Significance
The use of deltamethrin, a crystalline contact insecticide and a leading tool in combatting malaria vectors, faces an uncertain future, threatened by developing resistance of mosquitoes. A more active crystalline polymorph of deltamethrin, discovered here, speeds the knockdown of susceptible mosquitoes by a factor of up to 12 compared with the currently used crystalline form. The faster-acting deltamethrin polymorph is predicted to suppress malaria transmission and associated human mortality while reducing environmental exposure because less agent is required to achieve the same effect. The outstanding performance of form II promises increased serviceable use of deltamethrin crystals for indoor residual spraying. Metastable forms of contact insecticides should be considered generally for public health applications.
Keywords: deltamethrin, polymorphism, malaria, epidemiological modeling, mosquito
Abstract
Pyrethroid contact insecticides are mainstays of malaria control, but their efficacies are declining due to widespread insecticide resistance in Anopheles mosquito populations, a major public health challenge. Several strategies have been proposed to overcome this challenge, including insecticides with new modes of action. New insecticides, however, can be expensive to implement in low-income countries. Here, we report a simple and inexpensive method to improve the efficacy of deltamethrin, the most active and most commonly used pyrethroid, by more than 10 times against Anopheles mosquitoes. Upon heating for only a few minutes, the commercially available deltamethrin crystals, form I, melt and crystallize upon cooling into a polymorph, form II, which is much faster acting against fruit flies and mosquitoes. Epidemiological modeling suggests that the use of form II in indoor residual spraying in place of form I would significantly suppress malaria transmission, even in the presence of high levels of resistance. The simple preparation of form II, coupled with its kinetic stability and markedly higher efficacy, argues that form II can provide a powerful, timely, and affordable malaria control solution for low-income countries that are losing protection in the face of worldwide pyrethroid resistance.
Combatting malaria, a disease afflicting >200 million people annually, requires effective insecticides as part of integrated vector management control strategies (1). Interventions, including indoor residual spraying (IRS) of insecticides and insecticide-treated bed nets (ITNs), are estimated to have reduced malaria cases in Africa by 80% between 2000 and 2015 (2). Pyrethroids are the most widely used insecticides for malaria control today because of their high insect lethality and low mammalian toxicity (3). Among pyrethroids, deltamethrin (DM, (S)-cyano(3-phenoxyphenyl)methyl(1R,3R)-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropane-1-carboxylate; Fig. 1A) is one of the foremost insecticides for IRS, providing malaria control for millions (3, 4). Unfortunately, resistance to pyrethroids has become widespread in Anopheles mosquito populations, rapidly reducing the efficacy of DM and threatening the substantial progress in malaria control during the 21st century (5, 6). Consequently, new insecticides and mosquito-targeted antimalarials have been proposed (7, 8). The development, evaluation, and introduction of new chemical agents for malaria control, however, requires substantial investment and evaluation, and it incurs potential risks. Improving the effectiveness of compounds currently in use is preferable (9). Such improvements are needed as urgently as ever during the global COVID-19 crisis (10). The number of deaths from malaria in Africa this year is projected to double as a result of supply chain limitations due to coronavirus-related disruptions (11). More effective interventions are needed even more.
Fig. 1.
(A) Molecular structure of DM. (B) Microcrystals in suspension concentrate (Suspend SC, DM concentration: 5%), a formulation used for IRS. (C and D) Melt grown DM (C) form I and (D) form II spherulites observed between crossed polarizers. PXRD confirmed that the fibers in the spherulite were oriented along the crystallographic <010> direction. (E) PXRD data for form I (blue) and form II (orange) from their respective films. (F and G) Single crystal structures of forms (F) I (12) and (G) II determined at 100 K.
DM is a contact insecticide; its active form for IRS is crystalline (Fig. 1B) (13). As such, its activity depends upon the interface between the crystals and whole organisms. Herein, we describe the discovery of a more lethal DM crystal polymorph, form II, produced by cooling melts of the commercial form I from ca. 110 °C to room temperature. This polymorph is approximately 12 times faster acting in laboratory assays against disease vectors than the commercially available crystals of form I that are used in the field. Epidemiological modeling suggests that substitution of DM form II for commercial form I would reduce malaria transmission. The use of more-active crystal polymorphs is a simple and powerful strategy for improving the efficacy of existing compounds for malaria control, obviating the limitations associated with the introduction of new compositions of matter. Using polymorphs of existing insecticides circumvents the need for developing new compounds, thereby mitigating the cost of new manufacturing processes and regulatory testing.
