Significance
Previous studies have shown a transiently higher risk of acute cardiovascular disease (CVD) events within minutes to hours after behavioral, psychosocial, and environmental triggers. Research is limited examining acute CVD surrounding sociopolitical events. We compared hospitalization rates for acute CVD before and immediately after the date of the 2016 presidential election among patients in an integrated healthcare delivery system. The rate of CVD hospitalizations in the 2 d after the 2016 presidential election was 1.62 times higher compared to the rate in the same 2 d the week prior. Transiently heightened cardiovascular risk around the 2016 election may be attributable to sociopolitical stress. Further research is needed to understand the intersection between major sociopolitical events, perceived stress, and acute CVD.
Keywords: epidemiology, sociopolitical, stress, elections, cardiovascular disease
Abstract
Previous research suggests that stressors may trigger the onset of acute cardiovascular disease (CVD) events within hours to days, but there has been limited research around sociopolitical events such as presidential elections. Among adults ≥18 y of age in Kaiser Permanente Southern California, hospitalization rates for acute CVD were compared in the time period immediately prior to and following the 2016 presidential election date. Hospitalization for CVD was defined as an inpatient or emergency department discharge diagnosis of acute myocardial infarction (AMI) or stroke using International Classification of Diseases, 10th revision codes. Rate ratios (RR) and 95% confidence intervals (CIs) were calculated comparing CVD rates in the 2 d following the 2016 election to rates in the same 2 d of the prior week. In a secondary analysis, AMI and stroke were analyzed separately. The rate of CVD events in the 2 d after the 2016 presidential election (573.14 per 100,000 person-years [PY]) compared to the rate in the window prior to the 2016 election (353.75 per 100,000 PY) was 1.62 times higher (95% CI 1.17, 2.25). Results were similar across sex, age, and race/ethnicity groups. The RRs were similar for AMI (RR 1.67, 95% CI 1.00, 2.76) and stroke (RR 1.59, 95% CI 1.03, 2.44) separately. Transiently heightened cardiovascular risk around the 2016 election may be attributable to sociopolitical stress. Further research is needed to understand the intersection between major sociopolitical events, perceived stress, and acute CVD events.
Many studies have shown that there is a transiently higher risk of acute cardiovascular disease (CVD) events within minutes to hours after behavioral, psychosocial, and environmental triggers (1). Exposures associated with triggering acute CVD events include physical activity, sexual activity, respiratory tract infection, caffeinated beverage consumption, alcohol, heavy meals, cocaine and marijuana use, work stress, anger, and depressed mood (1). The risk of onset of acute myocardial infarction (AMI) (2–4) and stroke (5, 6) is increased within hours to days after psychological triggers, including anger, depression, anxiety, and stress.
Population-based events, including earthquakes (7–10), industrial accidents (11), war and terror attacks including the 2001 September 11 attack (12, 13), sporting events (14–16), and global pandemics (17) have also been associated with a higher risk of acute CVD in the immediate time period following these events. For example, a Kaiser Permanente Northern California study showed that on the day of the September 11 attacks, there were 70% more evaluations for angina and myocardial infarction (MI) than expected, while total urgent and emergent medical evaluations were reduced during the 6-wk period immediately following September 11 (13). It is possible that the stress surrounding the 2016 presidential election may have led to positive or negative emotions that can impact acute cardiovascular risk (18, 19). In a 2017 American Psychological Association survey, over half of the respondents noted the current political climate as a significant source of stress, and two-thirds of the respondents noted that concerns about the future of the nation were a significant source of stress (20).
There is limited research examining acute CVD surrounding elections, which have become increasingly divisive in recent cycles. Therefore, we compared rates of hospitalization for acute CVD before and immediately after the date of the 2016 United States presidential election among patients in an integrated healthcare delivery system.
Methods
KPSC is an integrated healthcare delivery system providing care to ∼4.6 million people. The membership is highly representative of the Southern California population with respect to age, sex, race/ethnicity, and socioeconomic status (21). For the present study, we included data from active members ≥18 y of age at the time of hospitalization. This project was approved by the Institutional Review Board at KPSC and a waiver of informed consent was obtained due to the data-only nature of the study.
