or minimize injury. However, reports for the majority of these adverse sports-related effects are isolated from one another (ie, studies on reduced sport performance after sleep restriction only; studies on impaired visual tracking follow sleep deprivation only) and therefore multivariate effects are not fully explained. Furthermore, prior investigations into injury risk have not specifically identified sports-related concussion risk, and therefore any translation of this hypothesis requires further corroboration.
Despite the lack of comprehensive analyses of sleep-related influences on injury risk, the present findings contribute to a larger body of work identifying poor or insufficient sleep as a contributory factor to sports-related injury risk [42–44,68]. Additionally, these findings contribute a critically absent piece of information to this literature base, highlighting the specific risk of sports-related concussion in the presence of poor sleep (eg, insomnia) and daytime sleepiness. Furthermore, these findings identify a plausible means by which prior sports-related concussions increase future risk. Namely, prior sports-related concussions often result in increased incidences of insomnia and daytime sleepiness, which, on the basis of the present findings, likely contribute to increased risk of future concussions. This, therefore, may create a feed-forward cycle where sports-related concussions increase the risk of poor sleep outcomes that, in turn, increase the risk of future concussions. While unconfirmed at present, this hypothesis merits further investigation.
Apart from the noted effects of sports-related concussions on sleep, collegiate and professional athletes are more generally at increased risk for insomnia [69] and sleep disordered breathing [70,71] compared to sub-elite and non-athlete populations. Evidence suggests that training schedules [72], travel requirements [73,74], and competition [71,72] may all contribute to poor quality sleep and insomnia, plausibly resulting in daytime sleepiness. Likewise, the prevalence of sleep-disordered breathing is higher in athletes, particularly football players, and is associated with increased complaints of daytime sleepiness and poor sleep quality [71,75,76].
Within the present sample, approximately 60% of the athletes reported daytime sleepiness or sleepiness on two or more days per month and approximately 12% reported moderate-to-severe insomnia symptom severity (Supplementary Tables). Despite a limited number of observed sports-related concussions in the follow-up period (n = 19), individuals reporting frequent daytime sleepiness accounted for the majority (n = 17; 89.4%). Furthermore, almost one-third of the sustained sports-related concussions occurred in individuals reporting both frequent daytime sleepiness and moderate-to-severe insomnia severity (n = 6; 31.6%). Combined with previously reported detrimental effects of sleep disruption on athletic performance and overall health, the present findings highlight the need for assessing and improving sleep, either quantitatively or qualitatively, as means of reducing sports-related concussion risk as well as supporting high-quality sport performance. Collegiate and professional athletes, in particular, may benefit from assessment for and treatment of sleep-disordered breathing, sleep hygiene education, and developing habits to obtain sufficient, high-quality sleep and manage daytime sleepiness.
4.3. Limitations
Several limitations must be considered when interpreting the present findings. First, a limited number of individuals sustained a sports-related concussion in the follow-up time-frame (n = 19; 10% of the sample). This limited proportion of our sample may have resulted in overestimating the relative risk and odds ratios and limited our ability to detect statistically significant effects. To minimize this concern, we used small sample size adjustments for the univariate risk ratio confidence intervals to provide conservative estimates of overall risk and the stepwise variable selection method restricted multivariate models to variables containing model information, rather than strictly statistical significance, while limiting multi-collinearity. However, all of the univariate (Table 3) and multivariate models (Table 4) as well as simple descriptive cross-tabulation (Supplemental Tables) converge on the interpretation that frequent daytime sleepiness, both apart from and in conjunction with moderate-to-severe insomnia severity, is associated with an increased occurrence of sports-related concussions.
Second, our analyses relied on diagnosed sports-related concussions. Historically, as many as 50% of sports-related concussions go unreported and therefore undiagnosed [4–6]. Independent of the research presented here, athletes and coaches in the present sample received routine education on concussion symptoms and reporting. Additionally, the sports medicine team maintains a high vigilance for detecting possible concussions, including the addition of independent medical spotter during football games. Therefore, we have reasonable confidence that the majority of the sustained concussions were diagnosed and recorded. However, given the vast number of sports-related concussions that do go unreported, it is possible that participants under-reported prior sports-related concussions to research staff and medical providers, potentially confounding the results of the multiple logistic regression models. Consequently, our findings may not reflect the true relative risk of sports-related concussion based on self-reported sleep-related outcomes when considering both diagnosed and unreported injuries.
