Abstract
Purpose
In 2007, the American Society of Clinical Oncology and the College of American Pathologists had established a human epidermal growth factor receptor 2 (HER2) testing guideline, which was updated in 2013 and subsequently in 2018. We assessed the clinical impact of the recent update by comparing the in situ hybridization (ISH) results based on the 2007, 2013, and 2018 guidelines.
Methods
We assessed 2 cohorts. The first cohort included 1,161 primary invasive breast cancer (IBC) samples including 18 bilateral IBC cases, with both immunohistochemistry (IHC) and silver-enhanced ISH (SISH) results available for the HER2 status. The second cohort included 160 IBC cases with equivocal HER2 IHC, assessed using SISH. We retrospectively evaluated and compared the HER2 SISH results.
Results
There were 22 (1.9%) and 20 (12.5%) cases with altered SISH results according to the 2013 guidelines in cohorts 1 and 2, respectively. As per the 2018 guidelines, final HER2 statuses of 16 (1.4%) and 14 (8.5%) cases changed in cohorts 1 and 2, respectively. The 2013 guidelines increased the positive rate compared to the 2007 guidelines, in both cohorts (0.6% and 6.2%, respectively). Most equivocal cases in cohorts 1 (92.3%) and 2 (100%) as per the 2013 guidelines were reclassified as HER2-negative according to the 2018 guidelines. The 2018 guidelines increased the negative rates (1.3% in cohort 1 and 8.7% in cohort 2) and slightly decreased the positive rates (−0.2% in cohort 1 and −3.1% in cohort 2), compared to the 2013 guidelines. With each update, minor changes in the positive and negative rates were observed in whole breast cancer samples (cohort 1). However, the 2018 guidelines affected previously defined HER2-positive IBC with equivocal IHC results.
Conclusion
Under the 2013 guidelines, the positive and equivocal cases increased. However, the 2018 guidelines eliminated ambiguous cases by reclassifying them as HER2-negative.
Keywords: Breast neoplasms, c-erbB-2 protein, Guideline, Immunohistochemistry, In situ hybridization
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2) gene, which encodes a transmembrane tyrosine kinase, is located on the chromosome 17q21. HER2 gene amplification and/or protein overexpression have been reported in 15%–20% of patients with invasive breast cancer (IBC) [1].
HER2-positive IBC is associated with poor prognoses, and a decreased sensitivity to anthracycline-based chemotherapy and HER2-targeted therapy using drugs such as trastuzumab, lapatinib, and pertuzumab [2,3]. It is therefore necessary to evaluate the HER2 status in IBC patients at the time of their initial diagnosis and metastasis [4], since the accurate HER2 status in tumor specimens is crucial for patients, clinicians, and pathologists.
The HER2 gene amplification can be assessed by in situ hybridization (ISH), including fluorescence ISH (FISH), chromogenic ISH and silver-enhanced ISH (SISH). Moreover, the HER2 protein overexpression can be evaluated by immunohistochemistry (IHC) [1]. In Korea, IHC is applied as the primary test and SISH has been used for the confirmation of HER2 IHC equivocal cases since October 2013 [5]. In 2007, the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) published the first set of recommendations for the optimal performance of HER2 testing [4]. The recommendations include the cutoff values of HER2 ISH interpretation, definition of the IHC results, and signposts for ambiguous cases. Since the issuance of the first major update in 2013 [6], ASCO/CAP released a focused update, in 2018, regarding the HER2 testing algorithm for a subset of ambiguous and difficult cases that may be observed using a dual-probe ISH assay [7]. The previous 2007 and 2013 guidelines suggested that IHC or ISH alone could establish the HER2 status, except for equivocal cases; the repeat or reflex IHC/ISH testing not performed on primary workup, was recommended to clarify the results. Compared to the 2007 guidelines, the 2013 guidelines include changes in the cut-offs for positive HER2, in both IHC and ISH. However, as per the latest guidelines, it is important that the integrated results of IHC and ISH are considered for deciding the accurate HER2 status in IBC patients.
This study aims to evaluate the impact of changes in the HER2 status categorization of IBC patients using SISH, according to the 2007, 2013, and 2018 ASCO/CAP guidelines.
METHODS
Patient selection
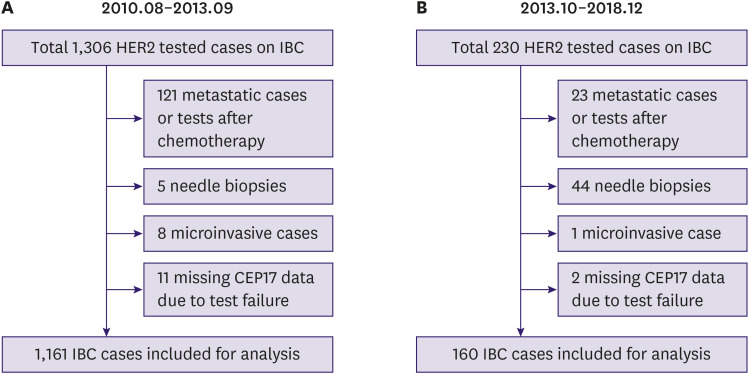
After receiving approval from the Institutional Review Board of Yeungnam University Hospital, Daegu, Korea (YUMC 2019-09-083), we retrospectively collected the data of primary IBC patients who had undergone surgical resection and HER2 evaluation using both IHC and SISH, at Yeungnam University Hospital. The requirement for the informed consent of the patient was waived. Patients who received neoadjuvant chemotherapy or underwent HER2 testing using needle biopsy specimens were excluded. Based on the national health insurance policy, we included 2 large study cohorts. The first cohort comprised 1,161 IBC cases from 1,143 patients, including 18 bilateral IBC patients who underwent surgical resection between August 2010 and September 2013 (Figure 1A). During this period, all IBC cases in Korea were eligible for both IHC and ISH testing for determining the HER2 status at the time of diagnosis. All IHC and SISH tests were interpreted according to the 2007 ASCO/CAP guidelines [4]. The second cohort comprised patients diagnosed between October 2013 and December 2018 and included 160 IHC 2+ IBC cases with corresponding SISH results (Figure 1B). Since October 2013, the Health Insurance Review and Assessment Service in Korea released a new guideline which stated that IHC should be performed as an initial test for screening the HER2-targeted therapy eligibility and that only equivocal IHC results would be subjected to additional alternative tests by ISH [5]. At the time of diagnosis for this cohort, IHC and SISH results were interpreted using the 2013 ASCO/CAP guidelines.
