SARS-CoV-2 spike (S) glycoprotein is the key target of current vaccine development efforts to combat COVID-19; neutralizing antibodies bind S and interfere with S binding to its receptor, angiotensin-converting enzyme 2. Recent work reveals the molecular basis of increased infectivity of the globally prevailing D614G S mutation.
The SARS-CoV-2 pandemic is a global crisis causing countless deaths and economic damage worldwide, vastly surpassing the previous SARS-CoV outbreak in 2002–2004. SARS-CoV-2 S glycoprotein is cleaved into S1 and S2 subunits enabling fusion of virus and host cell membranes. SARS-CoV-2 S has higher affinity for angiotensin-converting enzyme 2 (ACE2) as compared to SARS-CoV S due to six mutations in the receptor-binding motif (RBM).1,2 Moreover, SARS-CoV-2 S acquired a furin cleavage site between S1 and S2 (Fig. 1), thought to promote pathogenecity.3 ACE2 binding triggers conformational changes that allow host proteases to further cleave S2, followed by shedding of S1 and activation of drastic S2 refolding into a post-fusion state.4
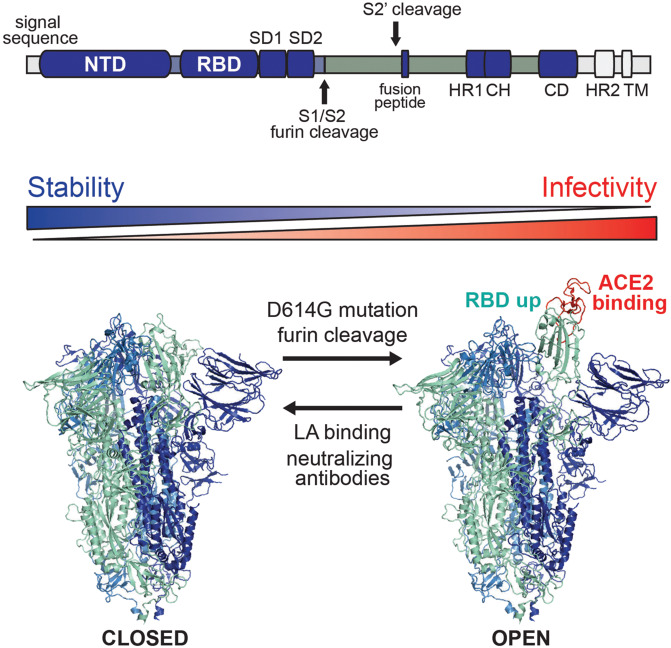
Fig. 1. SARS-CoV-2 spike prefusion structure in a dynamic equilibrium between closed and open states.
Above: Domain arrangement of SARS-CoV-2 S glycoprotein. S1 comprises a signal sequence, NTD, RBD, SD1 and SD2. S2 comprises a second cleavage site upstream of the fusion peptide, HR1, CH, CD, HR2, the TM domain and cytoplasmic C-terminus. NTD, N-terminal domain; RBD, receptor-binding domain; SD1, subdomain 1; SD2, subdomain 2; S2’, second protease cleavage site; HR1, heptad repeat 1; CH, central helix; CD, connector domain; HR2, heptad repeat 2; TM, transmembrane domain. Cleavage sites are marked with arrow. Below: Closed (PDB ID: 6zb58) and open (PDB ID: 6zgg3) conformations of S are in a dynamic equilibrium which is shifted by mutations, proteolytic cleavage, binding of LA or antibodies. The open conformations, with one or more RBDs up, expose the RBM (colored in red) required for ACE2 binding and subsequent host cell infection.
For cryo-EM and functional studies, different strategies were followed to stabilize the prefusion SARS-CoV-2 S trimer: mutation or deletion of the furin cleavage site, two proline (2P) mutations in S2 (K986P, V987P) and a C-terminal trimerization domain which replaces the transmembrane domain and cytoplasmic C-terminus of wild-type S.1,2 Numerous S structures were determined, revealing a highly dynamic protein: S receptor-binding domains (RBDs) undergo hinge-like movements to switch between “down” and “up” conformations; “up” is compatible with ACE2 binding while the RBM is tucked away in the “down” conformation. 50%–70% open conformations (RBDs up-down-down) were observed for S, along with 30%–50% closed trimer (RBDs all-down) in cryo-EM samples, with the open form thought to mediate infectivity.1,2 The structure of S with a native furin site, stabilized by the 2P mutation, highlighted the impact of cleavage3: while in the uncleaved form ~83% of S were in a closed conformation, only 34% were closed in furin-cleaved S. The remaining particles adopted intermediate (one RBD disordered) and open conformations (up-down-down). Overall, furin cleavage facilitates the movement of S1 RBDs and N-terminal domains (NTDs), leading to lower thermal stability and more receptor binding-competent forms of S.
