Significance
The mammary gland is functional for only a brief period of a female’s lifetime. During this time, it operates not for the survival of the individual, but for the survival of her species. Here, we visualize the nature of alveolar contractions in the functionally mature mammary gland, revealing how specialized epithelial cells, which possess the ability to behave like smooth muscle cells, undergo Ca2+-dependent contractions. We demonstrate that individual oscillators can be electrically coupled to achieve global synchrony, a phenomenon that has not yet been observed in the mammary gland. By imaging activity across scales, we provide a window into the organization, dynamics, and role of epithelial Ca2+ oscillations in the organ principally responsible for sustaining neonatal life in mammals.
Keywords: calcium signaling, GCaMP6, mammary gland, lactation, oxytocin
Abstract
The mammary epithelium is indispensable for the continued survival of more than 5,000 mammalian species. For some, the volume of milk ejected in a single day exceeds their entire blood volume. Here, we unveil the spatiotemporal properties of physiological signals that orchestrate the ejection of milk from alveolar units and its passage along the mammary ductal network. Using quantitative, multidimensional imaging of mammary cell ensembles from GCaMP6 transgenic mice, we reveal how stimulus evoked Ca2+ oscillations couple to contractions in basal epithelial cells. Moreover, we show that Ca2+-dependent contractions generate the requisite force to physically deform the innermost layer of luminal cells, compelling them to discharge the fluid that they produced and housed. Through the collective action of thousands of these biological positive-displacement pumps, each linked to a contractile ductal network, milk begins its passage toward the dependent neonate, seconds after the command.
The mammary gland has a central role in the health and survival of all mammals (1, 2). Development of this organ is a multistep process that begins as the female embryo develops in her mother’s uterus (2, 3) and culminates as she nurtures the next generation of offspring in her own (2, 4). In mice, the postpubertal female mammary gland consists of an elaborate network of evenly spaced branching ducts embedded within an adipocyte-rich stroma (4). Each mammary duct consists of an inner layer of heterogeneous luminal epithelial cells, which include both estrogen receptor (ER)-positive and -negative cell lineages (5, 6). These cells are surrounded by a layer of basal epithelial cells, which express the basal cytokeratins K5 and K14 as well as smooth muscle actin (SMA) (7, 8). Heterogeneity also exists within the basal cell compartment, with recent single-cell RNA sequencing confirming clusters of cells with high levels of the genes encoding SMA, oxytocin receptor (OXTR), and K15 (termed basal myoepithelial cells) as well as a population of cells with high levels of Procr, Gng11, and Zeb2 (termed basal Procr+ cells) (7).
During alveologenesis in pregnancy, adult mammary stem and progenitor cells rapidly proliferate to generate the millions of new cells that are required to produce, store, and expel milk during lactation (9, 10). These cells are arranged in mammary alveoli, with each alveolar unit broadly consisting of an inner layer of secretory luminal cells and an outer network of contractile basal cells (4). Many alveolar units cluster to form large lobuloalveolar complexes, which connect to each other and to the nipple via the tubular ductal network. The development and function of epithelial cells in the mammary gland during pregnancy and lactation are governed by a range of local and systemic factors (11). A greater appreciation of these factors, and the molecular pathways that link signal reception to cellular outcomes, would greatly improve our understanding of this fundamental process in mammalian biology.
The ability to visualize how a single living cell, in its native environment, translates an extracellular message into an intracellular signal to execute a defined task at the cell level and cooperatively achieve a biological outcome at the organ level is revolutionizing our understanding of multicellular systems. Such an approach has provided new insights into a range of biological phenomena, including how plants defend against herbivory (12), how fish escape looming predators (13, 14), and how mammals store memories (15). The rational design and continued refinement of genetically encoded Ca2+ indicators (GECIs) has fueled these advances (16). However, the use of GECIs for in situ activity mapping in adult vertebrates, has largely remained an achievement of neuroscience, where neural activity is tightly coupled to intracellular Ca2+ ([Ca2+]i) signaling (17).
Efforts to map activity networks in specific populations of nonexcitable cells in other solid organs is lagging. Indeed, our understanding of signal transduction in many epithelial tissue types (including the mammary gland) has principally arisen through analysis of isolated cells (often serially propagated under physiologically extraneous conditions), retrospective examination of fixed tissue, and interrogation of genetic knockout models (where biological function is inferred in the absence of physiological redundancy or compensation). The ability to visualize signal–response relationships in mammary epithelial cells in situ and across scales will shed important new light on both structure–function relationships and patterns of cellular connectivity in this important epithelial organ.
When young offspring suckle, maternally produced oxytocin (OT) binds to its cognate receptor (the OXTR) on mammary basal cells, causing them to contract (18). Activity is likely to be tightly coupled to [Ca2+]i in these cells via a phospholipase C (PLC)-inositol trisphosphate (InsP3) signaling pathway (18–22). The absence of physiological redundancy in the mammary OT/OXTR system—highlighted by the inability of both OT ligand- and receptor-null mice to adequately nurse their pups (23–25) [a phenotype that can be rescued in ligand-null animals through administration of exogenous OT (24)]—facilitates the direct visualization of this specific epithelial signal–response relationship at this important stage of development.
In this study, we engineered mice with directed expression of a GECI to basal epithelial cells in the mammary gland. This enabled us to quantitatively probe the organization and function of real-time [Ca2+]i signaling events in individual cells within this complex living tissue, at a level of rigor that has only previously been achieved in the adult brain.
Results
Basal Cell [Ca2+]i Oscillations Signal to Repetitively Deform Mammary Alveoli and Force Milk Out.
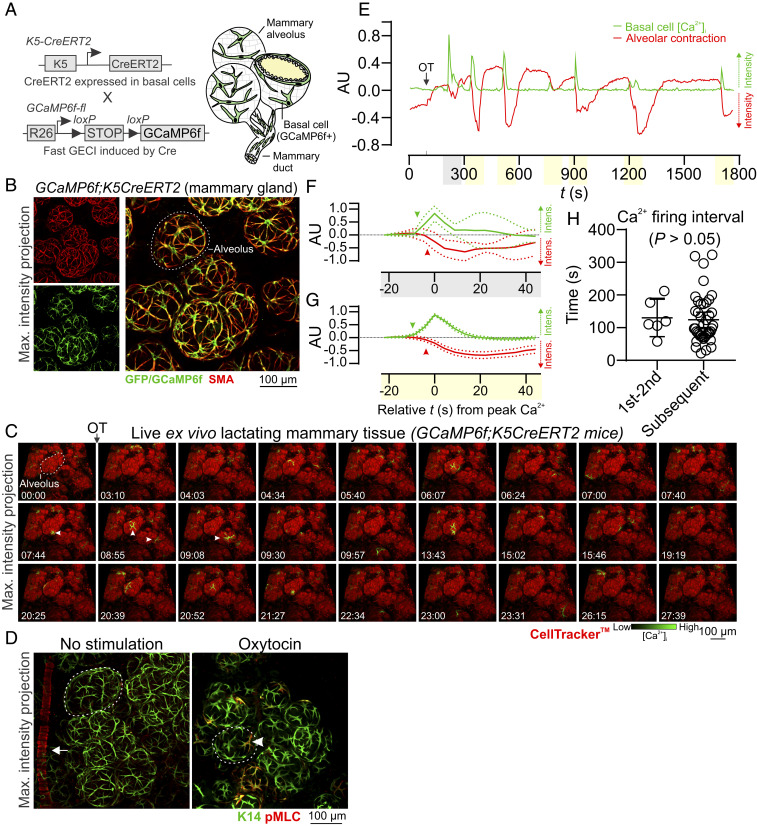
We developed transgenic mice that express the fast, ultrasensitive GECI GCaMP6f (16) under the inducible control of the K5 gene promoter (8) (GCaMP6f;K5CreERT2 mice) (Fig. 1A). The relatively high baseline fluorescence of this GECI is well suited for the quantitative assessment of [Ca2+]i responses in alveolar basal cells, which are sparsely distributed with thin cellular processes (16, 26) (SI Appendix, Fig. S1 A and B). GCaMP6f consists of a circularly permuted green fluorescent protein (GFP), enabling three-dimensional (3D) assessment of its expression and lineage-specific localization using an anti-GFP antibody (27) and optimized methods for tissue clearing (28). Genetic recombination in this model was high (SI Appendix, Fig. S2 A and B) and showed lineage restriction to basal epithelial cells (Fig. 1B).