Age, sex, and race/ethnicity were obtained from patient electronic health records. Race/ethnicities were categorized into groups including Hispanic (regardless of race) and non-Hispanic racial groups, including White, Black, Asian/Pacific Islander (API), and other. The outcome of interest was hospitalization for acute CVD, defined as an inpatient principal discharge diagnosis of AMI (International Classification of Diseases, 10th revision, Clinical Modification [ICD-10-CM] codes I21.x and I22.x), or an inpatient principal or emergency department discharge diagnosis of stroke (ICD-10-CM codes I60.x to I67.x). Hospitalization counts were determined based on the time of admission. If an individual experienced multiple events occurring within 7 d of the index event, they were counted as the same event. Secondary outcomes of interest were AMI and stroke, separately. We additionally examined hospitalizations with an inpatient principal or emergency department diagnosis for chest pain and unstable angina (UA). ICD-10 code definitions for chest pain and UA are listed in SI Appendix, Table S1.
Statistical Analysis.
Using prospectively collected data in a cohort of members enrolled in KPSC, we calculated daily rates of CVD 1 mo before and 1 mo after the date of election as the number of events per 100,000 person years (PY) and a smoothing trend of daily event rates using a 7-d moving average for descriptive purposes. We calculated the 7-d moving average by taking the arithmetic mean of daily event rates over 7 d prior to the date under observation. Using Poisson regression, rate ratios (RR) and 95% confidence intervals (CI) were calculated comparing rates of hospitalization for acute CVD per 100,000 PY in the 2 d following the 2016 United States presidential election (November 9–10) to the same 2 d in the prior week (November 2–3). A 2-d time window was chosen based on prior literature showing acute CVD risk transiently heightened within hours to days of a triggering event, as previously noted. Given the close proximity of the time windows pre- and postelection, potential confounding by seasonality is likely negligible. Furthermore, it is assumed that characteristics of the study population (e.g., demographics, comorbidities, and healthcare-seeking behaviors) are not appreciably different within members across the two time periods. We calculated RRs for the entire cohort and within subgroups by age (18 to 54, 55 to 74, ≥75 y), sex, and race/ethnicity, with Wald tests for heterogeneity. We additionally compared the rate of hospitalization for CVD in the 2 d following the election to the same 2 d of the week in each of the 2 wk prior to the election (October 26–27 and November 2–3, combined). These analyses were repeated for AMI and stroke, separately, as well as chest pain and UA. To control for potential confounding due to seasonality, in a sensitivity analysis we calculated a ratio of RRs comparing 2016 results to the equivalent calendar period for the combined nonelection years of 2015 and 2017. To compare our results with an outcome that should not be influenced by a stress trigger, in a separate analysis we examined rates of acute appendicitis (definition in SI Appendix, Table S1) in the 2-d period after the 2016 presidential election with the same two weekdays in the week prior and, separately, 2 wk prior. All statistical tests were two-sided with a P < 0.05 considered statistically significant. Analyses were conducted using SAS statistical software, v9.4.