Since the outbreak of the COVID-19 pandemic, only a few mutations reached high global frequency. One of these mutations is the D614G mutation in S which became dominant during the pandemic, virtually replacing the ancestral S. D614G S is associated with increased infectivity and viral load in patients with COVID-19.5 Yurkovetskiy et al.5 shed light on the molecular mechanism leading to dramatic changes in S structure and dynamics, caused by just a single mutation: the D614G S-trimer structure, stabilized in this study by deletion of the furin site and 2P mutations, revealed a preponderance of open conformations (~95%). In addition to the up-down-down open conformation, 2RBDs-up and 3RBDs-up conformations were observed.5 The authors determined a decreased affinity of D614G for ACE2, which appears to be compensated by the presence of many more receptor binding-competent RBDs. Residue D614 is positioned in subdomain SD2 and forms a hydrogen bond with T859 in S2,5 stabilizing the S1–S2 interface. In D614G, the inter-protomer hydrogen bond is replaced by an intra–SD2 interaction. Globally, destabilization of the S1–S2 interface by D614G triggers movement of S1 domains away from the S-trimer axis and RBD opening, likely leading to increased ACE2 binding and elevated viral infectivity.
These findings agree with studies describing subdomains SD1 and SD2 of S1 as a hinge for RBD up-movement.6,7 Henderson et al. introduced four hydrophobic mutations at the SD1–S2 interface to destabilize the interface and increase S1 mobility. The structure of this mutant indeed showed a prevalence of 1RBD-up and 2RBDs-up conformations.6 Similarly, structures of ACE2-bound S suggest a weakening of the S1–S2 interface upon ACE2 binding and destabilization of the S1-trimer.7 In the presence of ACE2, more open RBD conformations (2RBDs-up and 3RBDs-up) were observed and are thought to be on pathway for virion–host cell membrane fusion and cell entry. In addition, ACE2 binding was shown to induce SD2 refolding, which disrupts sidechain π-stacking interactions between SD2 and S2 and a salt bridge between D614 and K854 (rather than T8595).7 Moreover, a region in S2 proximal to the S2’ cleavage site becomes disordered, potentially exposing the cleavage site.7 Based on this, the D614G mutation likely facilitates SD2 rearrangements and destabilizes the closed conformation, thus increasing the likelihood of S trimers to adopt open conformations.5,7
While furin cleavage and D164G mutation destabilize S, binding of a free fatty acid, linoleic acid (LA) to a pocket in the RBD stabilizes the trimer.8 The cryo-EM structure of LA-bound S, stabilized by mutation of the furin cleavage site, revealed a compact, locked S conformation adopted by ~70% of the trimers. In S, LA binds to three bipartite binding pockets, formed by adjacent RBDs, leading to RBD-trimer compaction incompatible with ACE2 binding. Consequently, LA-bound S had reduced affinity for ACE2 in vitro. In cell culture, LA synergized with remdesivir, an RNA-dependent RNA polymerase inhibitor, markedly suppressing SARS-CoV-2 replication in human epithelial cells,8 which conveys that supplementation of LA or LA-mimic could be utilized as an antiviral to block virus replication.
Neutralizing antibodies primarily interfere with ACE2 binding by S.9 However, several potent neutralizing antibodies were reported to use a different mechanism, i.e., stabilizing the closed conformation by binding the NTD or the RBD–RBD interface,9 rather than directly targeting the RBM. In comparison to the above single-particle cryo-EM studies, cryo-tomography of intact SARS-CoV-2 virions, fixed with paraformaldehyde, revealed on average 26 ± 15 S-trimers per virion of which ~54% were in the closed conformation.10
Taken together, the current findings support the view that S proteins have evolved finely tuned mechanisms to reside in a metastable prefusion state, balancing RBM masking to avoid neutralization by the host immune response and its exposure which is necessary to enable ACE2 interaction and subsequent host cell infection. Mutations, proteolytic cleavage, or LA binding all affect the degrees of freedom of RBD movement. In agreement, differences between different β-coronavirus strains are clustered to domain interfaces,6 underscoring that subtle changes in interdomain contacts determine RBD conformations with repercussions on SARS-CoV-2 infectivity and thus COVID-19 disease phenotypes.
References
- 1.Wrapp D, et al. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walls AC, et al. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrobel AG, et al. Nat. Struct. Mol. Biol. 2020;27:763–767. doi: 10.1038/s41594-020-0468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Y, et al. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurkovetskiy L, et al. Cell. 2020 doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson R, et al. Nat. Struct. Mol. Biol. 2020;27:925–933. doi: 10.1038/s41594-020-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benton DJ, et al. Nature. 2020 doi: 10.1038/s41586-020-2772-0. [DOI] [PubMed] [Google Scholar]
- 8.Toelzer C, et al. Science. 2020 doi: 10.1126/science.abd3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, et al. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 10.Yao H, et al. Cell. 2020 doi: 10.1016/j.cell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]