Fig. 1.
Basal cell Ca2+ oscillations precede alveolar contractions. (A) Schematic representation of GCaMP6f;K5CreERT2 model. (B) Maximum intensity z-projection of cleared lactating mammary tissue immunostained with smooth muscle actin (SMA) to reveal basal cells and anti-GFP antibody to detect GCaMP6f. (C) Three-dimensional time-lapse imaging of live mammary tissue from GCaMP6f;K5CreERT2 lactating mice stimulated with OT (85 nM) at 01:33 (min:s). Images show maximum intensity z-projection. Arrowheads point to Ca2+ events in single cells. See Movie S1. (D) Maximum intensity z-projections of cleared mammary tissue immunostained with K14 to reveal basal cells and pMLC to show sites of contractile activity. Arrow shows pMLC+ blood vessel in control tissue, arrowhead shows pMLC+ basal cell in tissue stimulated with OT (85 nM) prior to fixation; dotted lines surround alveolar units. (E) Quantification of [Ca2+]i responses (green) and alveolar unit contraction (red) in lactating mammary tissue from GCaMP6f;K5CreERT2 mice. [Ca2+]i measurements are ΔF/F0. Alveolar unit contractions shown by negative deflections (CellTracker fluorescence). (F and G) Average (±SEM) peak [Ca2+]i and contractile responses. Highlighting (x axis) corresponds with events linked in E; arrowheads show initiation of the response. (H) Interval between the first and second, and all subsequent [Ca2+]i events (P > 0.05, Student’s t test). AU, arbitrary unit; n = 3 mice.
To assess OT-mediated basal cell [Ca2+]i responses, we performed four-dimensional (4D) (x, y, z, and t) imaging of ex vivo mammary tissue pieces from lactating GCaMP6f;K5CreERT2 mice—a method similar to the preparation of acute brain slices for neural imaging (SI Appendix, Methods) (29). Tissue was loaded with the live cell permeable dye CellTracker Red to visualize alveolar luminal (milk producing) cells (SI Appendix, Fig. S1A). A coordinated wave of [Ca2+]i, due to InsP3-mediated endoplasmic reticulum (ER) Ca2+ store release (18, 19, 22), was observed in mammary basal cells following OT stimulation and its diffusion through the tissue (Fig. 1C and Movie S1). This initial transient [Ca2+]i elevation was followed by a phase of stochastic [Ca2+]i oscillations (Fig. 1C arrowheads and Movie S1) that were likely to be sustained in part by Ca2+ influx across the plasma membrane (19, 21, 30).
The organization of basal cell contractions was also examined using 3D, deep tissue imaging of myosin light chain (MLC) phosphorylation. In tissue treated with OT prior to fixation, phospho-MLC (pMLC)-positive and -negative basal cells were observed to be interspersed throughout alveolar clusters (Fig. 1D), supporting the ostensibly stochastic nature of the mammary contractile response. Regions containing clusters of pMLC-positive cells, however, were also observed in OT-treated tissue (see SI Appendix, Fig. S3A asterisk and SI Appendix, Fig. S3B). Intravital imaging of OT-mediated [Ca2+]i responses (31) supported observations in acute ex vivo tissue preparations (SI Appendix, Fig. S4 A–C and Movie S2).
To determine whether increases in [Ca2+]i are temporally correlated with alveolar unit contractions, we quantified Ca2+-contraction responses in alveolar tissue. While cell- and tissue-level movement is physiologically relevant and important, it poses additional computational challenges to the analysis of single-cell Ca2+ responses in 4D image sequences. To overcome this, we utilized the diffeomorphic registration approach of Advanced Normalization Tools for motion correction (32, 33) (SI Appendix, Methods). This approach corrected major tissue movements; however, alveolar unit contractions remained largely intact, enabling quantification of [Ca2+]i responses in basal cells and analysis of the physical distortions to the alveolar units that these cells embrace. These analyses confirmed that increases in [Ca2+]i in individual basal cells were temporally correlated with physical distortions to the mechanically compliant luminal cell layer (Fig. 1E and see SI Appendix, Fig. S5A). For both the first InsP3 response and the subsequent oscillatory phase, increases in [Ca2+]i preceded alveolar unit contractions (Fig. 1 F and G and see SI Appendix, Fig. S5B). No statistical difference in the firing interval for [Ca2+]i was observed between the first and second events and all subsequent events (Fig. 1H). No [Ca2+]i oscillations or contractions were observed in live tissue in the absence of OT stimulation (SI Appendix, Fig. S5C). These results reveal that each mammary alveolar unit, acting downstream of a basal cell OT/OXTR/InsP3/Ca2+ signaling axis, serves as a biological positive-displacement pump, repeatedly forcing milk out of its central lumen for passage through the ductal network.
Basal Cell Contractions Are Ca2+ Signal Dependent.
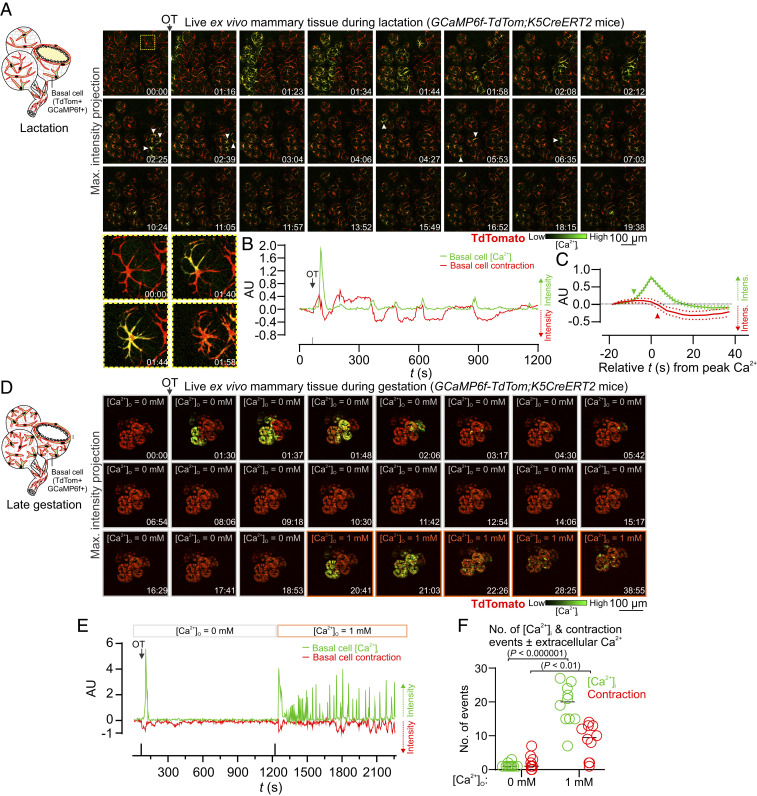
To directly assess Ca2+-contraction coupling in mammary basal cells, we engineered triple transgenic mice that express GCaMP6f and the red fluorescent protein TdTomato (34) in basal cells (GCaMP6f-TdTom;K5CreERT2 mice) (Fig. 2A). Using this model, we observed large increases in [Ca2+]i in single TdTomato-positive basal cells in response to OT, which immediately preceded their contraction (Fig. 2 A–C, see SI Appendix, Fig. S6, and Movie S3). These data reveal with greater optical clarity how basal cells contract to deform the inner luminal cell layer for milk ejection and show unequivocally, using a second model to measure basal cell contraction, a temporal relationship between the Ca2+ signal and the contractile response (SI Appendix, Fig. S6B).