Results
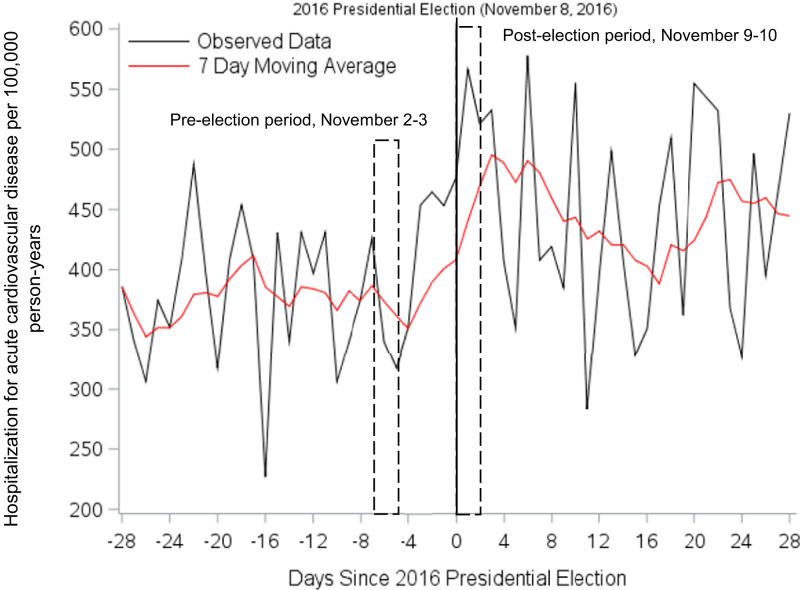
Table 1 presents characteristics of active KPSC members ≥18 y of age on the date of the 2016 presidential election. Among ∼3 million members, a majority were aged 18 to 54 y (63.9%) and female (53.2%), with large proportions of White (37.8%) and Hispanic (41.4%) individuals. Daily rates of hospitalization for CVD per 100,000 PY in the month before and month after the election are plotted with a moving average in Fig. 1. Compared to the rate of CVD in the same 2 d the week prior to the 2016 election date (58 hospitalizations; rate = 353.75 per 100,000 PY), the rate of CVD was 1.62 times higher (95% CI 1.17, 2.25, P = 0.004) in the 2 d following the election (94 hospitalizations; rate = 573.14 per 100,000 PY) (Table 2). Compared to the same 2 d in the week prior, the rates of CVD in the 2 d following the election among men and women were 1.33 and 2.21 times higher, respectively (P interaction = 0.15). Rates of CVD were also higher among White, Black, Hispanic, and API individuals following the election (RR 1.81, 2.33, 1.20, and 1.33, respectively; P interaction = 0.60). Similar results were also observed when we compared the rate in the 2 d following the 2016 election to the same 2 d of the week in each of the prior 2 wk (RR 1.45, 95% CI 1.11, 1.88) (Table 3).
Table 1.
Demographic characteristics of active KPSC members ≥18 y of age on the date of the 2016 presidential election (November 8, 2016)
| Characteristic | n (%) |
| Age, y | |
| 18–54 | 1,915,986 (63.9) |
| 55–74 | 861,732 (28.7) |
| 75+ | 219,690 (7.4) |
| Sex | |
| Male | 1,400,595 (46.7) |
| Female | 1,596,813 (53.2) |
| Race/ethnicity | |
| White | 1,132,280 (37.8) |
| Black | 272,073 (9.0) |
| Hispanic | 1,239,555 (41.4) |
| API | 334,574 (11.2) |
| Other | 18,926 (0.6) |
Fig. 1.
Hospitalization for acute cardiovascular disease events per 100,000 PY in the month preceding and after the 2016 presidential election among KPSC members ≥18 y of age.
Table 2.
Hospitalization for acute CVD events in the 2 d following the 2016 presidential election (November 9–10) compared to the same 2 d in the prior week (November 2–3)
| CVD events in the 2 d following the 2016 presidential election (November 9–10) | CVD events on the same weekdays in the prior week (November 2–3) | Rate ratio (95% CI) | P value | |||||
| Events, n | PY | Rate per 100,000 PY | Events, n | PY | Rate per 100,000 PY | |||
| Overall | 94 | 16,401 | 573.1 | 58 | 16,396 | 353.8 | 1.62 (1.17, 2.25) | |
| Age, y | 0.94 | |||||||
| 18–54 | 13 | 10,481 | 124.0 | 7 | 10,481 | 66.8 | 1.86 (0.74, 4.65) | |
| 55–74 | 39 | 4,717 | 826.9 | 24 | 4,714 | 509.1 | 1.62 (0.98, 2.70) | |
| 75+ | 42 | 1,203 | 3,490.3 | 27 | 1,201 | 2,248.4 | 1.55 (0.96, 2.52) | |
| Sex | 0.15 | |||||||
| Male | 52 | 7,662 | 678.7 | 39 | 7,659 | 509.2 | 1.33 (0.88, 2.02) | |
| Female | 42 | 8,739 | 480.6 | 19 | 8,737 | 217.5 | 2.21 (1.29, 3.80) | |
| Race/ethnicity | 0.60 | |||||||
| White | 47 | 6,197 | 758.4 | 26 | 6,197 | 419.6 | 1.81 (1.12, 2.92) | |
| Black | 14 | 1,489 | 940.2 | 6 | 1,489 | 403.1 | 2.33 (0.90, 6.07) | |
| Hispanic | 24 | 6,782 | 353.9 | 20 | 6,778 | 295.1 | 1.20 (0.66, 2.17) | |
| API | 8 | 1,829 | 437.3 | 6 | 1,829 | 328.1 | 1.33 (0.46, 3.84) | |
| Other | 1 | 104 | 966.0 | 0 | 103 | 0.0 | ||
Table 3.