Figure 1. Flowchart showing the case selection process and exclusion reasons. (A) Case selection process for cohort 1. (B) Case selection process for cohort 2.
HER2 = human epidermal growth factor receptor 2; IBC = invasive breast cancer; CEP17 = chromosome 17.
All IHC and SISH tests at the time of diagnosis were interpreted by a board-certified senior pathologist with a subspecialty in breast pathology. The patient demographic data, tumor characteristics, HER2 IHC results, and HER2 SISH results, including the average HER2 signals per cell, average CEP17 signals per cells, and the HER2/CEP17 ratio, were obtained from the pathology reports.
HER2 IHC
The IHC staining for HER2 protein was performed on 4 μm thick formalin-fixed paraffin-embedded (FFPE) tissue sections using a Benchmark® automatic immunostaining device (Ventana Medical Systems, Tucson, USA), according to the manufacturer's recommendations. Rabbit anti-HER2 antibody (CONFIRM anti-HER2/neu (4B5) rabbit monoclonal antibody; Ventana Medical Systems) and an UltraView™ DAB detection kit (Ventana Medical Systems) were used. The diagnosis of the IHC results was interpreted according to the 2007 ASCO/CAP guidelines (cohort 1 and part of cohort 2 until the release of the 2013 guidelines) or the 2013 guidelines (cohort 2).
HER2 SISH
SISH was performed using INFORM® HER2 and chromosome 17 (CEP17) DNA probes (Ventana Medical Systems) on two 3 μm thick, consecutive FFPE tissue sections, using the Benchmark® automatic immunostaining device until March 2012, as described previously [8]. In these assays, the black silver signals represented the copy number of each target. The SISH signals for HER2 and CEP17 were counted in more than 20 non-overlapping nuclei per sample in the same areas on separate slides, using a conventional Nikon Eclipse 80i Microscope (× 600 magnification; Nikon, Tokyo, Japan). On observing the intratumoral heterogeneity in the signal distribution, more than 100 nuclei per sample were counted. A discrete dot was counted as a single copy of HER2 and CEP17, and individual dot sizes were used as reference to determine the relative number of amplified copies in cancer cell nuclei. As per the manufacturer's instructions, a small cluster of multiple signals was counted as 6 signals and a large cluster was counted as 12 signals. The average HER2 and CEP17 signals per cell, and the HER2/CEP17 ratio, were obtained for each case.
Since April 2012, HER2 gene amplification in our laboratory was confirmed using the INFORM HER2 Dual ISH DNA Probe Cocktail Assay (Ventana Medical Systems). In dual-probe SISH, the red signals refer to CEP17 and black small dots denote the HER2 probe. The original SISH results of the cohort 2 cases were interpreted according to the 2007 ASCO/CAP guidelines, until the release of the 2013 guidelines. The SISH was approved by the U.S. Food and Drug Administration (FDA) only in June 2011 additionally, the 2007 ASCO/CAP guidelines did not include the scoring algorithms for SISH. Hence, we followed the algorithm for FISH at the time of diagnosis, as recommended by the manufacturer.
For this study, the SISH results of all the cases were categorized using the scoring thresholds and definitions of the 2007, 2013, and 2018 ASCO/CAP guidelines [4,6,7] and the final diagnostic categories were compared. The 2007, 2013, and 2018 HER2 scoring criteria are presented in Table 1. In addition to the cases with discrepant HER2 SISH results according to the 2007, 2013 and 2018 guidelines, cases with discrepant results between IHC and SISH were selectively reviewed.
Table 1. Interpretation of ISH assay for HER2 status according to the 2007, 2013, and 2018 ASCO/CAP guidelines.
| 2007 guideline | 2013 guideline | 2018 guideline | ||||
|---|---|---|---|---|---|---|
| ISH criteria | Result | ISH criteria | Result | ISH criteria | IHC | Result |
| HER2/CEP17 > 2.2 or HER2 gene copy > 6.0 | Positive | HER2/CEP17 ≥ 2.0 and HER2 gene copy ≥ 4.0 | Positive | Group 1: HER2/CEP17 ≥ 2.0 and HER2 gene copy ≥ 4.0 | Any | Positive |
| HER2/CEP17 ≥ 2.0 and HER2 gene copy < 4.0 | Positive | Group 2: HER2/CEP17 ≥ 2.0 and HER2 gene copy < 4.0 | 0, 1+, 2+ | Negative† | ||
| 3+ | Positive | |||||
| HER2/CEP17 < 2.0 and HER2 gene copy ≥ 6.0 | Positive | Group 3: HER2/CEP17 < 2.0 and HER2 gene copy ≥ 6.0 | 0, 1+ | Negative | ||
| 2+, 3+ | Positive* | |||||
| HER2/CEP17 1.8–2.2 or HER2 gene copy 4.0–6.0 | Equivocal | HER2/CEP17 < 2.0 and HER2 gene copy ≥ 4.0 and < 6.0 | Equivocal | Group 4: HER2/CEP17 < 2.0 and HER2 gene copy ≥ 4.0 and < 6.0 | 0, 1+, 2+ | Negative† |
| 3+ | Positive | |||||
| HER2/CEP17 2.2 or HER2 gene copy < 4.0 | Negative | HER2/CEP17 < 2.0 and HER2 gene copy < 4.0 | Negative | Group 5: HER2/CEP17 ratio < 2.0 and HER2 gene copy < 4.0 | Any | Negative |
ISH = in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists; HER2 = human epidermal growth factor receptor 2; CEP17 = chromosome 17; IHC = immunohistochemistry.
*An additional observer blinded to previous results recounts ISH. If the HER2/CEP17 ratio remains < 2.0 with ≥ 6.0 HER2 signals/cell, diagnosis is HER2 positive; †An additional observer blinded to previous results recounts ISH. If the repeated ISH result is designated to the same ISH group, it is finally regarded as HER2 negative.
RESULTS
Cohort 1
A total of 1,161 surgically resected IBC samples from 1,143 patients, including 18 bilateral IBCs, were collected for this study. The patient age ranged from 25 years to 85 years, with a median age of 50 years, at the time of diagnosis. During the study period, 585 samples (50.4%) underwent single-probe SISH for HER2 and CEP17 in 2 consecutive slides, and 576 samples (49.6%) were subjected to dual-probe SISH. The distribution of HER2 IHC results were 0 in 673 (58%), 1+ in 182 (15.7%), 2+ in 103 (8.9%), and 3+ in 203 (17.5%) cases.