Fig. 2.
Ca2+-contraction coupling. (A) Three-dimensional time-lapse imaging of live mammary tissue from GCaMP6f-TdTom;K5CreERT2 mice stimulated with OT (85 nM) at 01:09 (min:s). Images show maximum intensity z-projection. Box (frame 1) expanded in panel Below; arrowheads point to Ca2+ events in single cells. See Movie S3. (B) Quantification of [Ca2+]i responses (green) and alveolar unit contraction (red) in lactating mammary tissue from GCaMP6f-TdTom;K5CreERT2 mice. [Ca2+]i measurements are ΔF/F0. Basal cell contractions shown by negative deflections (TdTomato fluorescence). (C) Average (±SEM) peak [Ca2+]i response and contractile response in mammary tissue isolated from lactating GCaMP6f-TdTom;K5CreERT2 mice. Values averaged from both the first response and the oscillatory phase. (D) Three-dimensional time-lapse imaging of live mammary tissue from GCaMP6f-TdTom;K5CreERT2 mice (15.5 to 16.5 days postcoitus (d.p.c.) stimulated with OT (85 nM) at 01:08 (min:s) under extracellular Ca2+ free conditions. Images show maximum intensity z-projection. Ca2+ (1 mM free Ca2+) was added back at 20:23 (min:s). See Movie S5. (E) Quantification of [Ca2+]i responses and alveolar unit contraction in mammary tissue from pregnant GCaMP6f-TdTom;K5CreERT2 mice stimulated with OT under extracellular Ca2+-free conditions and with Ca2+ addback. [Ca2+]i measurements are ΔF/F0. Basal cell contractions shown by negative deflections (TdTomato fluorescence). (F) Number of [Ca2+]i and contraction events ± extracellular Ca2+ ([Ca2+]O). Graph shows individual measurements and median. P value in parentheses is from multiple t tests. n = 3 mice.
To determine whether Ca2+ forms an essential component of the signal transduction pathway linking OXTR engagement to basal cell contraction, we examined [Ca2+]i and contraction events under extracellular Ca2+-free conditions. Tissue was isolated from pregnant GCaMP6f-TdTom;K5CreERT2 mice and incubated in a Ca2+-free physiological salt solution supplemented with the Ca2+ chelator BAPTA. By performing experiments using mammary tissue harvested prior to secretory activation (gestation day 15.5 to 16.5), when Ca2+-contraction coupling is observed (Movie S4), we were able to avoid the exceedingly high (>90 mM) extracellular Ca2+ concentrations present in secreted milk (19). Under these experimental conditions, addition of OT resulted in intracellular Ca2+ store release associated with cell contraction (Fig. 2 D and E and Movie S5). Ensuing spike trains, however, were absent and subsequent contractions were abolished. Readdition of extracellular Ca2+ led to the resumption of Ca2+ firing and basal cell contractions (Fig. 2 D–F). These data demonstrate that both Ca2+ release from InsP3-sensitive intracellular Ca2+ stores (22) and Ca2+ influx across the plasma membrane are sufficient for basal cell contraction but that influx across the membrane is necessary to sustain cell and tissue contractions.
Both Ducts and Alveoli Contract to Expel Milk in the Mature Gland.
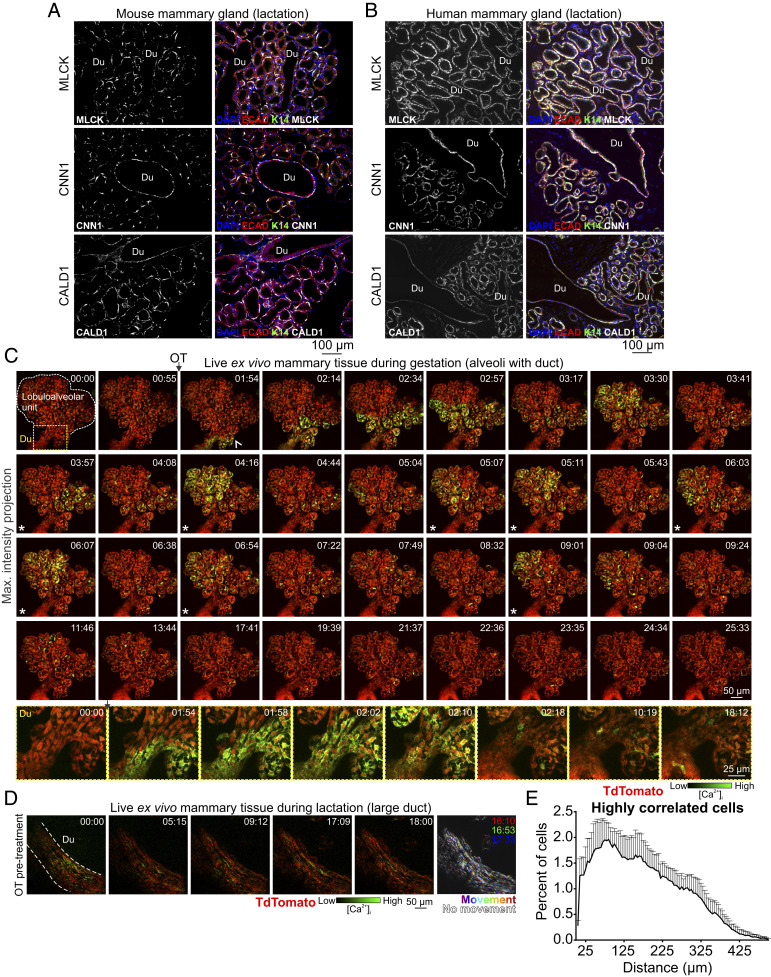
The lactating mouse mammary gland consists of milk producing alveoli that are connected to the nipple via a branching ductal network (Fig. 1A). Heterogeneity in the expression of contractile markers in basal cells of ducts and alveoli has led to speculation that these two related (but spatially and morphologically distinct) cell populations are functionally divergent (35). We compared expression of myosin light chain kinase (MLCK), calponin (CNN1), and caldesmon (CALD1)—key components of the vascular smooth muscle contraction pathway that are up-regulated in the mammary gland during lactation (SI Appendix, Fig. S7)—in ducts versus alveoli of lactating mice (Fig. 3A) and humans (Fig. 3B). Our analyses reveal that these proteins are expressed at comparable levels in basal cells of both structures (Fig. 3 A and B and see SI Appendix, Fig. S8).