Materials and Methods
Details of the materials and methods are included in SI Appendix. These include methods for preparing and/or distinguishing DM forms I and II as single crystals (SI Appendix, Table S1), in thin films and in commercial dusts. Methods are provided for determining average velocity and lethality of DM exposed to Drosophila raised locally as well as susceptible female Anopheles quadrimaculatus and Aedes aegypti purchased as adults. The model of malaria transmission (14) is described briefly in Lethality Comparison of Polymorphs in addition to SI Appendix. Methods of chemical analysis are more fully described in SI Appendix, including descriptions of optical microscopy, Raman spectroscopy, and X-ray crystallography.
Results and Discussion
Polymorph of DM.
Female mosquitoes resting on DM-treated walls contact the surfaces of DM crystals (13), absorbing the toxin through their tarsi. Despite the considerable study and widespread application of DM, only one crystal structure, measured at room temperature, has been reported (in 1975), designated here as form I (Cambridge Structural Database reference code PXBVCP10) (12). The structure of DM form I was redetermined at 100 K (Fig. 1F and SI Appendix, Fig. S1C and Table S1).
Commercial DM (form I) confined between glass slides was melted at ca. 110 °C to 120 °C (Tm = 99 °C), either by direct conduction heating or with a conventional microwave oven, and then cooled to 25 °C. This protocol resulted in the growth of polycrystalline spherulites (15) consisting of fine fibrils (Fig. 1D). On rare occasions, fields of chaotic polycrystalline textures also were observed (Fig. 1C). Powder X-ray microdiffraction (PXRD) and micro-Raman spectroscopy confirmed that the chaotic textures were DM (form I), whereas the spherulites were a polymorph, herein designated as form II (Fig. 1E and SI Appendix, Fig. S1 A and B). The single crystal structure of form II was determined at 100 K (Fig. 1G and SI Appendix, Fig. S1D and Table S1). The melting point of form II (68 °C) is much lower than form I, suggesting form II is metastable. Unconfined films of form II nucleate form I within 3 h, but the transformation is slow (SI Appendix, Fig. S2). However, crystalline DM form II confined between the glass slides is stable for at least 12 mo at 25 °C and at least 6 mo at 40 °C.
Lethality Comparison of Polymorphs.
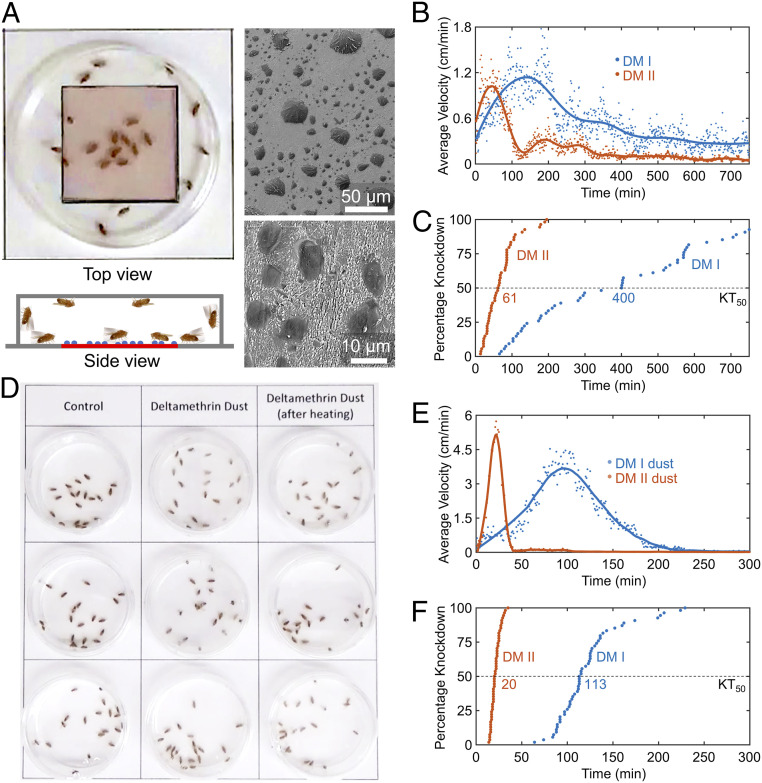
A comparison of the lethality of DM forms I and II was difficult for neat films owing to their exceptionally short knockdown times (KTs, the time required for a fly to become immobile in a supine position); insects were knocked down within minutes of exposure to either form. Therefore, films of DM form I or II were partially covered with nebulized, micrometer-size droplets of polyethylene glycol (PEG) to reduce the surface area for contact of the insect tarsi with DM, thus bringing the KTs within a more conveniently measured range (Fig. 2A and SI Appendix, Fig. S3). Female fruit flies (Drosophila melanogaster), an accepted model for testing insecticide lethality against mosquitoes (16), were exposed to these films (Fig. 2A), following a procedure similar to that previously reported (17–19). Fly hyperactivity, the first symptom of poisoning (20), and KTs were analyzed by measurement of the average speed of fly motion, using a video camera and a custom MATLAB program (SI Appendix, Fig. S4). Peak hyperactivity occurred after 160 min of exposure to form I compared with 40 min for form II (Fig. 2B). The KT50 values—the knockdown times required for 50% of the insects to become immobile and supine—for the PEG-treated crystalline films were 400 min for form I compared with 61 min for form II (Fig. 2C), corresponding to a ratio of 6.6 for Drosophila, undoubtedly a kinetic difference associated with uptake of DM molecules from the crystal surface through the insect tarsi.