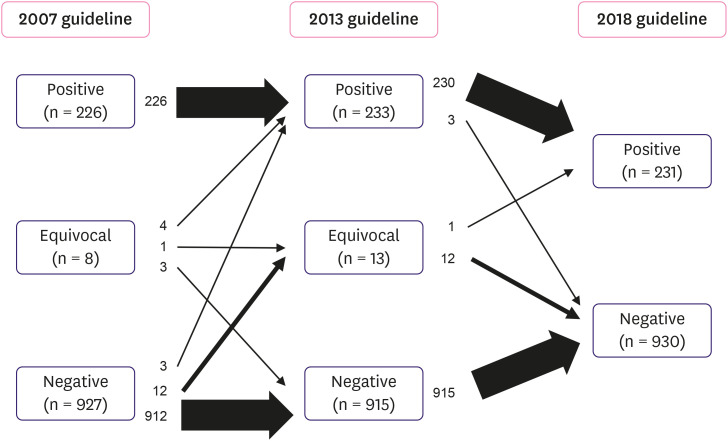
Based on the 2007 guidelines, the initial SISH results revealed 226 (19.5%) positive, 8 (0.7%) equivocal, and 927 (79.8%) negative cases (Table 2). Reviewing the same cases as per the updated 2013 guidelines altered the SISH results of 22 (1.9%) samples, classifying a total of 233 (20.1%) positive, 13 (1.1%) equivocal, and 915 (78.8%) negative cases (Table 2). Among 8 equivocal cases as determined by the 2007 guidelines, 4 cases (50%) were altered to HER2 positive and 3 cases (37.5%) were classified as HER2 negative as per the 2013 guidelines. One case (12.5%) remained equivocal. Of the 927 negative cases, 3 cases (0.3%) were reclassified as HER2 positive, and 12 cases (1.3%) were reclassified as equivocal cases based on the 2013 guidelines (Figure 2). Therefore, compared with the use of the 2007 guidelines, the use of 2013 guidelines resulted in small increases in HER2 positive (20.1% vs. 19.5%) and equivocal (1.1% vs. 0.7%) cases. Detailed information of the cases in cohort 1 with discrepant HER2 SISH results according to the 2007, 2013, and 2018 guidelines are listed in Table 3.
Table 2. Distribution of HER2 SISH results according to the 2007, 2013 and 2018 ASCO/CAP guidelines in cohort 1.
| Result | 2007 | 2013 | 2018 |
|---|---|---|---|
| Positive | 226 (19.5) | 233 (20.1) | 231 (19.9) |
| Equivocal | 8 (0.7) | 13 (1.1) | 0 (0) |
| Negative | 927 (79.8) | 915 (78.8) | 930 (80.1) |
| Total | 1,161 | 1,161 | 1,161 |
Values are presented as number (%).
HER2 = human epidermal growth factor receptor 2; SISH = silver-enhanced in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists.
Figure 2. Flowchart of cases according to the 2007, 2013, and 2018 ASCO/CAP guidelines in cohort 1. The left column denotes the distribution of cases under the 2007 criteria. The application of the 2013 recommendations is depicted in the middle column. The right column indicates the distribution of cases using the 2018 guidelines. Each arrow shows the movement of cases. The thicknesses of arrows denote the amount of cases. The details of cases affected by each guideline are summarized in Table 3.
ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists.
Table 3. Details of cases with discrepant HER2 SISH results according to the 2007, 2013 and 2018 ASCO/CAP HER2 testing guidelines in cohort 1.
| Case number | IHC result | Average HER2 signal/cell | Average CEP17 signal/cell | HER2/CEP17 ratio | SISH results | |||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2013 | 2018 | ||||||
| Group | Result | |||||||
| 174 | 2+ | 4.45 | 3.30 | 1.35 | Negative | Equivocal | 4 | Negative |
| 204 | 2+ | 4.70 | 4.05 | 1.16 | Negative | Equivocal | 4 | Negative |
| 288 | 2+ | 4.45 | 3.05 | 1.46 | Negative | Equivocal | 4 | Negative |
| 306 | 2+ | 4.00 | 2.70 | 1.48 | Negative | Equivocal | 4 | Negative |
| 317 | 2+* | 4.00 | 2.50 | 1.60 | Negative | Equivocal | 4 | Negative |
| 504 | 2+ | 5.10 | 3.15 | 1.62 | Negative | Equivocal | 4 | Negative |
| 601 | 2+ | 4.30 | 3.80 | 1.13 | Negative | Equivocal | 4 | Negative |
| 845 | 0 | 4.30 | 3.05 | 1.41 | Negative | Equivocal | 4 | Negative |
| 1,087 | 2+* | 4.20 | 3.10 | 1.35 | Negative | Equivocal | 4 | Negative |
| 1,147 | 1+ | 4.00 | 3.80 | 1.05 | Negative | Equivocal | 4 | Negative |
| 1,158 | 1+ | 4.05 | 3.15 | 1.29 | Negative | Equivocal | 4 | Negative |
| 328 | 3+ | 5.70 | 3.40 | 1.68 | Negative | Equivocal | 4 | Positive |
| 37 | 3+ | > 20.00 | > 20.00 | 1.00 | Negative | Positive | 3 | Positive |
| 214 | 3+ | 7.20 | 4.85 | 1.48 | Negative | Positive | 3 | Positive |
| 749 | 2+ | 6.85 | 5.80 | 1.18 | Negative | Positive | 3 | Positive |
| 308 | 3+ | 8.75 | 4.00 | 2.19 | Equivocal | Positive | 1 | Positive |
| 569 | 3+ | 7.35 | 3.55 | 2.07 | Equivocal | Positive | 1 | Positive |
| 648 | 0 | 2.05 | 1.00 | 2.05 | Equivocal | Positive | 2 | Negative |
| 699 | 2+ | 2.80 | 1.33 | 2.11 | Equivocal | Positive | 2 | Negative |
| 369 | 0 | 3.60 | 2.00 | 1.80 | Equivocal | Negative | 5 | Negative |
| 693 | 1+ | 3.40 | 1.85 | 1.84 | Equivocal | Negative | 5 | Negative |
| 913 | 3+ | 3.80 | 1.95 | 1.95 | Equivocal | Negative | 5 | Negative |
| 1,115 | 0 | 4.28 | 2.30 | 1.86 | Equivocal | Equivocal | 4 | Negative |
| 62 | 2+ | 2.80 | 1.10 | 2.55 | Positive | Positive | 2 | Negative |
HER2 = human epidermal growth factor receptor 2; SISH = silver-enhanced in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists; IHC = immunohistochemistry; CEP17 = chromosome 17.