Fig. 3.
Functional differentiation and Ca2+-contraction coupling in ducts and alveoli. (A and B) Immunostaining of paraffin-embedded mouse and human lactating tissue. MLCK, CNN1, and CALD1 are expressed in both ducts (Du) and alveoli. E-cadherin shows the luminal cell lineage; K14 shows the basal cell lineage. Nuclei are stained with DAPI; n = 3 samples, mouse and human. (C) Three-dimensional time-lapse imaging of live mammary tissue from a pregnant (15.5 to 16.5 d.p.c.) GCaMP6f-TdTom;K5CreERT2 mouse stimulated with OT (85 nM) at 01:15 (min:s). Images show maximum intensity z-projection of live tissue; box (frame 1) shows subtending duct (Du, magnified at Bottom), extending deeper into the tissue. Arrowhead at 01:54 shows direction of OT diffusion; asterisks show coordinated firing; n = 3. See Movie S6. (D) Three-dimensional time-lapse imaging of a large duct from a lactating GCaMP6f-TdTom;K5CreERT2 mouse stimulated with OT (85 nM) immediately prior to imaging. Images show maximum intensity z-projection of live tissue; n = 3. See Movie S7. (E) Percent of cells with a high correlation coefficient (>0.5) in Ca2+ firing and the Euclidean distance of correlated events. Graph shows average ± SEM (n = 4 mice, gestation).
Next, we used our model to examine possible Ca2+-contraction coupling in ductal cells of pregnant GCaMP6f-TdTom;K5CreERT2 mice. At this developmental stage, contractile proteins are already up-regulated (SI Appendix, Fig. S7C), Ca2+-contraction coupling is observed in alveolar structures (Movies S4 and S5), and the visualization of ducts is not completely obscured by light scattering and/or absorptive properties of interposing structures. Although oriented deep within the tissue, ductal basal cells responded to OT with a transient increase in [Ca2+]i (Fig. 3C) and Ca2+-contraction coupling was clearly observed in live recordings (Movie S6). Although more challenging to visualize, large ducts that were positioned deep within the mammary tissue of lactating animals were captured (Fig. 3D and Movie S7), confirming these findings in the fully mature state. In mammary ducts, basal cells adopt a spindle-like morphology and are collectively oriented along the length of the duct (Fig. 1A). Our data reveal that contraction of ductal basal cells generates longitudinal motion, facilitating the continued flow of milk. We also demonstrate that differences in the type of motion generated by ductal and alveolar contractions arise from organizational heterogeneity, rather than divergent functional differentiation or signal transduction.
Mammary Epithelial Cells In Situ Exhibit Both Stochastic and Coordinated Behaviors.
Our model enables us to visualize molecular events in single cells, to observe how these events control an individual cell’s behavior, and to understand how individual behaviors produce tissue-level outcomes. In mammary tissue, basal epithelial cells primarily exhibit stochastic activity (Figs. 1 and 2 and see SI Appendix, Figs. S4 and S5). Individual oscillatory behavior, however, was observed to be temporarily entrained across large lobuloalveolar structures (Fig. 3C asterisks and see SI Appendix, Figs. S3 and S4 asterisks and Movies S2, S4, and S6), suggesting that this organ can generate both synchronized and unsynchronized motion for optimal milk ejection. To determine the degree of lobuloalveolar cooperativity in firing, we employed two agnostic approaches to analyze the functional connectivity in Ca2+ signaling events. First, we analyzed correlations in the firing pattern of individual basal cells in the postdiffusion phase and graphed the Euclidean distances between highly correlated (>0.5) cells. Highly correlated responses exhibited a short Euclidean distance (Fig. 3E). We also analyzed network topologies by connecting highly correlated cells within a single field of view. This method confirmed high clustering associated with short internodal distances in some lobular structures (small worldness) (SI Appendix, Fig. S9) (36, 37). These analyses suggest some cooperativity in firing and, by extension, contraction.
Distinct Signaling Pathways Underpin the Passage of Milk, Tears, and Sperm.
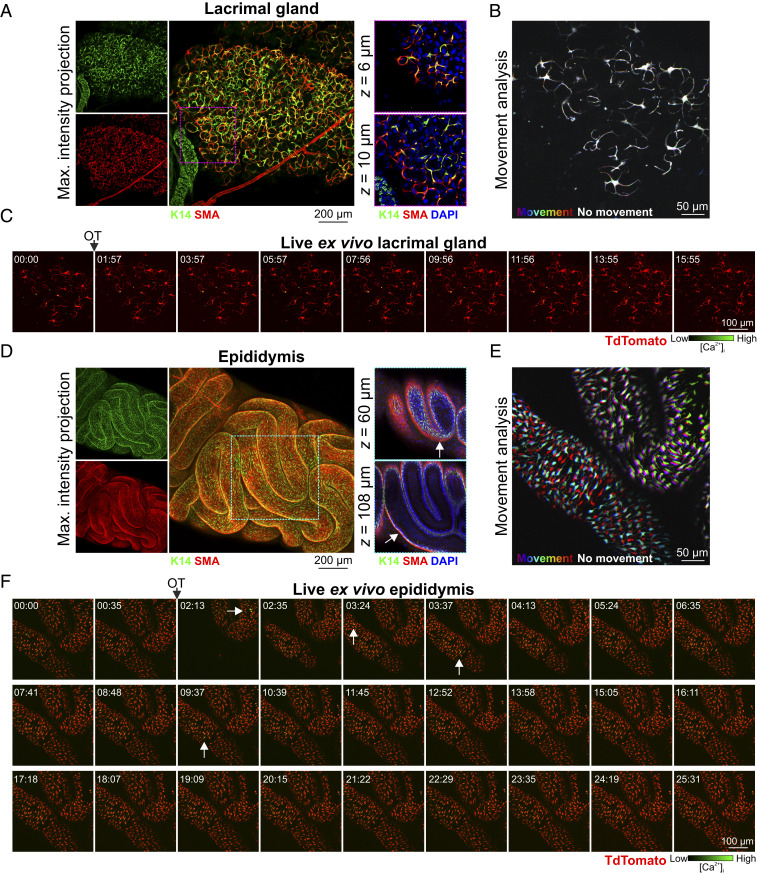
To assess potential conservation in the signaling pathways that operate in basal cells of other OT-sensitive, fluid-transporting epithelia, we assessed OT-mediated responses in the lacrimal glands and epididymides of GCaMP6f-TdTom;K5CreERT2 mice. In the lacrimal gland, basal cells have a similar morphology, arrangement, and function to mammary basal cells (38). They have previously been shown to undergo OT-dependent contractions (39), and diminished OT/OXTR signaling in these cells has been linked to dry eye disease (39). Like the mammary gland, dual expression of basal and smooth muscle markers was confirmed in lacrimal acini (Fig. 4A); however, no OT-mediated [Ca2+]i or contractile responses were detected in these cells in this study (Fig. 4 B and C and Movie S8).