Fig. 2.
Lethality comparison of DM forms I and II against D. melanogaster. (A) Experimental setup for lethality comparisons using crystalline films of DM form I or II covered with microislands of PEG (sprayed on films to reduce their activity so that the KTs could be compared more readily), schematically illustrated by the blue dots in the side view and revealed in scanning electron micrographs. At higher magnification (10-mm scale bar), form II crystallites were observed between PEG islands. (B) The motions of D. melanogaster exposed to crystalline films. The dots represent the average speed of all fruit flies in 1 min, smoothed (solid line) by a Savitzky−Golay filter. (C) KTs for D. melanogaster exposed to crystalline films. KT50 values are indicated, as in F. (D) Setup for lethality comparisons of dusts. Form I dust was converted to form II dust by heating, either using a microwave oven or convective heating (SI Appendix, Fig. S5). (E) Motions of D. melanogaster exposed to dusts. (F) KTs for D. melanogaster exposed to dusts.
Some of the most widely used formulations of DM are so-called dusts, microcrystals dispersed on inert carriers (e.g., chalk and silica). Commercial DM dust (D-fence Dust; 0.05 wt% DM) was heated for 5 min above the melting point of DM form I either in a microwave oven or in a 150 °C oil bath. Although the DM polymorphs could not be identified at the low 0.05 wt % loading, the lethality assays described below for heat-treated DM dust parallel the results for the crystalline films of forms I and II, corroborating the conversion of form I in the commercial dust to form II. Commercial and heat-treated dusts are denoted as form I and form II dusts, respectively. Female fruit flies were exposed to 2.0 mg of forms I and II dusts (equivalent to 1 μg of DM microcrystals each) (Fig. 2D). The fruit flies exposed to form II were immediately hyperactive (Fig. 2E), while the onset of hyperactivity was 40 min for form I. The KT50 values for forms I and II were 113 and 20 min, respectively (Fig. 2F), a ratio of 5.7, comparable to the corresponding DM films described above. Both microwave and convective heating produced equivalent results (SI Appendix, Fig. S5). The remarkably stability of form II in dusts can be attributed to the low probability of the nucleation of form I in minute crystals of form II.
Female A. quadrimaculatus and A. aegypti mosquitoes exposed to 2.0 mg of either DM form I or form II dust also were more susceptible to form II, as indicated by the onset of hyperactivity (Fig. 3 A and C and SI Appendix, Fig. S6A). The KT50 values for Aedes exposed to forms I and II dust were 192 and 21 min, respectively (Fig. 3B), a ratio of 9.1. The corresponding KT50 values for Anopheles mosquitoes were 282 and 24 min, respectively (Fig. 3D), a ratio of 11.8. Anopheles mosquitoes exposed to DM form II were virtually inactive after the hyperactivity peak (SI Appendix, Fig. S6).
Fig. 3.