Hospitalization for acute CVD events in the 2 d following the 2016 presidential election (November 9–10) compared to the same 2 d of the week in each of the 2 wk prior to the election (October 26–27 and November 2–3)
| CVD events in the 2 d following the 2016 presidential election (November 9–10) | CVD events on the same weekdays in the prior 2 wk (October 26–27, November 2–3) | Rate ratio (95% CI) | P value | |||||
| Events, n | PY | Rate per 100,000 PY | Events, n | PY | Rate per 100,000 PY | |||
| Overall | 94 | 16,401 | 573.1 | 130 | 32,784 | 396.5 | 1.45 (1.11, 1.88) | |
| Age, y | 0.91 | |||||||
| 18–54 | 13 | 10,481 | 124.0 | 16 | 20,958 | 76.3 | 1.62 (0.78, 3.38) | |
| 55–74 | 39 | 4,717 | 826.9 | 57 | 9,423 | 604.9 | 1.37 (0.91, 2.05) | |
| 75+ | 42 | 1,203 | 3,490.3 | 57 | 2,404 | 2,371.2 | 1.47 (0.99, 2.19) | |
| Sex | 0.81 | |||||||
| Male | 52 | 7,662 | 678.7 | 74 | 15,318 | 483.1 | 1.40 (0.99, 2.00) | |
| Female | 42 | 8,739 | 480.6 | 56 | 17,466 | 320.6 | 1.50 (1.00, 2.24) | |
| Race/ethnicity | 0.52 | |||||||
| White | 47 | 6,197 | 758.4 | 55 | 12,396 | 443.7 | 1.71 (1.16, 2.52) | |
| Black | 14 | 1,489 | 940.2 | 18 | 2,979 | 604.3 | 1.56 (0.77, 3.13) | |
| Hispanic | 24 | 6,782 | 353.9 | 45 | 13,548 | 332.2 | 1.07 (0.65, 1.75) | |
| API | 8 | 1,829 | 437.3 | 12 | 3,655 | 328.3 | 1.33 (0.54, 3.26) | |
| Other | 1 | 104 | 966.0 | 0 | 207 | 0.0 | ||
In secondary analyses we evaluated hospitalization for AMI and stroke, separately (Table 4 and SI Appendix, Fig. S1). The rate of AMI in the 2 d following the election was 1.67 times higher (95% CI 1.00, 2.76, P = 0.05) compared to the same 2 d the week prior to the election. The rate of stroke in the 2 d following the election was 1.59 times higher (95% CI 1.03, 2.44, P = 0.03) compared to the same 2 d the week prior to the election. Similar results for AMI and stroke, examined separately, were observed comparing the rate in the 2 d following the 2016 election to the same 2 d of the week in each of the prior 2 wk (RR 1.57 and 1.37, respectively) (SI Appendix, Table S2). There was no evidence of higher hospitalization rates for chest pain or UA in the 2 d following the presidential election compared to the same 2 d in the prior week (RR 0.96 [95% CI 0.83, 1.10] and RR 0.59 [95% CI 0.27, 1.28], respectively) or prior 2 wk (RR 1.00 [95% CI 0.88, 1.13] and RR 0.56 [95% CI 0.28, 1.12], respectively) (SI Appendix, Table S3). In a sensitivity analysis, the RR for CVD hospitalizations in 2016 (RR 1.67) compared to 2015 and 2017 (combined RR 1.16) resulted in a ratio of RRs of 1.39 (95% CI 0.93, 2.08) (SI Appendix, Table S4). Similar results were obtained for other outcomes comparing RR in 2016 to the combined RR for 2015/2017. Separately, rates of acute appendicitis were not higher in the 2 d after the 2016 presidential election compared to the same 2 d in the week prior (RR 1.00, 95% CI 0.49, 2.04) and 2 wk prior (RR 0.97, 95% CI 0.52, 1.79) (SI Appendix, Table S5).