Fig. 4.
OT responses in basal epithelial cells of other fluid-moving organs. (A) Maximum intensity z-projection and optical slices of lacrimal tissue. Lacrimal acinar basal cells express K14 and SMA. (B) Analysis of tissue movement created by the overlay of three images (approximately 43 s apart). Each image has been assigned a primary color. Regions that do not move during the 90-s window have R-G-B (red, green, and blue) pixels superimposed and are white. Regions where significant movement has occurred appear R, G, B or a combination of two colors. See Movie S8. (C) Three-dimensional time-lapse imaging of lacrimal tissue from GCaMP6f-TdTom;K5CreERT2 mice. Tissue was stimulated with OT (85 nM, 00:45). Image series show maximum intensity z-projection. (D) Maximum intensity z-projection and optical slices of cleared mouse caput epididymis. Basal K14 positive cells are surrounded by SMA positive cells (arrow). (E) Tissue movement analysis of three images (approximately 45 s apart) as per B. (F) Three-dimensional time-lapse imaging of epididymal tissue from GCaMP6f-TdTom;K5CreERT2 mice. Tissue was stimulated with OT (850 nM, 01:38); arrows show single-cell calcium responses. See Movie S9. n = 3 mice.
In males, a large burst of OT is released into the bloodstream at ejaculation (18, 40). This produces contractions of the male reproductive tract and, by assisting with the passage of fluid along this tract, these contractions are thought to reduce postejaculatory refractoriness and improve reproductive readiness (40, 41). Epididymal basal cells express basal cell markers; however, unlike the lacrimal and mammary glands, they do not coexpress smooth muscle markers (Fig. 4D). Instead, movement of fluid through this organ appears to rely on a layer of smooth muscle surrounding the inner tubular epithelium (Fig. 4D). To assess the transport of sperm through this organ, its OT responsiveness and its relationship to basal cell [Ca2+]i elevations, we stimulated acute epididymal tissue pieces with a large bolus dose of OT. OT stimulation triggered marked peristaltic-like movements of the epididymal tubes (Fig. 4E) and a suprabasal pattern of phosphorylation of MLC (SI Appendix, Fig. S10A). Low-frequency Ca2+ firing in basal cells was observed before and after OT stimulation (Fig. 4F arrows and see SI Appendix, Fig. S10B and Movie S9). Basal cell Ca2+-contraction signaling can therefore be selectively uncoupled in different fluid moving epithelia.
Pharmacological Inhibitors of Regulatory Proteins of Myosin Light Chain Phosphorylation Are Unable to Block Mammary Contractions.
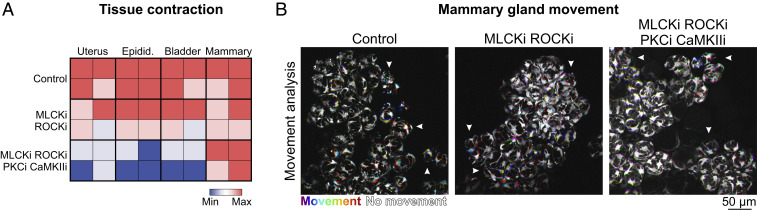
Mammary basal cells typically express smooth muscle actin (SI Appendix, Fig. S1A) and strongly up-regulate elements of the vascular smooth muscle contraction pathway during gestation and early lactation (SI Appendix, Fig. S7) (7). Our group and others have therefore hypothesized that basal cell contraction is principally controlled by Ca2+/calmodulin-dependent phosphorylation of the myosin light chain (MLC) by MLCK and subsequent dephosphorylation by myosin light chain phosphatase (MLCP) (19, 20, 42). This hypothesis is supported in the current study by a pattern of pMLC immunostaining in OT-treated tissue that is consistent with the organization of its Ca2+ firing activity (Fig. 1 C and D and see SI Appendix, Fig. S3). To explore this further, we treated uterine, bladder, epididymal, and mammary tissue pieces with pharmacological inhibitors of both MLCK and the MLCP inhibitor rho-associated protein kinase (ROCK) (SI Appendix, Fig. S11A). Inhibition of MLCK and ROCK did not significantly reduce the intensity of tissue contraction in any organ examined (Fig. 5 and Movie S10) (P > 0.05, one-way ANOVA, n = 4). This is in contrast to a previous study, which scored contraction based on basal cell morphology in mammary tissue treated prior to fixation with this ROCKi (43). When tissue was incubated with a mixture of pharmacological inhibitors against MLCK, ROCK, protein kinase C (PKC) (44), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (45), however, contraction was robustly inhibited in uterine, epididymal, and bladder preparations (53 ± 13%, 69 ± 12.5%, and 60 ± 15% reduction, respectively, P < 0.05, one-way ANOVA, n = 4), but persisted in the mammary gland (Fig. 5 A and B and Movie S10), suggesting that other pathways are responsible for mammary basal cell contractions or that they may compensate when these pathways are transiently disrupted.
Fig. 5.
Pharmacological inhibition of the contractile pathway. (A) Matrix of contractile activity in tissue pieces isolated from uterus, epididymis, bladder, and mammary gland and treated with either buffer (control), a combination of inhibitors of MLCK (ML-9) and ROCK (Y27632), or a combination of inhibitors of MLCK (ML-9), ROCK (Y27632), PKC (calphostin-C), and CaMKII (KN93). Contractions were induced with oxytocin (85 nM, uterus and mammary gland; 850 nM epididymis) or carbachol (10 μM, bladder). See Movie S10. (B) Analysis of tissue movement in mammary tissue pieces created by the overlay of three images (30 s apart). Each image has been assigned a primary color. Regions that do not move during the 60-s window have R-G-B pixels superimposed and are white. Regions where significant movement has occurred appear R, G, B, or a combination of two colors. n = 4 mice.
It is also conceivable, however, that some pharmacological inhibitors are unable to effectively and consistently bind to their intracellular targets when applied to intact, lipid-rich mammary tissue. We therefore interrogated Ca2+-contraction coupling in dissociated primary mammary basal cells in a 2D assay. Cells from pregnant GCaMP6f-TdTom;K5CreERT2 mice were isolated, plated in coculture on a nanopatterned surface (SI Appendix, Fig. S11B), and imaged within 12 h of dissection. These conditions were optimal for 1) maintaining cell health and stage-specific differentiation, and 2) achieving anisotropy in the arrangement of contractile elements for the experimental measurement of force generation along a single axis (46). Under these conditions, OT stimulation produced [Ca2+]i responses, which were coupled to contraction at the first (InsP3) phase (SI Appendix, Fig. S11C and Movie S11). Later phase Ca2+-contraction coupling, however, was not able to be assessed in this model, due to the intensity of the first contraction (even at picomolar concentrations of OT) and the relatively low strength of the newly formed surface adhesions (22). Nevertheless, as Ca2+-contraction coupling is observed at this phase, we proceeded to use this system to examine this initial event in primary cells.