Third, the a priori data collection methods in the present study did not allow for follow-up or on-going sleep tracking throughout the course of the season. Therefore, it was not possible to track in-season sleep changes, correlate sleep changes with academic or sport demands, or quantify the extent to which poor sleep outcomes immediately prior to injury may have predisposed individuals to subsequently poorer sleep post-injury. This is an important consideration in light of the prevalence of sleep disruption following sports-related concussions.
Lastly, all athletes completed surveys prior to the start of the fall semester but may not have had practice or competition until the spring. Consequently, sleep characteristics leading up to the injury (eg, sleep patterns, habits, daytime sleepiness in the days and weeks preceding injury, advanced or delayed sleep phase due to travel), rather than more remote sleep outcomes, may be a more sensitive indicator of sports-related concussion risk.
4.4. Future research
The findings from the present study highlight a critical need for further research in this area. Specific research efforts should include the following:
Fine-grained sleep assessments leading up to, and following, injury: Wearable activity trackers (eg, actigraphs) can objectively quantify sleep and sleep habits over time and are appropriate for sleep tracking in numerous populations. Prior investigations have included actigraphy-related findings following concussions. In the present study, we were unable to follow athletes during their sport season to quantify how self-reported and measured sleep may change. Prospective sleep tracking in athletes, with a particular emphasis on preinjury risks and post-injury outcomes, would provide essential, quantifiable data on the role of sleep leading up to injury. Likewise, sleep tracking following sports-related concussion would provide needed clarity on how sleep changes with injury as well as the extent to, and timing at, which recovery occurs.
Sport and academic demands, and travel as risk factors for concussion. Sport and academic demands – such as travel to and from competitions, early morning or late evening practices, and studying for exams – may negatively impact sleep (eg, self-imposed sleep restriction; phase advanced/delayed sleep) prior to and following a concussion. Consequently, sleep-related risks for, and outcomes from, a concussion may change throughout the sport and academic year as a function of these factors and requires further examination.
The interplay between sleep and biopsychosocial risk factors for sports-related concussions. Further studies are needed to more fully identify biopsychosocial (eg, training response, age, sex and/or gender, self-esteem, stress, nutrition, training load) risk factors in conjunction with sleep that may more fully identify individuals at increased risk for injury. Of particular interest would be the identification of modifiable risk factors – such as stress, nutrition, sleep and sleep hygiene, daytime sleepiness – that can be leveraged to prevent avoidable injury.
5. Conclusions
The results of this study indicate that self-reported poor sleep outcomes, specifically moderate-to-severe insomnia severity and frequent excessive daytime sleepiness, are independently associated with an increased risk for subsequent sports-related concussions. Further research is needed to more completely identify the bidirectional relationship between sports-related concussion and both objective and subjective sleep disruption. Additionally, our findings highlight the need for both clinicians and athletes to be cognizant of the relationship between sleep and sports-related concussions and to take proactive measures to improve athletes’ sleep – quantitatively, qualitatively, or both e in order to reduce sports-related concussion risk as well as support and improve overall athletic performance.
Supplementary Material
Acknowledgements
We are thankful for the help of Mckenzie Rafferty and Jonathan Charest for the assistance in extracting and compiling the dataset.
Funding
The study was funded by an Innovations grant from the National Collegiate Athletic Association. Dr. Grandner is also supported by R01 MD011600. Drs. Raikes and Killgore are also supported by a US Army Medical Research and Materiel Command grant (W81XWH-14-1-0571) to Dr. Killgore.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2019.03.008.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sleep.2019.03.008.