Table 4.
Hospitalization for AMI and stroke in the 2 d following the 2016 presidential election (November 9–10) compared to the same 2 d in the prior week (November 2–3)
| CVD events in the 2 d following the 2016 presidential election (November 9–10) | CVD events on the same weekdays in the prior week (November 2–3) | Rate ratio (95% CI) | |||||
| Events, n | PY | Rate per 100,000 PY | Events, n | PY | Rate per 100,000 PY | ||
| AMI | 40 | 16,399 | 243.9 | 24 | 16,393 | 146.4 | 1.67 (1.00, 2.76) |
| Stroke | 54 | 16,412 | 329.0 | 34 | 16,407 | 207.2 | 1.59 (1.03, 2.44) |
Discussion
In the present study among KPSC members ≥18 y of age, we observed a higher rate of hospitalization for acute CVD in the 2 d following the 2016 presidential election compared to the same 2 d in the prior week and prior 2 wk. Similar results were observed regardless of age, sex, race/ethnicity, and for AMI and stroke, considered separately. These data suggest that sociopolitical stress from elections may contribute to transiently heightened risk of acute CVD hospitalization.
At an individual level, acute psychological stressors, including anger (3, 22), anxiety and depression (23), and emotional upset (24) have all been associated with a sudden and transient increase in the risk of acute CVD events in the hours to days following exposure (1). For example, in a cohort of 1,194 adults, emotional upset was associated with a 2.7-fold increased risk of (MI) within 24 h (24). Furthermore, several studies have documented that population-wide exposure to stressors, including natural disasters (7–10), industrial accidents (11), and sporting events (14–16) among others, have also been associated with a heightened risk of acute CVD events in the hours to days following the potential stressor. For example, emotional upset associated with the loss of a World Cup match led to 25% increased risk for AMI hospitalization in the days following (16). More recently, among patients with acute coronary syndrome who tested negative for COVID-19, the rate of stress cardiomyopathy was 4.51 times higher in the COVID-19 pandemic period of March–April 2020 compared to a composite of multiple prepandemic periods (17), suggesting that the stress and anxiety of the pandemic can manifest as this stress-related disorder (25).
Episodes of stress and anxiety or population-wide stressors may cause acute CVD events in susceptible individuals, including those with chronic macrovascular and microvascular disease, preexisting atherosclerotic plaque, and disorders of the cardiac conduction system (1). Potential physiological mechanisms facilitating these types of responses have been proposed, including vasoconstrictive and prothrombotic effects, lowering of the threshold for cardiac conduction system instability, increased cardiac sympathetic activation, inflammation, oxidative stress, and coagulation regulation (26–30). Gullette et al. (23) demonstrated that among a sample of patients with coronary artery disease and documented exercise-inducible ischemia, periods of self-reported anxiety or sadness were associated with a twofold higher incidence of electrocardiographically documented episodes of myocardial ischemia. In a study of healthy female participants undergoing functional magnetic resonance imaging, Muscatell et al. found that increased neural activity in the left amygdala in response to negative social feedback was related to greater stressor-evoked changes in IL-6 levels, an inflammatory cytokine and marker of CVD risk (31). Furthermore, the authors suggested that as few as one negative experience was sufficient to alter gene expression, leading to transiently increased production of proteins associated with inflammation. While these negative experiences of stress and anxiety are not limited to political events, it is reasonable to speculate that those targeted by divisive rhetoric in politics may be at a higher risk of acute CVD. However, we cannot rule out the potential that positive stress responses (e.g., neurochemical responses to feelings of euphoria) may also increase the risk of acute CVD. Behavioral interventions emphasizing coping mechanisms for stressful triggers may be important in assessing whether these interventions can lower the risk of acute CVD.