Intracellular Ca2+ chelation with BAPTA completely blocked [Ca2+]i responses to OT (SI Appendix, Fig. S11D and Movie S12). Cell contractions were also attenuated, demonstrating, unequivocally, their Ca2+ dependence. To gauge the distance between the Ca2+ source (in this case InsP3 receptors) and sensor, we compared OT-mediated basal cell contractions in cells loaded with two different [Ca2+]i chelators (BAPTA-AM and EGTA-AM), with different Ca2+ binding rates but comparable binding affinities (47, 48). Both intracellular BAPTA and EGTA were able to capture Ca2+ between the channel and the sensor (SI Appendix, Fig. S11D), suggestive of “loose” Ca2+-contraction coupling in these cells that is not strictly dependent on nanodomain signaling (where EGTA is ineffective) (48). Similar to whole tissue preparations, however, treatment of cells with MLCK and ROCK inhibitors failed to block OT-mediated basal cell contraction (SI Appendix, Fig. S11D). These data are not dissimilar to previous studies, where in vitro contraction was inhibited by only 30% in basal cells isolated from mice deficient for the gene encoding smooth muscle actin (42), and support a level of functional redundancy in the mammary contraction pathway.
Coupled Oscillator-Based Synchronization in the Mammary Gland.
Ca2+-activation mechanisms in smooth muscle cells are incredibly diverse and are uniquely adapted to match the developmental stage-specific function of the biological structure on which they exert their force. Additional complexity arises when the mechanisms responsible for generating and propagating [Ca2+]i signals in “smooth muscle-like” epithelial lineages are considered (49). Here, we demonstrate in mammary basal cells that OXTR engagement produces initial release of Ca2+ from intracellular stores, sufficient to generate cell and tissue contraction. Initial [Ca2+]i responses have been shown to be sensitive to PLC inhibition in in vitro assays (22) and similar [Ca2+]i responses are observed with InsP3 infusion (22), consistent with coupling via Gq proteins to PLCβ (18). In some smooth muscle cells, [Ca2+]i signals are propagated along the length of the cell via the regenerative release of stored Ca2+ by ryanodine receptors (RYRs) (50, 51). As cytosolic Ca2+ waves were also observed in mammary basal cells (Fig. 2A), we investigated novel roles for RYRs in this tissue. Ryr1 (but not -2 or -3) was expressed in lysates that were prepared from homogenized mammary tissue during lactation (SI Appendix, Fig. S12A) and enriched in functionally mature basal cells (SI Appendix, Fig. S12B). To determine the role of RYR1 channels in these cells, we treated mammary tissue from GCaMP6f-TdTom;K5CreERT2 mice with the ryanodine receptor inhibitor dantrolene (52). Dantrolene did not inhibit the initial release of Ca2+ from intracellular stores (Fig. 6A temporal sequence 1 and Movie S13). However, to our surprise, [Ca2+]i oscillations became entrained in some regions and tissue exhibited rhythmic and sustained pulses of activity that resembled smooth muscle phase waves, with a periodicity (time between waves) of 104.2 ± 16.38 s and a velocity (speed of wave through the tissue) of 10.62 ± 2.64 μm⋅s−1 (Fig. 6A temporal sequence 2, Fig. 6B and Movie S13). A similar effect was observed with inhibiting concentrations of the plant alkaloid ryanodine (53) (Movie S14). These data, together with our observation that [Ca2+]i oscillations could be temporarily entrained under physiological conditions (Fig. 3C and see SI Appendix, Fig. S4 and Movies S2 and S6), support a model whereby mammary basal cells can alternate between unsynchronized movements and coupled oscillator-based lobuloalveolar synchronization, modulated in part by the mechanism of ER Ca2+ release.
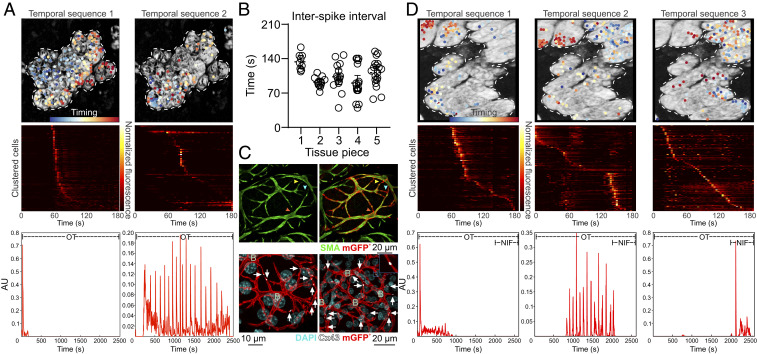
Fig. 6.
Dantrolene-induced tissue synchronization. (A) Sequential non-negative matrix factorization (seqNMF) was used for unsupervised discovery of repeated temporal sequences of activation and to cluster cells accordingly. Temporal sequence 1 corresponds to the initial InsP3 response; temporal sequence 2 corresponds to the dantrolene-dependent synchronized oscillations. Dots (Top) are cells color coded (see timing colorbar) according to the order of their activation in the sequence (Middle, each row is one cell) and overlaid on a maximum intensity z-projection of the green channel. The times at which each temporal sequence of [Ca2+]i activity is repeated for each cluster is represented by a spike at the Bottom; n = 3 mice. (B) Interval between each synchronized oscillation in ex vivo dantrolene-treated mammary tissue (mean ± 95% CI); n = 5 tissue pieces from at least 3 mice. (C) Optically cleared mammary tissue from lactating mice showing SMA immunostaining (green, Top) and cells expressing a membrane-targeted fluorescent protein (red, Top). Colored arrowheads point to sites of cell–cell contact that are revealed by the membrane fluorescent protein (Lck-GCaMP6f/mGFP, detected using an anti-GFP antibody). Immunostaining for Cx43 (white, Bottom) in cells expressing the membrane-targeted fluorescent protein (red, Bottom). White arrows show Cx43 staining at sites where basal cells are connected; B, basal cell; n = 3 mice. (D) seqNMF as in A, where temporal sequence 1 corresponds to the initial InsP3 response; temporal sequence 2 corresponds to dantrolene-dependent synchronized oscillations; and temporal sequence 3 corresponds to addition of nifedipine. After addition of nifedipine, the synchronized activity disappears and switches to a stochastic activity distributed through the tissue, as can be seen by the lack of repeated spikes in the bottom pane. See Movie S15. n = 3 mice.
A key factor of coupled oscillator-based synchronization is intercellular communication via gap junctions (50, 54). Mammary basal cells express Cx43 (55, 56) and mice with severely compromised Cx43 function have impaired milk ejection (57). However, it is often difficult to appreciate how stellate basal cells are physically coupled to their neighbors when visualized using thin tissue sections (SI Appendix, Fig. S13). Similarly, due to their size and exclusion from near plasma membrane domains, the true extent of basal cell connectivity has not yet been captured using three-dimensional imaging of conventional basal cell markers (SI Appendix, Fig. S1A). To overcome this, we developed mice that express a membrane localized fluorescent protein in basal cells and assessed Cx43 localization in optically cleared tissue. Using this approach, basal cell boundaries were readily identified, enabling us to visualize how thin processes of adjacent cells are physically connected (Fig. 6 C, Top). Cx43 was enriched at sites of homotypic cell contact (Fig. 6 C, Bottom arrows). These data confirm that the cytoplasms of adjacent basal cells are linked, enabling individual cells to coordinate the activity of the larger system.