*Changes in HER2 IHC results according to the 2018 guideline.
The classification of the SISH results as per the 2018 guidelines determined 226 cases (19.5%) in group 1, 4 cases (0.3%) in group 2, 3 cases (0.2%) in group 3, 13 cases (1.1%) in group 4, and 915 cases (78.8%) in group 5 (Table 4). The original HER2 IHC slides were reviewed and redefined according to the 2018 criteria, and 2 cases showed different results. The cases 317 and 1,087 (Table 3) were interpreted as 1+ IHC according to the 2007 guidelines; however, because of weak, incomplete staining of more than 10% tumor cells according to the 2013 and 2018 ASCO/CAP HER2 IHC guidelines, they were interpreted as 2+. The final SISH results obtained as per the 2018 guidelines demonstrated 231 (19.9%) positive cases, 930 (80.1%) negative cases, and no equivocal case (Table 2). Compared to the 2013 diagnosis, the SISH results of 16 (1.4%) cases were changed with the updated guidelines of 2018. Three cases (1.3%) of the 233 HER2-positive cases (2013 classification) were reclassified as negative because of the absence of HER2 overexpression. These cases were equivocal (2 cases) and positive (1 case) on application of the 2007 guidelines. Moreover, 12 (92.3%) of 13 cases classified as equivocal by the 2013 guidelines were reclassified as HER2-negative, and the remaining 1 case was reclassified as HER2-positive, according to the 2018 guidelines (Table 3 and Figure 2). Diagnosis as per the latest guidelines revealed that 14 cases (1.2%) were discordant between the HER2 IHC and SISH; additionally, and only 5 of these patients received HER2-targeted therapy (Table 5 and Figure 3).
Table 4. Classification of HER2 result groups by the 2013 and 2018 ASCO/CAP HER2 testing guidelines in cohort 1.
| Group | 2013 ASCO/CAP guideline | 2018 ASCO/CAP guideline | |||
|---|---|---|---|---|---|
| Number | SISH result | Number | IHC result | SISH result | |
| 1 | 226 | Positive | 11 | 0 or 1+ | Positive |
| 19 | 2+ | Positive | |||
| 196 | 3+ | Positive | |||
| 2 | 4 | Positive | 1 | 0 or 1+ | Negative |
| 2 | 2+ | Negative | |||
| 1 | 3+ | Positive | |||
| 3 | 3 | Positive | 0 | 0 or 1+ | Negative |
| 1 | 2+ | Positive | |||
| 2 | 3+ | Positive | |||
| 4 | 13 | Equivocal | 6 | 0 or 1+ | Negative |
| 6 | 2+ | Negative | |||
| 1 | 3+ | Positive | |||
| 5 | 915 | Negative | 837 | 0 or 1+ | Negative |
| 75 | 2+ | Negative | |||
| 3 | 3+ | Negative | |||
HER2 = human epidermal growth factor receptor 2; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists; IHC = immunohistochemistry; SISH = silver-enhanced in situ hybridization.
Table 5. Cases with discordant results between HER2 IHC and SISH according to the 2018 ASCO/CAP HER2 testing guideline.
| Case number | HER2 IHC result | Average HER2 signal/cell | Average CEP17 signal/cell | HER2/CEP17 ratio | Anti-HER2 therapy | SISH result | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2007 | 2013 | 2018 | ||||||||
| Group | Result | |||||||||
| Positive IHC/negative SISH | ||||||||||
| 572 | 3+ | 2.00 | 1.90 | 1.05 | Yes | Negative | Negative | 5 | Negative | |
| 797 | 3+ | 1.15 | 8.70 | 0.13 | Yes | Negative | Negative | 5 | Negative | |
| 913 | 3+ | 3.80 | 1.95 | 1.95 | Yes | Equivocal | Negative | 5 | Negative | |
| Negative IHC/positive SISH | ||||||||||
| 492 | 0 | 4.15 | 1.75 | 2.37 | No | Positive | Positive | 1 | Positive | |
| 676 | 0 | 5.10 | 1.85 | 2.76 | No | Positive | Positive | 1 | Positive | |
| 816 | 0 | 6.20 | 1.90 | 3.26 | No | Positive | Positive | 1 | Positive | |
| 834 | 1+ | 6.60 | 1.65 | 4.00 | No | Positive | Positive | 1 | Positive | |
| 862 | 0 | 5.15 | 1.60 | 3.21 | Yes | Positive | Positive | 1 | Positive | |
| 917 | 1+ | 10.00 | 3.15 | 3.17 | Yes | Positive | Positive | 1 | Positive | |
| 924 | 1+ | 20.00 | 1.85 | 10.80 | No | Positive | Positive | 1 | Positive | |
| 940 | 1+ | 6.10 | 1.30 | 4.69 | No | Positive | Positive | 1 | Positive | |
| 962 | 1+ | 7.00 | 1.75 | 4.00 | No | Positive | Positive | 1 | Positive | |
| 1,023 | 1+ | 8.10 | 2.50 | 3.24 | No | Positive | Positive | 1 | Positive | |
| 1,110 | 1+ | 4.95 | 2.18 | 2.28 | Yes | Positive | Positive | 1 | Positive | |
HER2 = human epidermal growth factor receptor 2; IHC = immunohistochemistry; SISH = silver-enhanced in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists.
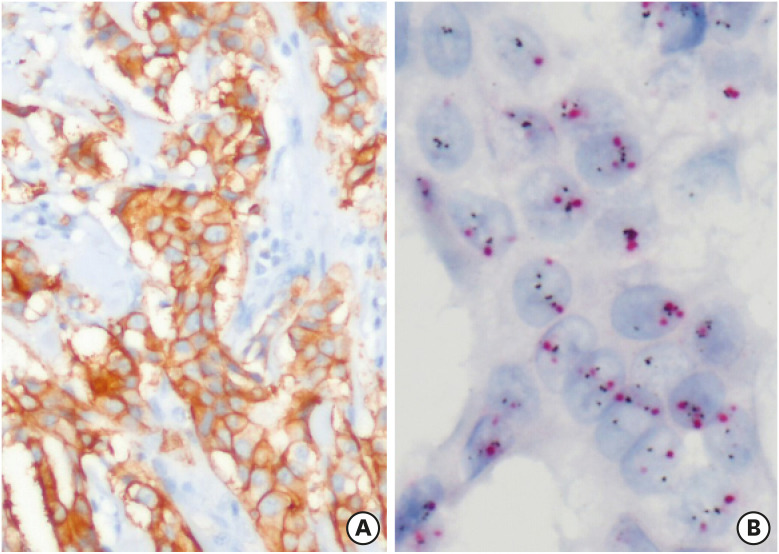
Figure 3. Representative images of cases with discordant HER2 IHC (A, × 100) and SISH (B, × 400) results. HER2 IHC shows strong and membranous staining; however, SISH results were negative, showing 3.8 average HER2 signals per cell and an HER2/CEP17 ratio of 1.95 (red, CEP17; black, HER2).