As noted previously, in a 2017 survey by the American Psychological Association, a large portion of adults noted the current political climate as a significant source of stress (20). There is a growing body of evidence that stress from elections can negatively impact health. A recent population-based study found that preterm births were elevated among Latina women compared with other women in the 9-mo period after the 2016 presidential election (19). In the present study, we found consistent associations of higher risks of cardiovascular hospitalization within 48 h following the 2016 presidential election compared to the same days of the week 1 and 2 wk earlier. There was no evidence of statistical heterogeneity by age, sex, and race/ethnicity. However, qualitatively, the association was higher among women compared with men, and among Black and White compared with Hispanic individuals. Since the number of events each day is low, we cannot definitively make conclusions about the rates in these subgroups. Similar patterns may be observed in other nationally representative populations.
Strengths of the present study include the use of a large, diverse population within an integrated healthcare network that contains extensive electronic health record information. Furthermore, to our knowledge, no prior studies have compared hospitalization rates for acute CVD immediately before and after presidential elections. This analysis is timely given the polarized political climate preceding the 2020 presidential election. We also acknowledge some limitations. Deaths prior to hospitalization were not captured in the present study. Given the short time frame for assessing acute CVD, small numbers of hospitalizations may have limited our statistical power to detect differences within subgroups. In addition, results may not be fully generalizable to patients in less-integrated health systems, uninsured individuals, or those in other regions of the country, emphasizing the importance of reproducibility in other populations. Furthermore, measures of stress are not captured in the patient electronic health record and we cannot make direct connections between sociopolitical stress and risk of acute CVD. We included time periods only after the transition to ICD-10 coding in October 2015, since the transition between ICD-9 and ICD-10 coding could influence trends. We conducted a sensitivity analysis comparing rates in 2016 to similar time periods in 2015 and 2017, but future work is also warranted to investigate these associations in future election cycles. Finally, seasonal effects due to daylight savings time have been shown to increase the risk of CVD events (32), and we cannot rule out the potential influence of seasonality on our results. However, confounding is of minimal concern given the short event windows chosen for the present analysis.
In conclusion, transiently heightened cardiovascular risk around the 2016 election may be attributable to sociopolitical stress. Further research is needed to understand the intersection between major sociopolitical events, perceived stress, and acute CVD events.
Supplementary Material
Acknowledgments
The authors thank Ran Liu of the Kaiser Permanente Southern California Department of Research & Evaluation for her programming and statistical contributions to this study. Funding for the present study was provided by Grant P0131281 from the W.K. Kellogg Foundation.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2012096117/-/DCSupplemental.
Data Availability.
Anonymized data that support the findings of this study may be made available from the investigative team in the following conditions: 1) agreement to collaborate with the study team on all publications, 2) provision of external funding for administrative and investigator time necessary for this collaboration, 3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and 4) agreement to abide by the terms outlined in data use agreements between institutions.
References
- 1.Mittleman M. A., Mostofsky E., Physical, psychological and chemical triggers of acute cardiovascular events: Preventive strategies. Circulation 124, 346–354 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smyth A.et al.; INTERHEART Investigators , Physical activity and anger or emotional upset as triggers of acute myocardial infarction: The INTERHEART study. Circulation 134, 1059–1067 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Mittleman M. A.et al.; Determinants of Myocardial Infarction Onset Study Investigators , Triggering of acute myocardial infarction onset by episodes of anger. Circulation 92, 1720–1725 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Mostofsky E., Maclure M., Tofler G. H., Muller J. E., Mittleman M. A., Relation of outbursts of anger and risk of acute myocardial infarction. Am. J. Cardiol. 112, 343–348 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koton S., Tanne D., Bornstein N. M., Green M. S., Triggering risk factors for ischemic stroke: A case-crossover study. Neurology 63, 2006–2010 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Sharma A.et al., Prevalence of triggering factors in acute stroke: Hospital-based observational cross-sectional study. J. Stroke Cerebrovasc. Dis. 24, 337–347 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Leor J., Poole W. K., Kloner R. A., Sudden cardiac death triggered by an earthquake. N. Engl. J. Med. 334, 413–419 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Kloner R. A., Leor J., Poole W. K., Perritt R., Population-based analysis of the effect of the Northridge Earthquake on cardiac death in Los Angeles County, California. J. Am. Coll. Cardiol. 