References
- [1].Bryan MA, Rowhani-Rahbar A, Comstock RD, et al. Collaborative on behalf of the SSCR. Sports- and recreation-related concussions in US youth. Pediatrics 2016;138(1):e20154635 10.1542/peds.2015-4635. [DOI] [PubMed] [Google Scholar]
- [2].Faul M, Xu L, Wald MM, et al. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. http://origin.glb.cdc.gov/traumaticbraininjury/pdf/blue_book.docx. [Accessed 25 April 2015]. [Google Scholar]
- [3].Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21(5): 375–8. 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- [4].Kerr ZY, Register-Mihalik JK, Kroshus E, et al. Motivations associated with nondisclosure of self-reported concussions in former collegiate athletes. Am J Sports Med 2016;44(1):220–5. 10.1177/0363546515612082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Register-Mihalik JK, Guskiewicz KM, McLeod TCV, et al. Knowledge, attitude, and concussion-reporting behaviors among high school athletes: a preliminary study. J Athl Train 2013;48(5):645–53. 10.4085/1062-6050-48.3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McCrea M, Hammeke T, Olsen G, et al. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med 2004;14(1): 13–7. 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- [7].Abrahams S, Fie SM, Patricios J, et al. Risk factors for sports concussion: an evidence-based systematic review. Br J Sports Med 2014;48(2):91–7. 10.1136/bjsports-2013-092734. [DOI] [PubMed] [Google Scholar]
- [8].Brett BL, Kuhn AW, Yengo-Kahn AM, et al. Risk factors associated with sustaining a sport-related concussion: an initial synthesis study of 12,320 student-athletes. Arch Clin Neuropsychol 2018;33(8):984–92. 10.1093/arclin/acy006. [DOI] [PubMed] [Google Scholar]
- [9].Emery CA, Kang J, Schneider KJ, et al. Risk of injury and concussion associated with team performance and penalty minutes in competitive youth ice hockey. Br J Sports Med 2011;45(16):1289–93. 10.1136/bjsports-2011-090538. [DOI] [PubMed] [Google Scholar]
- [10].Hollis SJ, Stevenson MR, McIntosh AS, et al. Incidence, risk, and protective factors of mild traumatic brain injury in a cohort of Australian nonprofessional male rugby players. Am J Sports Med 2009;37(12):2328–33. 10.1177/0363546509341032. [DOI] [PubMed] [Google Scholar]
- [11].Schneider KJ, Meeuwisse WH, Kang J, et al. Preseason reports of neck pain, dizziness, and headache as risk factors for concussion in male youth ice hockey players. Clin J Sport Med 2013;23(4):267 10.1097/JSM.0b013e318281f09f. [DOI] [PubMed] [Google Scholar]
- [12].Covassin T, Moran R, Elbin RJ. Sex differences in reported concussion injury rates and time loss from participation: an update of the national collegiate athletic association injury surveillance program from 2004–2005 through 2008–2009. J Athl Train 2016;51(3):189–94. 10.4085/1062-6050-51.3.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Emery CA, Tyreman H. Sport participation, sport injury, risk factors and sport safety practices in Calgary and area junior high schools. Paediatr Child Health 2009;14(7):439–44. 10.1093/pch/14.7.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kontos AP, Elbin RJ, Collins MW. Aerobic fitness and concussion outcomes in high school football In: Slobounov S, Sebastianelli W, editors. Foundations of sport-related brain injuries. Boston, MA: Springer US; 2006. p. 315–39. 10.1007/0-387-32565-4_14. [DOI] [Google Scholar]
- [15].Lincoln AE, Caswell SV, Almquist JL, et al. Trends in concussion incidence in high school sports: a prospective 11-year study. Am J Sports Med 2011;39(5): 958–63. 10.1177/0363546510392326. [DOI] [PubMed] [Google Scholar]
- [16].Lincoln AE, Hinton RY, Almquist JL, et al. Head, face, and eye injuries in scholastic and collegiate lacrosse: a 4-year prospective study. Am J Sports Med 2007;35(2):207–15. 10.1177/0363546506293900. [DOI] [PubMed] [Google Scholar]
- [17].Nation AD, Nelson NG, Yard EE, et al. Football-related injuries among 6- to 17-year-olds treated in US emergency departments, 1990–2007. Clin Pediatr (Phila) 2011;50(3):200–7. 10.1177/0009922810388511. [DOI] [PubMed] [Google Scholar]