In other tissue types that exhibit rhythmic contractions, e.g., vascular, lymphatic, and airway smooth muscle, periodic release of Ca2+ from the ER produces membrane depolarization and activation of L-type Ca2+ channels (50). Current flow through gap junctions enables depolarization to spread rapidly into neighboring cells, synchronizing large numbers of cells potentially over millimeter distances (50, 54). To determine whether L-type calcium channels are involved in synchronization events in the mammary gland, we treated rhythmically contracting tissue with the L-type Ca2+ channel blocker nifedipine. Nifedipine rapidly and consistently resulted in the reversion to stochastic activity (Fig. 6D [absence of repeated sequences of activation for temporal sequence 3] and Movie S15). Collectively, these data reveal that mammary basal cells are physically and electrically coupled, enabling Ca2+ to control both the behavior of individual cells as well as the system as a whole.
Discussion
Real-time, in situ activity monitoring provides important insights into how individual cells behave in multidimensional and multicellular environments (12–15). This approach was used to describe and quantify the mechanism by which milk is transported through the hollow mammary epithelium, making it available on demand and with minimal delay to the nursing neonate (2, 18). Our data support a number of conclusions that could not have been obtained using conventional methods.
We revealed that transient [Ca2+]i elevations precede and are required for basal cell contractions in the functionally mature gland. We extended this finding to demonstrate how Ca2+-contraction coupling in a single basal cell can physically warp the layer of alveolar luminal cells that it encircles. Structure, function, and expression were examined in the adjoining ductal epithelium, previously relegated to a role akin to a biological drinking straw. Instead, our analyses revealed active participation of the ductal epithelium in the process of milk ejection. Differences in the type of motion generated by basal cell contractions in ducts and alveoli were ascribed to heterogeneity in cellular organization, rather than expression or function of contractile elements.
We explored components of the contractile network downstream of Ca2+ activation in mammary basal cells. A pattern of pMLC positivity was observed in mammary cell ensembles, which mirrored the Ca2+ activity of the tissue. Pharmacological inhibition of the Ca2+-dependent MLCK and the Ca2+-sensitizer ROCK, however, failed to block mammary contractions in our study. While MLCK is widely considered to be the primary Ca2+-dependent regulator of MLC phosphorylation in smooth muscle, this model is based on reductionist principles, does not fit all smooth muscle cell types, and fails to acknowledge the growing complexity in regulatory kinases known or hypothesized to govern smooth muscle contraction in vivo (58–60). Indeed, embryonic blood vessels from MLCK knockout mice remain responsive to cytosolic Ca2+ elevations (61). Our data reveal that, similar to aortic smooth muscle cells, smooth muscle-like epithelial cells in the mammary gland also display considerable complexity and diversity in their biomechanical behavior. Complexity in the pathways downstream of Ca2+ activation may extend beyond Ca2+-contraction coupling to Ca2+-transcription coupling (62), an aspect of signaling that has not been considered here but which may be relevant for the interpretation of genetic knockout models (19).
In addition to the diversity in signal transduction downstream of Ca2+ activation in mammary basal cells, our study and others (19, 22, 63) have demonstrated that a number of Ca2+ channels—with distinct activation mechanisms and cellular localizations—participate in its encoding. These include channels that regulate Ca2+ release from intracellular stores, influx from the extracellular environment, and movement between the cytosol of adjacent cells. In this sense, [Ca2+]i acts as a central node in a type of bow-tie motif in basal cells (64), whereby multiplicity in its encoding and decoding enable this evolutionarily essential organ to engage local and global motions to ensure adequate nutrition for the dependent offspring, while on the other hand remaining vulnerable at this crucial point of convergence.
The dynamic nature of the oscillatory Ca2+ signal enables basal cells to rapidly cycle between contracted and relaxed states. We posit that the spatiotemporal properties of this signal are important insomuch as its oscillation intensity and interval match the activation threshold and decay rate of the downstream effector to permit efficient switching between cycles of contraction and relaxation. Coupling of the Ca2+ sensor within nanometer distance to the channel pore, however, appears unlikely based on the following observations: 1) both ER Ca2+ release and plasmalemmal Ca2+ influx were sufficient for in situ basal cells to develop and bear tension; and 2) BAPTA-AM and EGTA-AM were equally effective in inhibiting in vitro contractions, despite EGTA’s slower binding kinetics. Although not essential for Ca2+-contraction coupling, highly spatially regulated [Ca2+]i signals may be an important factor for Ca2+-transcription coupling for the long-term maintenance of the contractile phenotype (62) or Ca2+ wave generation at the tissue level.
Finally, our data, together with published work, suggest that mammary basal cells are able to shift between store- (19) and voltage-dependent modes of operation, a phenomenon that appears to be moderated, at least in part, by the mechanism of ER Ca2+ release. It is currently unclear how basal cells coordinate the activity of these two, often reciprocally regulated (65, 66), influx pathways under physiological conditions. However, our observation that pharmacological inhibition of RYR1 promoted dihydropyridine-sensitive signal synchronization, corresponds with accounts of RYR activity in bona fide smooth muscle cells (62). Here, RYR-mediated Ca2+ sparks can activate nearby BKCa channels, producing spontaneous transient outward currents (STOCs), membrane hyperpolarization, and reduced Cav1.2 activity (50, 62). Optical monitoring of voltage in three dimensions using genetically encoded voltage indicators (GEVIs) (67) and examination of population dynamics in Cacna1c-, Ryr1-, and Kcnma1-conditional knockout mice remain aims for the future. It is also unclear at this time whether spatial synchronicity can be initiated by any oscillating basal cell (alveolar or ductal) within the mammary epithelium or whether basal cells lock into the frequency of a putative population of epithelial (68) or interstitial (69) mammary “pacemaker” cells. This question may be addressed by future studies using light-sheet fluorescence microscopy and quantitative image analysis to create a spatial footprint of the frequency dynamics of individual oscillators and phase advanced cells.
In summary, by imaging activity in the mammary gland across scales, we were able to visualize and describe in unprecedented detail how the repetitive and collective effort of thousands of mammary basal cells facilitate the transport of a thick biological emulsion through a narrow passage in a manner that is both consistent and persistent. Moreover, the system presented here represents a physiologically relevant model for studying the collective nature of mammalian biological processes.
Materials and Methods
Mice.
Animal experimentation was carried out in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes and the Queensland Animal Care and Protection Act (2001), with local animal ethics committee approval. Strain, genotyping, and reporter induction methods are detailed in SI Appendix.
Human Subjects.
Healthy tissue biopsies from consented lactating women were obtained from the Susan G. Komen Tissue Bank at the IU Simon Cancer Center; see SI Appendix.
Ex Vivo Tissue Imaging.
Mammary glands and uteri were harvested from lactating wild-type, GCaMP6f;K5CreERT2, or GCaMP6f-TdTom;K5CreERT2 mice, diced into 3- to 4-mm3 pieces and loaded with CellTracker (1.5 μM) in complete media for at least 20 min at 37 °C and 5% CO2 (19). Under these conditions CellTracker preferentially labels luminal cells (SI Appendix, Fig. S1A). Images were acquired using an Olympus FV3000 laser scanning microscope; see SI Appendix for details and intravital imaging conditions.
Statistical Analysis.
Statistical analysis was performed in GraphPad Prism (v7.03). Details of statistical tests are outlined in the figure legends.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council (1141008, 1138214), The University of Queensland, the Mater Foundation (Equity Trustees/AE Hingeley Trust), and the National Stem Cell Foundation of Australia. We thank Dr. Jerome Boulanger for the 3D denoising algorithm and Mr. Karsten Bach for assistance with accessing and analyzing RNA-sequencing data. Samples from the Komen Tissue Bank at the IU Simon Cancer Center were used in this study; we thank contributors, donors, and their families.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2016905117/-/DCSupplemental.