Lethality of DM crystalline forms I and II against A. aegypti and A. quadrimaculatus mosquitoes. (A) The motions of A. aegypti mosquitoes exposed to dusts. The dots represent the average speed of all mosquitoes in 1 min. Solid lines are smoothed trend lines (as in C), See also SI Appendix, Fig. S6. (B) Comparison of A. aegypti KTs for forms I and II dusts. KT50 values are indicated, as in D. (C) Motions of A. quadrimaculatus mosquitoes exposed to dusts. (D) Comparison of A. quadrimaculatus KTs using dusts.
Impact on Current Malaria Control Intervention.
A model of malaria transmission (SI Appendix, Fig. S7) previously developed by Catteruccia, Childs, and coworkers was employed using a variable to account for the lethality difference between DM forms I and II (8, 14). The mosquito population dynamics and human malaria infection prevalence were incorporated into this model to evaluate the potential consequences of the application of form II in IRS. We assumed that the mosquito population is homogenous, and 1 unit of form II was equal to 12 units of form I. The introduction of form II was predicted, by modeling, to increase the effectiveness of IRS under a broad range of human infection prevalence, intervention coverage, and insecticide resistance conditions within the model (Fig. 4A and SI Appendix, Figs. S8 and S9). This suggests that application of form II may suppress malaria in endemic regions where formulations of form I suffer from pyrethroid resistance.
Fig. 4.
Simulation of malaria transmission dynamics. (A) Effectiveness of IRS for inhibition of malaria transmission, using the same doses of DM form I (Left) or II (Right) at varying levels of coverage and insecticide resistance with an initial human infection prevalence of 45%. Insecticide resistance corresponds to the percentage of mosquitoes that survive after contacting the applied dose of form I. Coverage corresponds to the probability of a mosquito contacting the insecticide during a single resting episode. (B) Predicted reduction in population of infected mosquitoes and human prevalence upon application of DM form I and then switching to form II (coverage = 75%; resistance = 70%; infection prevalence = 45%, denoted in Fig. 4A as red squares). Both the population of infected mosquitoes and human disease prevalence dropped precipitously.
We then modeled the effect of switching from form I to form II on the infectious mosquito population and human malaria infection dynamics. The simulation was performed for hypothetical regions (marked in Fig. 4A as red squares) characterized by high resistance (70%), moderate infection prevalence (45%), and high IRS coverage (75%) (Fig. 4B). The simulation was performed in the absence of insecticide intervention in the first 3 years, when the mosquito population and human prevalence converged to 370 and 45%, respectively. During the following 3 years, the simulation was performed under the condition that DM form I was used via IRS. Due to high levels of resistance, however, the introduction of form I only marginally reduced both the population of infected mosquitoes (298) and the human infection prevalence (40%). Starting from the seventh year, DM form II was substituted for DM form I. Both the population of infected mosquitoes and the human infection prevalence experienced a significant drop and converged to 18 and 4%, respectively. This decrease, arising from the introduction of DM II in simulation, would translate to suppression of malaria transmission in hot spots where resistance to pyrethroids is as high as 70%. A simulation performed with a lower initial infection prevalence (20%) indicated that the switch from form I to form II would decrease the human infection prevalence from 18 to 0.3% at even higher resistance (80%) and lower IRS coverage (60%) (SI Appendix, Fig. S8). We emphasize in Fig. 4A that the gains in effectiveness of form II from IRS can be relatively small, even if the coverage is 100%, for mosquito population with extremely high-level resistance.
DM form II, because it is less thermodynamically stable than form I, favors uptake through dissolution of DM molecules by secretions or detachment from underlying crystal planes. Even for insects with cuticle thickness resistance (21), metabolic resistance (overexpressed detoxification enzymes) (22), knockdown resistance (23), and the newly discovered chemosensory protein SAP2 binding resistance (24), lethal doses may still reach the sodium ion channel target site, due to the significantly increased uptake of DM molecules from form II. DM form II dust continues to exhibit greater lethality than form I dust 3 mo after heating (SI Appendix, Fig. S10), consistent with the kinetic stability of form II crystals. As recommended by the World Health Organization (WHO), DM must be sprayed every 3 mo to 6 mo to maintain its residual effect (13). The 3-mo durability of DM form II formulations, combined with its higher activity, promises significant benefits for field applications.