HER2 = human epidermal growth factor receptor 2; SISH = silver-enhanced in situ hybridization; IHC = immunohistochemistry; CEP17 = chromosome 17.
Cohort 2
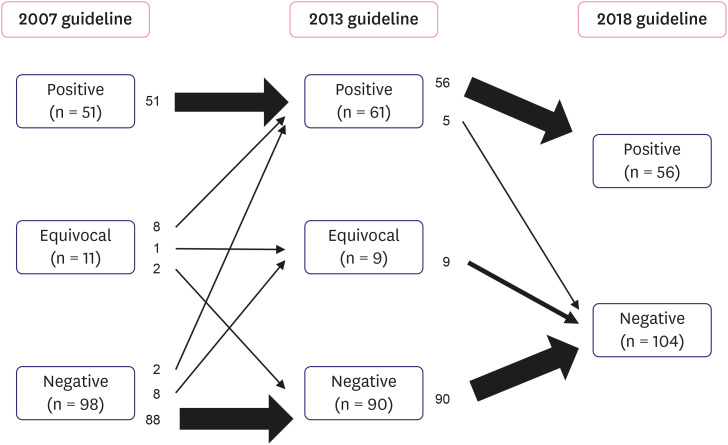
A total of 160 HER2 IHC equivocal samples from 159 patients, including 1 patient with bilateral IBC, were included in this group. The distributions of the HER2 SISH results according to the 2007, 2013, and 2018 guidelines are presented in Table 6. The application of the 2007 guidelines revealed 51 (31.9%) positive, 11 (6.9%) equivocal, and 98 (61.3%) negative cases, whereas the 2013 guideline revealed 61 (38.1%) positive, 9 (5.6%) equivocal, and 90 (56.3%) negative cases for HER2 SISH. Thus, the interpretation of 20 (12.5%) cases was different as per the 2013 guidelines, compared to the 2007 guidelines (Figure 4). Of the 11 equivocal cases determined by the 2007 guidelines, 8 were reclassified as positive and 2 were reclassified as negative by the 2013 guidelines. The equivocal status remained unchanged for 1 case. Two cases were changed from negative to positive and 8 changed from negative to equivocal as per the 2013 guidelines. All positive cases remained positive (Figure 4). The positive rate increased from 31.9% to 38.1% on the application of the 2013 guidelines, when compared to the 2007 guidelines; additionally, 10 (6.3% of IHC 2+ cases) additional patients who met the eligibility criteria for HER2-targeted therapy were identified.
Table 6. Distribution of HER2 SISH results in cohort 2 according to the 2007, 2013, and 2018 ASCO/CAP guidelines.
| Result | 2007 | 2013 | 2018 |
|---|---|---|---|
| Positive | 51 (31.9) | 61 (38.1) | 56 (35) |
| Equivocal | 11 (6.9) | 9 (5.6) | 0 (0) |
| Negative | 98 (61.3) | 90 (56.3) | 104 (65) |
| Total | 160 | 160 | 160 |
Values are presented as number (%).
HER2 = human epidermal growth factor receptor 2; SISH = silver-enhanced in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists.
Figure 4. Flowchart of cases according to the 2007, 2013, and 2018 ASCO/CAP guidelines in cohort 2. The left column showcases the distribution of cases under the 2007 criteria. Application of the 2013 recommendations is depicted in the middle column. The right column indicates the distribution of cases using the 2018 guidelines. Each arrow shows the movement of cases. The thicknesses of arrows present the amount of cases.
ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists.
Classification of the same cases according to the 2018 guidelines resulted in 56 (35%) positive and 104 (65%) negative cases. The interpretation of 14 (8.5%) cases differed on the application of the 2018 guidelines, compared to the 2013 guidelines (Figure 4). All equivocal cases were classified as negative and 5 positive cases were reclassified as negative (group 2 with < 4 HER2 signal per cell and ≥ 2 HER2/CEP17 ratio) by the 2018 guidelines. The 5 (3.1% of IHC 2+ cases) cases that were reclassified as HER2-negative from HER2-positive were therefore no longer eligible for HER2 targeted therapy. They were 4 equivocal cases and one positive case as per the 2007 guidelines. Detailed information on the cases in cohort 2 with discrepant HER2 SISH results according to the 2007, 2013, and 2018 guidelines is presented in Table 7.
Table 7. Details of cases with discrepant HER2 SISH results according to the 2007, 2013 and 2018 ASCO/CAP HER2 testing guidelines in cohort 2.