- [18].O’Connor KL, Baker MM, Dalton SL, et al. Epidemiology of sport-related concussions in high school athletes: national athletic treatment, injury and outcomes network (NATION), 2011–2012 through 2013–2014. J Athl Train 2017;52(3):175–85. 10.4085/1062-6050-52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang J, Phillips G, Xiang H, et al. Hospitalisations for sport-related concussions in US children aged 5 to 18 years during 2000–2004. Br J Sports Med 2008;42(8):664–9. 10.1136/bjsm.2007.040923. [DOI] [PubMed] [Google Scholar]
- [20].Davis GA, Anderson V, Babl FE, et al. What is the difference in concussion management in children as compared with adults? A systematic review. Br J Sports Med 2017;51(12):949–57. 10.1136/bjsports-2016-097415. [DOI] [PubMed] [Google Scholar]
- [21].Guskiewicz KM, Weaver NL, Padua DA, et al. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med 2000;28(5): 643–50. 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- [22].Knox CL, Comstock RD, McGeehan J, et al. Differences in the risk associated with head injury for pediatric ice skaters, roller skaters, and in-line skaters. Pediatrics 2006;118(2):549–54. 10.1542/peds.2005-2913. [DOI] [PubMed] [Google Scholar]
- [23].Iverson GL, Wojtowicz M, Brooks BL, et al. High school athletes with ADHD and learning difficulties have a greater lifetime concussion history. J Atten Disord July 2016. 10.1177/1087054716657410.1087054716657410. [DOI] [PubMed] [Google Scholar]
- [24].Nelson LD, Guskiewicz KM, Marshall SW, et al. Multiple self-reported concussions are more prevalent in athletes with ADHD and learning disability. Clin J Sport Med 2016;26(2):120 10.1097/JSM.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015;1(1):40–3. 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- [26].Keyes KM, Maslowsky J, Hamilton A, et al. The great sleep recession: changes in sleep duration among US adolescents, 1991–2012. Pediatrics 2015;135(3): 460–8. 10.1542/peds.2014-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu Y Prevalence of healthy sleep duration among adults d United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- [28].Mah CD, Kezirian EJ, Marcello BM, et al. Poor sleep quality and insufficient sleep of a collegiate student-athlete population. Sleep Health 2018;4(3): 251–7. 10.1016/j.sleh.2018.02.005. [DOI] [PubMed] [Google Scholar]
- [29].Ma J, Yao Y-J, Ma R-M, et al. Effects of sleep deprivation on human postural control, subjective fatigue assessment and psychomotor performance. J Int Med Res 2009;37(5):1311–20. 10.1177/147323000903700506. [DOI] [PubMed] [Google Scholar]
- [30].Renn RP, Cote KA. Performance monitoring following total sleep deprivation: effects of task type and error rate. Int J Psychophysiol 2013;88(1):64–73. 10.1016/j.ijpsycho.2013.01.013. [DOI] [PubMed] [Google Scholar]
- [31].Heaton KJ, Maule AL, Maruta J, et al. Attention and visual tracking degradation during acute sleep deprivation in a military sample. Aviat Space Environ Med 2014;85(5):497–503. 10.3357/ASEM.3882.2014. [DOI] [PubMed] [Google Scholar]
- [32].Smith CD, Cooper AD, Merullo DJ, et al. Sleep restriction and cognitive load affect performance on a simulated marksmanship task. J Sleep Res 2017. 10.1111/jsr.12637. [DOI] [PubMed] [Google Scholar]
- [33].Furtado F, Bruno da Salva BGonçalves, Abrantes ILL, et al. Chronic low quality sleep impairs postural control in healthy adults. PLoS One 2016;11(10): e0163310 10.1371/journal.pone.0163310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev 2017;31:6–16. 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- [35].Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res 2011;217(2):463–6. 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- [36].Palagini L, Caruso D, Mainardi C, et al. Lack of resilience is related to hyperarousal, emotion dysregulation and increased impulsivity in insomnia disorder. Sleep Med 2017;40:e47 10.1016/j.sleep.2017.11.130. [DOI] [Google Scholar]