30, 1174–1180 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Chan C.et al., Acute myocardial infarction and stress cardiomyopathy following the Christchurch earthquakes. PLoS One 8, e68504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niiyama M.et al., Population-based incidence of sudden cardiac and unexpected death before and after the 2011 earthquake and tsunami in Iwate, northeast Japan. J. Am. Heart Assoc. 3, e000798 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruidavets J. B.et al., Triggering of acute coronary syndromes after a chemical plant explosion. Heart 92, 257–258 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg J. S.et al., Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. J. Am. Coll. Cardiol. 44, 1261–1264 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Johnston S. C., Sorel M. E., Sidney S., Effects of the September 11th attacks on urgent and emergent medical evaluations in a Northern California managed care plan. Am. J. Med. 113, 556–562 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Witte D. R., Bots M. L., Hoes A. W., Grobbee D. E., Cardiovascular mortality in Dutch men during 1996 European football championship: Longitudinal population study. BMJ 321, 1552–1554 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilbert-Lampen U.et al., Modified serum profiles of inflammatory and vasoconstrictive factors in patients with emotional stress-induced acute coronary syndrome during World Cup Soccer 2006. J. Am. Coll. Cardiol. 55, 637–642 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Carroll D., Ebrahim S., Tilling K., Macleod J., Smith G. D., Admissions for myocardial infarction and World Cup football: Database survey. BMJ 325, 1439–1442 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabri A.et al., Incidence of stress cardiomyopathy during the coronavirus disease 2019 pandemic. JAMA Netw. Open 3, e2014780 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams D. R., Medlock M. M., Health effects of dramatic societal events—Ramifications of the recent presidential election. N. Engl. J. Med. 376, 2295–2299 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Gemmill A.et al., Association of preterm births among US Latina women with the 2016 presidential election. JAMA Netw. Open 2, e197084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychological Association , Stress in America: Coping with change. Part 1. Stress in AmericaTM Survey. https://www.apa.org/news/press/releases/stress/2016/coping-with-change.pdf. Accessed 16 February 2020.
- 21.Koebnick C.et al., Sociodemographic characteristics of members of a large, integrated health care system: Comparison with US Census Bureau data. Perm. J. 16, 37–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley T.et al., Triggering of acute coronary occlusion by episodes of anger. Eur. Heart J. Acute Cardiovasc. Care 4, 493–498 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Gullette E. C.et al., Effects of mental stress on myocardial ischemia during daily life. JAMA 277, 1521–1526 (1997). [PubMed] [Google Scholar]
- 24.Willich S. N.et al.; Triggers and Mechanisms of Myocardial Infarction Study Group , Physical exertion as a trigger of acute myocardial infarction. N. Engl. J. Med. 329, 1684–1690 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Ghadri J. R.et al., International expert consensus document on Takotsubo syndrome (part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 39, 2032–2046 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson S. M.et al., Prothrombotic effects of environmental stress: Changes in platelet function, hematocrit, and total plasma protein. Psychosom. Med. 57, 592–599 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Abisse S. S., Lampert R., Burg M., Soufer R., Shusterman V., Cardiac repolarization instability during psychological stress in patients with ventricular arrhythmias. J. Electrocardiol. 44, 678–683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hering D., Lachowska K., Schlaich M., Role of the sympathetic nervous system in stress-mediated cardiovascular disease. Curr. Hypertens. Rep. 17, 80 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Siegrist J., Sies H., Disturbed redox homeostasis in oxidative distress: A molecular link from chronic psychosocial work stress to coronary heart disease? Circ. Res. 121, 103–105 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Fioranelli M.et al., Stress and inflammation in coronary artery disease: A review psychoneuroendocrineimmunology-based. Front. Immunol. 9, 2031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muscatell K. A.et al., Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav. Immun. 43, 46–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfredini R.et al., Daylight saving time and myocardial infarction: Should we be worried? A review of the evidence. Eur. Rev. Med. Pharmacol. Sci. 22, 750–755 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data that support the findings of this study may be made available from the investigative team in the following conditions: 1) agreement to collaborate with the study team on all publications, 2) provision of external funding for administrative and investigator time necessary for this collaboration, 3) demonstration that the external investigative team is qualified and has documented evidence of training for human subjects protections, and 4) agreement to abide by the terms outlined in data use agreements between institutions.