| Case number | IHC result | Average HER2 signal/cell | Average CEP17 signal/cell | HER2/CEP17 ratio | SISH results | |||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2013 | 2018 | ||||||
| Group | Result | |||||||
| 79 | 2+ | 6.20 | 4.78 | 1.30 | Negative | Positive | 3 | Positive |
| 83 | 2+ | 6.30 | 4.45 | 1.42 | Negative | Positive | 3 | Positive |
| 32 | 2+ | 4.90 | 3.00 | 1.63 | Negative | Equivocal | 4 | Negative |
| 33 | 2+ | 4.50 | 3.00 | 1.50 | Negative | Equivocal | 4 | Negative |
| 52 | 2+ | 5.60 | 4.60 | 1.21 | Negative | Equivocal | 4 | Negative |
| 72 | 2+ | 4.35 | 2.60 | 1.67 | Negative | Equivocal | 4 | Negative |
| 81 | 2+ | 4.10 | 2.30 | 1.78 | Negative | Equivocal | 4 | Negative |
| 92 | 2+ | 4.52 | 3.45 | 1.31 | Negative | Equivocal | 4 | Negative |
| 94 | 2+ | 4.60 | 4.15 | 1.10 | Negative | Equivocal | 4 | Negative |
| 113 | 2+ | 4.00 | 2.90 | 1.38 | Negative | Equivocal | 4 | Negative |
| 48 | 2+ | 3.15 | 1.45 | 2.17 | Equivocal | Positive | 2 | Negative |
| 78 | 2+ | 2.25 | 1.07 | 2.10 | Equivocal | Positive | 2 | Negative |
| 115 | 2+ | 2.20 | 1.08 | 2.04 | Equivocal | Positive | 2 | Negative |
| 147 | 2+ | 3.78 | 1.86 | 2.03 | Equivocal | Positive | 2 | Negative |
| 82 | 2+ | 4.70 | 2.35 | 2.00 | Equivocal | Positive | 1 | Positive |
| 111 | 2+ | 5.50 | 2.50 | 2.20 | Equivocal | Positive | 1 | Positive |
| 114 | 2+ | 5.70 | 2.60 | 2.19 | Equivocal | Positive | 1 | Positive |
| 121 | 2+ | 4.90 | 2.40 | 2.04 | Equivocal | Positive | 1 | Positive |
| 116 | 2+ | 5.90 | 3.00 | 1.97 | Equivocal | Equivocal | 4 | Negative |
| 152 | 2+ | 3.58 | 1.90 | 1.88 | Equivocal | Negative | 5 | Negative |
| 154 | 2+ | 3.50 | 1.90 | 1.84 | Equivocal | Negative | 5 | Negative |
| 21 | 2+ | 3.25 | 1.00 | 3.25 | Positive | Positive | 2 | Negative |
HER2 = human epidermal growth factor receptor 2; SISH = silver-enhanced in situ hybridization; ASCO = American Society of Clinical Oncology; CAP = College of American Pathologists; IHC = immunohistochemistry; CEP17 = chromosome 17.
DISCUSSION
This study evaluated the impact of the updated ASCO/CAP guidelines on 2 different cohorts, by comparing the SISH results according to the 2007, 2013, and 2018 guidelines. There were slight changes in the overall rates of HER2 positivity as per the 2013 and 2018 guidelines, compared to the 2007 guideline. The positive rate was observed to increase under the 2013 guidelines, when compared with the 2007 guidelines, and it was observed to slightly decrease under the 2018 updates. The negative rate was considerably increased under the 2018 guidelines compared with the 2013 guidelines.
Our results coincide with the findings in previous studies. Martin et al. [9] compared the HER2 FISH results of 880 IBC samples using the 2007, 2013, and 2018 guidelines and reported that 95% of equivocal cases were reclassified as negative, whereas 5% positive cases were reclassified as negative, according to the 2018 guidelines, when compared to the 2013 guidelines. In line within our cohort 1, 92.3% of the equivocal and 1.3% of the positive cases were reclassified as negative. Murray et al. [10] assessed the impact of the ASCO/CAP guidelines on a series of 1,044 HER2 IHC 2+ cases, by comparing the HER2 FISH results according to the 2007, 2013, and 2018 guidelines. They reported that there was an increase in the positive cases (6.7%, from 10.7% to 17.4%) when comparing the 2007 and 2013 guidelines; additionally, 2.9% of the tumors were reclassified as HER2 negative under the 2018 updates. Similarly, in our cohort 2, positive cases increased by 6.3% under the 2013 updates and 3.1% of the cases were reclassified as HER2 negative under the 2018 guidelines. Zare et al. [11] compared the HER2 FISH results interpreted by the 2013 and 2018 guidelines in 1,542 consecutive primary and metastatic breast cancers, and demonstrated that the HER2 results of 10.7% cases were changed as per the 2018 guidelines, mainly by reclassifying previously equivocal results as negative results. Bethune et al. [12] reported that applying the 2013 ASCO/CAP guidelines altered the HER2 status obtained in 2007 in approximately 9.4% cases, with an increase in the equivocal results. Several researchers [13,14] demonstrated an increase in the equivocal and positive results under the 2013 guidelines, whereas others reported a decrease in the equivocal cases [15,16]. This discrepancy could be explained by the differences in the pre-analytical conditions, ISH methods, or sample size [16]. The proficiency of the HER2 test interpretation is also important when comparing the study results [15].
As the ASCO/CAP intended, the 2013 guidelines were successful in diminishing the false negative test results. When the first guideline was established in 2007, the positive cutoff value was higher than the entry criteria indicated for anti-HER2 therapy clinical trials. This led to an in increase in the number of false-negative cases, and HER2-targeted therapy could be missed in approximately 4% cases that were positive under the FDA criteria set for clinical trials [17]. Therefore, the 2013 guideline reverted the threshold for positive HER2 to a similar level that was originally set by the U.S. FDA.
The 2018 ASCO/CAP guidelines clarified the previous equivocal cases by simultaneously considering the HER2 ISH and IHC results. In general, the positive cases were altered to negative ones under the new guidelines, when compared with the 2013 guidelines. In our study, 16 (1.4%) cases in cohort 1 were reclassified under the 2018 guidelines, including a change from equivocal to negative (12 cases), or from positive to negative (3 cases). Three (0.3%) cases in cohort 1 became ineligible for HER2-targeted therapy. Our findings were consistent with the results of the previous studies. Gordian-Arroyo et al. [18] evaluated the impact of the 2018 ASCO/CAP guidelines on breast core biopsies, and identified that 6% of tumors were redistributed mainly to the negative group. Other previous studies also reported that the number of cases in the negative group was significantly increased by applying the 2018 criteria (range 8.2%–10.4%) [11,19,20]. Similarly, previous studies on IHC 2+ cases showed a decrease in positive cases (range 0%–3.6%) and an increase in the negative cases (range 3.0%–15.5%), using the 2018 guidelines [21,22,23].
As per the 2018 ASCO/CAP guidelines, the IHC and ISH results in combination should decide the final HER2 status. Based on the 2013 guidelines, groups 2 and 3 are considered positive, regardless of the HER2 IHC results, and group 4 is classified as equivocal. These groups account for only a small minority when compared to groups 1 (classical positive) and 5 (classical negative). In 2016, Press et al. [24] retrospectively reviewed these groups by applying the Breast Cancer International Research Group (BCIRG)-005, BCIRG-006, and BCIRG-007 data. They found that group 2 could be considered as HER2 negative because of the lack of HER2 expression and response to anti-HER2 treatment. In addition, group 4 did not show HER2 expression and had a similar prognosis as group 5; hence, group 4 could be presumed to have a negative HER2 status. Furthermore, group 3, originally positive as per the 2013 guidelines, heterogeneously consisted of HER2-positive and -negative cases. Therefore, the recent ASCO/CAP guidelines divided group 3 on the basis of IHC findings. Further studies are required to identify the clinical outcome of these changes.