- [37].Short MA, Weber N. Sleep duration and risk-taking in adolescents: a systematic review and meta-analysis. Sleep Med Rev 2018;41:185–96. 10.1016/j.smrv.2018.03.006. [DOI] [PubMed] [Google Scholar]
- [38].Fullagar HHK, Skorski S, Duffield R, et al. Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med 2015;45(2):161–86. 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- [39].Patrick Y, Lee A, Raha O, et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol Rhythms 2017;15(3): 217–25. 10.1007/s41105-017-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ben Cheikh R, Latiri I, Dogui M, et al. Effects of one-night sleep deprivation on selective attention and isometric force in adolescent karate athletes. J Sports Med Phys Fitness 2017;57(6):752–9. 10.23736/S0022-4707.16.06323-4. [DOI] [PubMed] [Google Scholar]
- [41].Knowles OE, Drinkwater EJ, Urwin CS, et al. Inadequate sleep and muscle strength: implications for resistance training. J Sci Med Sport 2018;21(9): 959–68. 10.1016/j.jsams.2018.01.012. [DOI] [PubMed] [Google Scholar]
- [42].Milewski MD, Skaggs DL, Bishop GA, et al. Chronic lack of sleep is associated with increased sports injuries in adolescent athletes. J Pediatr Orthop 2014;34(2):129 10.1097/BPO.0000000000000151. [DOI] [PubMed] [Google Scholar]
- [43].von Rosen P, Frohm A, Kottorp A, et al. Multiple factors explain injury risk in adolescent elite athletes: applying a biopsychosocial perspective. Scand J Med Sci Sports 2017;27(12):2059–69. 10.1111/sms.12855. [DOI] [PubMed] [Google Scholar]
- [44].von Rosen P, Frohm A, Kottorp A, et al. Too little sleep and an unhealthy diet could increase the risk of sustaining a new injury in adolescent elite athletes. Scand J Med Sci Sports 2017;27(11):1364–71. 10.1111/sms.12735. [DOI] [PubMed] [Google Scholar]
- [45].Bastien CH, Valliéres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4): 297–307. 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- [46].Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [47].Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46(10):1121–3. 10.1001/ARCHNEUR.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- [48].Fichtenberg NL, Putnam SH, Mann NR, et al. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh sleep quality Index. Am J Phys Med Rehabil 2001;80(5):339–45. 10.1097/00002060-200105000-00003. [DOI] [PubMed] [Google Scholar]
- [49].Centers for Disease Control and Prevention (CDC). In: Hyattsville MD, editor. National center for health statistics (NCHS) National health and nutrition examination survey sleep disorders questionnaire. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/questionnaires/SLQ_J.pdf. [Google Scholar]
- [50].Zuckerman SL, Kerr ZY, Yengo-Kahn A, et al. Epidemiology of sports-related concussion in NCAA athletes from 2009–2010 to 2013–2014: incidence, recurrence, and mechanisms. Am J Sports Med 2015;43(11):2654–62. 10.1177/0363546515599634. [DOI] [PubMed] [Google Scholar]
- [51].Kerr ZY, Roos KG, Djoko A, et al. Epidemiologic measures for quantifying the incidence of concussion in national collegiate athletic association sports. J Athl Train 2016;52(3):167–74. 10.4085/1062-6050-51.6.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/. [Google Scholar]
- [53].Wickham H, Francois R. Dplyr: a grammar of data manipulation. 2015. https://CRAN.R-project.org/package=dplyr.
- [54].Wickham H Tidyr: easily tidy data with `spread ( )` and `gather ( )` functions. 2016. https://CRAN.R-project.org/package=tidyr.
- [55].Wickham H Stringr: simple, consistent wrappers for common string operations. 2018. https://CRAN.R-project.org/package=stringr.
- [56].Grolemund G, Wickham H. Dates and times made easy with lubridate. J Stat Softw 2011;40(3):1–25. 10.18637/jss.v040.i03. [DOI] [Google Scholar]
- [57].Venables WN, Ripley BD. Modern applied Statistics with S. Fourth. New York: Springer; 2002. http://www.stats.ox.ac.uk/pub/MASS4. [Google Scholar]
- [58].Wickham H Ggplot2: elegant graphics for data analysis. Springer-Verlag; New York; 2016. http://ggplot2.org. [Google Scholar]
- [59].Aragon TJ. Epitools: epidemiology tools. 2017. https://CRAN.R-project.org/package=epitools.