Since the more active form II can be produced by simply melting form I, which is currently in use, the cost of its implementation would be low compared with the development of alternative interventions. IRS alternatives to pyrethroids, although recommended for insecticide resistance management (25), have proven expensive for scale-up (5). The use of DM form II in IRS, however, could be integrated into the extensive manufacturing and distribution pipelines of DM that are already in operation in malaria-endemic regions. Form II may even be less costly overall than form I because less compound would be needed to achieve the desired outcome, allowing for greater IRS coverage with limited funding. Moreover, the overall environmental exposure would be reduced.
The WHO recently recommended the use of the synergist piperonyl butoxide (PBO) for coincorporation in ITNs (26, 27). PBO doubles the speed of action of DM against Anopheles by neutralizing metabolic pyrethroid resistance (28, 29). ITNs with DM and PBO have improved the control of malaria transmission in areas where pyrethroid resistance is prevalent (30). The additional cost of PBO-incorporated ITNs poses a challenge for resource-poor countries, however (31, 32). DM form II, which improves knockdown speeds 12-fold, promises to be beneficial even in the absence of additives, while mitigating insecticide resistance wherever DM is used in its crystalline form, as in IRS.
Conclusion
A crystalline polymorph of DM, form II, is 12 times faster acting against disease-carrying mosquitoes than DM form I when used as microcrystals embedded in commonly sprayed dusts. Epidemiological modeling suggests that malaria control could be significantly improved by applying form II during IRS. The simple preparation of form II by cooling from the melt, coupled with its kinetic stability and markedly greater efficacy, argues that DM form II can serve as a powerful, timely, and affordable tool for controlling malaria and other infectious diseases for low-income countries that are losing protection in the face of worldwide pyrethroid resistance.
The Anopheles species that transmit malaria in sub-Saharan Africa belong to the gambiae and funestus species. Our observations of the activities of DM forms I and II are consistent across three insect species, D. melanogaster, A. aegypti, and A. quadrimaculatus. We anticipate that the DM forms would have comparable relative activities against susceptible gambiae and funestus. Notably, the differences in activities have been measured for common insecticides against the sibling species of one Anopheles organism, and DM was the least variable (ca. ±10%) (33). Nevertheless, the assays described here should be performed for the Anopheles species most relevant in Africa—in addition to the six major Anopheles vectors that are common in South Asia—by scientists who maintain these species. Both susceptible and pyrethroid-resistant organisms should likewise be compared, as the experimental results above do not address whether form II will exhibit the same increase in efficacy against insecticide-resistant mosquito species.
Polymorph discovery promises to improve the performance of other contact insecticides (17–19), as less thermodynamically stable crystal forms are expected to surrender their molecules to insects more readily. Greater attention to the crystal phases of contact insecticides, both existing compounds and new ones, can provide an opportunity to prevent vector-borne disease while reducing risks to the environment.
Code Availability.
The customized program used in this study for insect lethality measurement can be obtained from GitHub at the following URLs: https://github.com/Jingxiang-Yang/Deltamethrin-Average-Velocity-Calculation and https://github.com/Jingxiang-Yang/Deltamethrin-Polymorph-Malaria. The code also is available upon requests to the corresponding authors.
Supplementary Material
Acknowledgments
This work was supported primarily by the Materials Research Science and Engineering Center program of the NSF under Award DMR-1420073. The X-ray facility was supported partially by the NSF under Award CRIF/CHE-0840277.
Footnotes
Competing interest statement: New York University has applied for a patent on the use of form II of deltamethrin with J.Y., X.Z., M.D.W., and B.K. as inventors, to encourage development for malaria prophylaxis.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2013390117/-/DCSupplemental.
Data Availability.
Crystal structure data reported in this paper have been deposited at the Cambridge Crystallographic Data Centre with accession numbers 1985206 (form I) and 1985207 (form II).