We did not reassess all the HER2 IHC slides and only cases affected by algorithm updates were reviewed. Our SISH analysis required no additional consideration of other non-reviewed IHC results. Furthermore, the changes in the IHC criteria were minimal; hence, it was sufficient to reconsider only the affected cases. Two significant changes were determined in the IHC criteria between the 2007 and 2013/2018 guidelines: 1) Positive criteria in the 2007 guidelines required 30% complete membranous stained tumor cells, which lessened to 10% in the 2013 and 2018 guidelines and such cases retained their positive status. As per the 2007 guidelines, complete membrane staining in 10%–30% tumor cells was interpreted as 2+. Group 2 or 4 cases could be affected by this alteration; however, none of the cases were present in our analysis. 2) Considering the definition of negative IHC as per the 2007 guidelines, the cases with weak incomplete membrane staining of more than 10% were classified as 1+; however, the same cases were reclassified as 2+ under the 2013 and 2018 guidelines (cases 317 and 1,087 in Table 3).
According to ASCO/CAP, approximately 5% of the total HER2-tested cases are expected to fit in groups 2–4. In our study, 1.7% cases were in groups 2–4. This difference in the percentages from other studies is not clear; nevertheless, we assume that SISH could have a role in this classification. The greatest advantage of bright field ISH is that it is possible to co-evaluate the histologic morphologies under conventional light microscopy [1]. It helps in distinguishing the tumor cells from normal background cells, which provides a more accurate copy number count than FISH. In addition, one pathologist with a specialization in breast pathology at our center has been analyzing the HER2 status since 2003; therefore, we believe that the interpretations of HER2 would be consistent.
The health insurance system of Korea forces laboratories to use IHC as a primary test for determining the HER2 status. However, analyzing HER2 IHC is difficult because of the vulnerability to pretreatment conditions, and use of different antibody clones and detection systems between laboratories. A study by Gown et al. [25] revealed that the discordance rate between the HER2 IHC and ISH results could be up to 20% when the test was performed in low-volume laboratories. As shown in 1.2% of the cases (Table 5) in this study, there is a possibility of discordance between the IHC and ISH results. Gibbons-Fideler et al. [26] found that approximately 1.5% patients were HER2 IHC-negative FISH-positive. Solomon et al. [27] revealed 9 (2.4%) discrepant HER2 FISH/IHC cases among 368 IBC samples (8 cases were FISH-positive IHC-negative, and 1 was FISH-negative IHC-positive). The impact of this discordance is not yet understood. Varga et al. [28] reported that the IHC-negative FISH-positive patients exhibited similar survival as IHC-positive FISH-positive patients. In our study, 11 (78.6%) of the 14 discordant cases were IHC-negative and SISH-positive. Although further studies are required to identify the prognostic impact of this group, performing both HER2 IHC and ISH simultaneously would be necessary to ensure that the patients do not miss the chance to receive adequate therapy. Our study showed a similar proportion of HER2 IHC/SISH discordance seen in previous studies [29] and suggested that dual testing was more cost-effective than the current IHC-first strategy.
We did not evaluate the treatment effect in discrepant cases. The majority of cases had never received anti-HER2 therapy due to the HER2-negative status during the initial diagnosis. Only 3 cases in cohort 1 and one case in cohort 2 received HER2-targeted therapy, which show a negative HER2 status when applying the latest guideline. Due to the small number of cases included in this study, a direct treatment response comparison in discrepant cases was not conducted. Further larger studies are required to evaluate the treatment effect in discrepant HER2 groups.
Since the first ASCO/CAP guideline was revealed for HER2 interpretation in 2007, numerous clinical data have been generated and have helped in designing accurate criteria. Based on our data, the latest ASCO/CAP HER2 interpretation guideline will increase the number of negative cases; therefore, the treatment strategies for patients could be revised. Further, the 2018 guidelines allow for more proper decision-making on the therapeutic options for challenging breast cancer cases. Clinicians need to decide the appropriate therapeutic options for challenging breast cancer cases, by applying the new ASCO/CAP guidelines. Furthermore, we identified a few cases with discordant ISH and IHC results for HER2. Our nation's insurance policy could overlook IHC-negative SISH-positive IBC patients, although the clinical significance of such a result is still unknown.
Footnotes
Presentation: Presented in part at the 109th Annual Meeting of United States and Canadian Academy of Pathology (USCAP), February 29–March 5, 2020, Los Angeles, USA.
Funding: This study was supported by the Medical Research Center Program of the National Research Foundation of Korea (NRF), Ministry of Science, ICT and Future Planning (2015R1A5A2009124).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim MC, Bae YK.
- Data curation: Kang SH, Choi JE.
- Formal analysis: Kim MC;.
- Funding acquisition: Bae YK.
- Methodology: Kim MC, Kang SH.
- Supervision: Bae YK.
- Writing - original draft: Kim MC.
- Writing - review & editing: Kang SH, Choi JE, Bae YK.
References
- 1.Ahn S, Woo JW, Lee K, Park SY. HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. J Pathol Transl Med. 2020;54:34–44. doi: 10.4132/jptm.2019.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voigtlaender M, Schneider-Merck T, Trepel M. Lapatinib. Recent Results Cancer Res. 2018;211:19–44. doi: 10.1007/978-3-319-91442-8_2. [DOI] [PubMed] [Google Scholar]
- 3.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health and Welfare (KR) (Notice 2013-152) Guidance on the establishment and revision of details on the standards and methods of applying medical care benefits. [Accessed May 7th, 2020]. http://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=5131&pageIndex=1.