- [60].Gosselin N, Duclos C. Insomnia following a mild traumatic brain injury: a missing piece to the work disability puzzle? Sleep Med 2016;20:155–6. 10.1016/J.SLEEP.2015.10.011. [DOI] [PubMed] [Google Scholar]
- [61].Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Insomnia in patients with traumatic brain injury: frequency, characteristics, and risk factors. J Head Trauma Rehabil 2006;21(3):199–212. 10.1097/00001199-200605000-00001. [DOI] [PubMed] [Google Scholar]
- [62].Ouellet M-C, Morin CM. Subjective and objective measures of insomnia in the context of traumatic brain injury: a preliminary study. Sleep Med 2006;7(6): 486–97. 10.1016/j.sleep.2006.03.017. [DOI] [PubMed] [Google Scholar]
- [63].Allan AC, Edmed SL, Sullivan KA, et al. Actigraphically measured sleep-wake behavior after mild traumatic brain injury: a case-control study. J Head Trauma Rehabil 2017;32(2):E35 10.1097/HTR.0000000000000222. [DOI] [PubMed] [Google Scholar]
- [64].Hoffman NL, O’Connor PJ, Schmidt MD, et al. Differences in sleep between concussed and nonconcussed college students: a matched caseecontrol study. Sleep 2019;42(2). 10.1093/SLEEP/ZSY222. [DOI] [PubMed] [Google Scholar]
- [65].Raikes AC, Satterfield BC, Killgore WDS. Evidence of actigraphic and subjective sleep disruption following mild traumatic brain injury. Sleep Med 2019;54: 62–9. 10.1016/J.SLEEP.2018.09.018. [DOI] [PubMed] [Google Scholar]
- [66].Raikes AC, Schaefer SY. Sleep quantity and quality during acute concussion: a pilot study. Sleep 2016;39(12):2141–7. 10.5665/sleep.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sufrinko AM, Howie EK, Elbin RJ, et al. A preliminary investigation of accelerometer-derived sleep and physical activity following sport-related concussion. J Head Trauma Rehabil 2018. 10.1097/HTR.0000000000000387. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- [68].Chau K Impact of sleep difficulty on single and repeated injuries in adolescents. Accid Anal Prev 2015;81:86–95. 10.1016/j.aap.2015.04.031. [DOI] [PubMed] [Google Scholar]
- [69].Gupta L, Morgan K, Gilchrist S. Does elite sport degrade sleep quality? A systematic review. Sports Med 2017;47(7):1317–33. 10.1007/s40279-016-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Luyster F, Dunn R, Lauderdale D, et al. Sleep-apnea risk and subclinical atherosclerosis in early-middle-aged retired National Football League players. Nat Sci Sleep 2017;9:31–8. 10.2147/NSS.S125228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Swinbourne R, Gill N, Vaile J, et al. Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes. Eur J Sport Sci 2016;16(7):850–8. 10.1080/17461391.2015.1120781. [DOI] [PubMed] [Google Scholar]
- [72].Juliff LE, Halson SL, Peiffer JJ. Understanding sleep disturbance in athletes prior to important competitions. J Sci Med Sport 2015;18(1):13–8. 10.1016/j.jsams.2014.02.007. [DOI] [PubMed] [Google Scholar]
- [73].Fowler P, Duffield R, Howle K, et al. Effects of northbound long-haul international air travel on sleep quantity and subjective jet lag and wellness in professional Australian soccer players. Int J Sports Physiol Perform 2015;10(5):648–54. 10.1123/ijspp.2014-0490. [DOI] [PubMed] [Google Scholar]
- [74].Fullagar HHK, Duffield R, Skorski S, et al. Sleep, travel, and recovery responses of national footballers during and after long-haul international air travel. Int J Sports Physiol Perform 2016;11(1):86–95. 10.1123/ijspp.2015-0012. [DOI] [PubMed] [Google Scholar]
- [75].Peck B, Renzi T, Peach H, et al. Examination of risk for sleep-disordered breathing among college football players. J Sport Rehabil 2018;28(2): 126–32. 10.1123/jsr.2017-0127. [DOI] [PubMed] [Google Scholar]
- [76].Dobrosielski DA, Nichols D, Ford J, et al. Estimating the prevalence of sleep-disordered breathing among collegiate football players. Respir Care March 2016:04520 10.4187/respcare.04520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