References
- 1.World Health Organization , World Malaria Report 2019, (World Health Organization, 2019). [Google Scholar]
- 2.Bhatt S.et al., The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casida J.-E., Michael Elliott’s billion dollar crystals and other discoveries in insecticide chemistry. Pest Manag. Sci. 66, 1163–1170 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Elliott M., Farnham A.-W., Janes N.-F., Needham P.-H., Pulman D.-A., Synthetic insecticide with a new order of activity. Nature 248, 710–711 (1974). [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization , World Malaria Report 2018, (World Health Organization, 2018). [Google Scholar]
- 6.Toé K.-H.et al., Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 20, 1691–1696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemingway J., The way forward for vector control. Science 358, 998–999 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Paton D.-G.et al., Exposing Anopheles mosquitoes to antimalarials blocks Plasmodium parasite transmission. Nature 567, 239–243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupferschmidt K., Pick your poison. Science 354, 171–173 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Wang J.et al., Preparedness is essential for malaria-endemic regions during the COVID-19 pandemic. Lancet 395, 1094–1096 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization , The Potential Impact of Health Service Disruptions on the Burden of Malaria: A Modelling Analysis for Countries in Sub-Saharan Africa, (World Health Organization, 2020). [Google Scholar]
- 12.Owen J.-D., Absolute configuration of the most potent isomer of the pyrethroid insecticide α-cyano-3-phenoxybenzyl-cis-3-(2,2-dibromovinyl)-2,2-dimethylcyclopropanecarboxylate by crystal structure analysis. J. Chem. Soc. Perkin Trans. 1 1975, 1865–1868 (1975). [PubMed] [Google Scholar]
- 13.World Health Organization , Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination, (World Health Organization, 2015). [Google Scholar]
- 14.Childs L.-M.et al., Disrupting mosquito reproduction and parasite development for malaria control. PLoS Pathog. 12, e1006060 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shtukenberg A.-G., Punin Y.-O., Gunn E., Kahr B., Spherulites. Chem. Rev. 112, 1805–1838 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Schneider D., Using Drosophila as a model insect. Nat. Rev. Genet. 1, 218–226 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Yang J.et al., DDT polymorphism and the lethality of crystal forms. Angew. Chem. Int. Ed. Engl. 56, 10165–10169 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Yang J.et al., Inverse correlation between lethality and thermodynamic stability of contact insecticide polymorphs. Cryst. Growth Des. 19, 1839–1844 (2019). [Google Scholar]
- 19.Zhu X.et al., Manipulating solid forms of contact insecticides for infectious disease prevention. J. Am. Chem. Soc. 141, 16858–16864 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Alzogaray R.-A., Fontán A., Zerba E.-N., Evaluation of hyperactivity produced by pyrethroid treatment on third instar nymphs of Triatoma infestans (Hemiptera: Reduviidae). Arch. Insect Biochem. Physiol. 35, 323–333 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Balabanidou V.et al., Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 113, 9268–9273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevenson B.-J.et al., Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 41, 492–502 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Torres D.et al., Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 7, 179–184 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Ingham V.-A.et al., A sensory appendage protein protects malaria vectors from pyrethroids. Nature 577, 376–380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization , Global Plan for Insecticide Resistance Management in Malaria Vectors, (World Health Organization, 2012). [Google Scholar]
- 26.Corbel V.et al., Field efficacy of a new mosaic long-lasting mosquito net (PermaNet 3.0) against pyrethroid-resistant malaria vectors: A multi centre study in Western and Central Africa. Malar. J. 9, 113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization , Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide (World Health Organization, 2017). https://apps.who.int/iris/bitstream/handle/10665/258939/WHO-HTM-GMP-2017.17-eng.pdf?sequence=5. Accessed 15 September 2020.
- 28.Gylnne-Jones D., PBO—The Insecticide Synergist, (Academic, 1998). [Google Scholar]
- 29.Darriet F., Chandre F., Combining piperonyl butoxide and dinotefuran restores the efficacy of deltamethrin mosquito nets against resistant Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 48, 952–955 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Protopopoff N.et al., Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet 391, 1577–1588 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Killeen G.-F., Ranson H., Insecticide-resistant malaria vectors must be tackled. Lancet 391, 1551–1552 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Sachs J., Malaney P., The economic and social burden of malaria. Nature 415, 680–685 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Surendran S.-N., Jude P.-J., Weerarathne T.-C., Parakrama Karunaratne S.-H.-P., Ramasamy R., Variations in susceptibility to common insecticides and resistance mechanisms among morphologically identified sibling species of the malaria vector Anopheles subpictus in Sri Lanka. Parasit. Vectors 5, 34 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystal structure data reported in this paper have been deposited at the Cambridge Crystallographic Data Centre with accession numbers 1985206 (form I) and 1985207 (form II).