- 6.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Allison KH, Harvey BE, Mangu PB, Bartlett JM, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 8.Bae YK, Gong G, Kang J, Lee A, Cho EY, Lee JS, et al. HER2 status by standardized immunohistochemistry and silver-enhanced in situ hybridization in Korean breast cancer. J Breast Cancer. 2012;15:381–387. doi: 10.4048/jbc.2012.15.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin V, Valera A, De Joffrey M, Banfi S, Mazzucchelli L. Implementation of the 2018 human epidermal growth factor receptor 2 guideline by American Society of Clinical Oncology/College of American Pathologists will reduce false-positive tests. Arch Pathol Lab Med. 2019;143:411–412. doi: 10.5858/arpa.2018-0904-LE. [DOI] [PubMed] [Google Scholar]
- 10.Murray C, Flanagan L, D'Arcy C, Gullo G, Quinn CM. Assessing the impact of the 2018 American Society of Clinical Oncology/College of American Pathologists recommendations on human epidermal growth factor receptor 2 testing by fluorescence in situ hybridization in breast carcinoma. Virchows Arch. 2020;476:367–372. doi: 10.1007/s00428-019-02636-3. [DOI] [PubMed] [Google Scholar]
- 11.Zare S, Rong J, Daehne S, Roma A, Hasteh F, Dell'Aquila M, et al. Implementation of the 2018 American Society of Clinical Oncology/College of American Pathologists Guidelines on HER2/neu assessment by FISH in breast cancers: predicted impact in a single institutional cohort. Mod Pathol. 2019;32:1566–1573. doi: 10.1038/s41379-019-0295-8. [DOI] [PubMed] [Google Scholar]
- 12.Bethune GC, Veldhuijzen van Zanten D, MacIntosh RF, Rayson D, Younis T, Thompson K, et al. Impact of the 2013 American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing of invasive breast carcinoma: a focus on tumours assessed as ‘equivocal’ for HER2 gene amplification by fluorescence in-situ hybridization. Histopathology. 2015;67:880–887. doi: 10.1111/his.12723. [DOI] [PubMed] [Google Scholar]
- 13.Varga Z, Noske A. Impact of modified 2013 ASCO/CAP guidelines on HER2 testing in breast cancer. One year experience. PLoS One. 2015;10:e0140652. doi: 10.1371/journal.pone.0140652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long TH, Lawce H, Durum C, Moore SR, Olson SB, Gatter K, et al. The new equivocal: changes to HER2 FISH results when applying the 2013 ASCO/CAP guidelines. Am J Clin Pathol. 2015;144:253–262. doi: 10.1309/AJCP3Q9WFOQTKUVV. [DOI] [PubMed] [Google Scholar]
- 15.Garbar C, Savoye AM, Mascaux C, Brabencova E, Curé H. The human epidermal growth factor receptor 2 screening tests for breast cancer suggested by the new updated recommendation of the american society of clinical oncology/college of american pathologists will involve a rise of the in-situ hybridization tests for the European laboratories of pathology. ISRN Oncol. 2014;2014:793695. doi: 10.1155/2014/793695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polónia A, Leitão D, Schmitt F. Application of the 2013 ASCO/CAP guideline and the SISH technique for HER2 testing of breast cancer selects more patients for anti-HER2 treatment. Virchows Arch. 2016;468:417–423. doi: 10.1007/s00428-016-1903-3. [DOI] [PubMed] [Google Scholar]
- 17.Perez EA, Dueck AC, McCullough AE, Reinholz MM, Tenner KS, Davidson NE, et al. Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst. 2012;104:159–162. doi: 10.1093/jnci/djr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordian-Arroyo AM, Zynger DL, Tozbikian GH. Impact of the 2018 ASCO/CAP HER2 guideline focused update. Am J Clin Pathol. 2019;152:17–26. doi: 10.1093/ajcp/aqz012. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZH, Wang K, Lin DY, Xu J, Chen J, Long XY, et al. Impact of the updated 2018 ASCO/CAP guidelines on HER2 FISH testing in invasive breast cancer: a retrospective study of HER2 FISH results of 2233 cases. Breast Cancer Res Treat. 2019;175:51–57. doi: 10.1007/s10549-019-05148-5. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Bai Q, Kong H, Zhou S, Lv H, Zhong S, et al. Impact of the updated 2018 American Society of Clinical Oncology/College of American Pathologists guideline for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2020;144:1097–1107. doi: 10.5858/arpa.2019-0369-OA. [DOI] [PubMed] [Google Scholar]
- 21.Curado M, Caramelo AS, Eloy C, Polónia A. What to expect from the 2018 ASCO/CAP HER2 guideline in the reflex in situ hybridization test of immunohistochemically equivocal 2+ cases? Virchows Arch. 2019;475:303–311. doi: 10.1007/s00428-019-02567-z. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract. 2019;215:251–255. doi: 10.1016/j.prp.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Hoda RS, Brogi E, Xu J, Ventura K, Ross DS, Dang C, et al. Impact of the 2018 American Society of Clinical Oncology/College of American Pathologists HER2 guideline updates on HER2 assessment in breast cancer with equivocal HER2 immunohistochemistry results with focus on cases with HER2/CEP17 ratio <2.0 and average HER2 copy number ≥4.0 and <6.0. Arch Pathol Lab Med. 2020;144:597–601. doi: 10.5858/arpa.2019-0307-OA. [DOI] [PubMed] [Google Scholar]
- 24.Press MF, Sauter G, Buyse M, Fourmanoir H, Quinaux E, Tsao-Wei DD, et al. HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group clinical trials. J Clin Oncol. 2016;34:3518–3528. doi: 10.1200/JCO.2016.66.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gown AM, Goldstein LC, Barry TS, Kussick SJ, Kandalaft PL, Kim PM, et al. High concordance between immunohistochemistry and fluorescence in situ hybridization testing for HER2 status in breast cancer requires a normalized IHC scoring system. Mod Pathol. 2008;21:1271–1277. doi: 10.1038/modpathol.2008.83. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons-Fideler IS, Nitta H, Murillo A, Tozbikian G, Banks P, Parwani AV, et al. Identification of HER2 immunohistochemistry-negative, FISH-amplified breast cancers and their response to anti-HER2 neoadjuvant chemotherapy. Am J Clin Pathol. 2019;151:176–184. doi: 10.1093/ajcp/aqy136. [DOI] [PubMed] [Google Scholar]
- 27.Solomon JP, Dell'Aquila M, Fadare O, Hasteh F. HER2/neu status determination in breast cancer: a single institutional experience using a dual-testing approach with immunohistochemistry and fluorescence in situ hybridization. Am J Clin Pathol. 2017;147:432–437. doi: 10.1093/ajcp/aqw224. [DOI] [PubMed] [Google Scholar]
- 28.Varga Z, Tubbs RR, Moch H. Concomitant detection of HER2 protein and gene alterations by immunohistochemistry (IHC) and silver enhanced in situ hybridization (SISH) identifies HER2 positive breast cancer with and without gene amplification. PLoS One. 2014;9:e105961. doi: 10.1371/journal.pone.0105961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hariri N, Zare S, Murphy J, Fadare O. Cost-effectiveness of a dual (immunohistochemistry and fluorescence in situ hybridization) HER2/neu testing strategy on invasive breast cancers. Appl Immunohistochem Mol Morphol. doi: 10.1097/PAI.0000000000000849. Forthcoming. [DOI] [PubMed] [Google Scholar]