Data Availability.
All data are available in the article and SI Appendix. Scripts are available on GitHub, https://github.com/NickCondon/FMD_Cell_Contraction_Script (70).
References
- 1.Victora C. G.et al.; Lancet Breastfeeding Series Group , Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Macias H., Hinck L., Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 1, 533–557 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowin P., Wysolmerski J., Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb. Perspect. Biol. 2, a003251 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lloyd-Lewis B., Harris O. B., Watson C. J., Davis F. M., Mammary stem cells: Premise, properties and perspectives. Trends Cell Biol. 27, 556–567 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Sleeman K. E.et al., Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J. Cell Biol. 176, 19–26 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Keymeulen A.et al., Lineage-restricted mammary stem cells sustain the development, homeostasis, and regeneration of the estrogen receptor positive lineage. Cell Rep. 20, 1525–1532 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bach K.et al., Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun. 8, 2128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Keymeulen A.et al., Distinct stem cells contribute to mammary gland development and maintenance. Nature 479, 189–193 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Lloyd-Lewis B., Davis F. M., Harris O. B., Hitchcock J. R., Watson C. J., Neutral lineage tracing of proliferative embryonic and adult mammary stem/progenitor cells. Development 145, 164079 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis F. M.et al., Single-cell lineage tracing in the mammary gland reveals stochastic clonal dispersion of stem/progenitor cell progeny. Nat. Commun. 7, 13053 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gjorevski N., Nelson C. M., Integrated morphodynamic signalling of the mammary gland. Nat. Rev. Mol. Cell Biol. 12, 581–593 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Toyota M.et al., Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Heap L. A. L., Vanwalleghem G., Thompson A. W., Favre-Bulle I. A., Scott E. K., Luminance changes drive directional startle through a thalamic pathway. Neuron 99, 293–301.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Dunn T. W.et al., Neural circuits underlying visually evoked escapes in larval zebrafish. Neuron 89, 613–628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cichon J., Gan W. B., Branch-specific dendritic Ca(2+) spikes cause persistent synaptic plasticity. Nature 520, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T.-W.et al., Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q.et al., Imaging neural activity using Thy1-GCaMP transgenic mice. Neuron 76, 297–308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimpl G., Fahrenholz F., The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 81, 629–683 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Davis F. M.et al., Essential role of Orai1 store-operated calcium channels in lactation. Proc. Natl. Acad. Sci. U.S.A. 112, 5827–5832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore D. M., Vogl A. W., Baimbridge K., Emerman J. T., Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J. Cell Sci. 88, 563–569 (1987). [DOI] [PubMed] [Google Scholar]
- 21.Olins G. M., Bremel R. D., Oxytocin-stimulated myosin phosphorylation in mammary myoepithelial cells: Roles of calcium ions and cyclic nucleotides. Endocrinology 114, 1617–1626 (1984). [DOI] [PubMed] [Google Scholar]
- 22.Nakano H., Furuya K., Yamagishi S., Synergistic effects of ATP on oxytocin-induced intracellular Ca2+ response in mouse mammary myoepithelial cells. Pflugers Arch. 442, 57–63 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Lee H. J., Caldwell H. K., Macbeth A. H., Tolu S. G., Young W. S. 3rd, A conditional knockout mouse line of the oxytocin receptor. Endocrinology 149, 3256–3263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimori K.et al., Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc. Natl. Acad. Sci. U.S.A. 93, 11699–11704 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takayanagi Y.et al., Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 16096–16101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dana H., et al. , High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan R.et al., New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron 92, 1181–1195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Lewis B.et al., Imaging the mammary gland and mammary tumours in 3D: Optical tissue clearing and immunofluorescence methods. Breast Cancer Res. 18, 127 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akemann W., Mutoh H., Perron A., Rossier J., Knöpfel T., Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat. Methods 7, 643–649 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Dupont G., Combettes L., Bird G. S., Putney J. W., Calcium oscillations. Cold Spring Harb. Perspect. Biol. 3, a004226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masedunskas A., Chen Y., Stussman R., Weigert R., Mather I. H., Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: Lipid droplet release is intermittently stimulated by oxytocin. Mol. Biol. Cell 28, 935–946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avants B. B.et al., A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avants B. B., Epstein C. L., Grossman M., Gee J. C., Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong T. X.et al., T-cell calcium dynamics visualized in a ratiometric tdTomato-GCaMP6f transgenic reporter mouse. eLife 6, e32417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moumen M.et al., The mammary myoepithelial cell. Int. J. Dev. Biol. 55, 763–771 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Stožer A.et al., Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLOS Comput. Biol. 9, e1002923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watts D. J., Strogatz S. H., Collective dynamics of “small-world” networks. Nature 393, 440–442 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Farmer D. T.et al., Defining epithelial cell dynamics and lineage relationships in the developing lacrimal gland. Development 144, 2517–2528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawley D.et al., Myoepithelial cell-driven acini contraction in response to oxytocin receptor stimulation is impaired in lacrimal glands of Sjögren’s syndrome animal models. Sci. Rep. 8, 9919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thackare H., Nicholson H. D., Whittington K., Oxytocin–Its role in male reproduction and new potential therapeutic uses. Hum. Reprod. Update 12, 437–448 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Arrighi S., Are the basal cells of the mammalian epididymis still an enigma? Reprod. Fertil. Dev. 26, 1061–1071 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Haaksma C. J., Schwartz R. J., Tomasek J. J., Myoepithelial cell contraction and milk ejection are impaired in mammary glands of mice lacking smooth muscle alpha-actin. Biol. Reprod. 85, 13–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raymond K.et al., Control of mammary myoepithelial cell contractile function by α3β1 integrin signalling. EMBO J. 30, 1896–1906 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo I. Y., Ehrlich B. E., Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 7, a006023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rokolya A., Singer H. A., Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 278, C537–C545 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Chaterji S.et al., Synergistic effects of matrix nanotopography and stiffness on vascular smooth muscle cell function. Tissue Eng. Part A 20, 2115–2126 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vyleta N. P., Jonas P., Loose coupling between Ca2+ channels and release sensors at a plastic hippocampal synapse. Science 343, 665–670 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Eggermann E., Bucurenciu I., Goswami S. P., Jonas P., Nanodomain coupling between Ca2+ channels and sensors of exocytosis at fast mammalian synapses. Nat. Rev. Neurosci. 13, 7–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson K. C., Contractile tissues in the mammary gland, with special reference to myoepithelium in the goat. 1949. J. Mammary Gland Biol. Neoplasia 14, 223–242 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Berridge M. J., Smooth muscle cell calcium activation mechanisms. J. Physiol. 586, 5047–5061 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collier M. L., Ji G., Wang Y., Kotlikoff M. I., Calcium-induced calcium release in smooth muscle: Loose coupling between the action potential and calcium release. J. Gen. Physiol. 115, 653–662 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi R. H., Koenig X., Launikonis B. S., Dantrolene requires Mg2+ to arrest malignant hyperthermia. Proc. Natl. Acad. Sci. U.S.A. 114, 4811–4815 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meissner G., Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 261, 6300–6306 (1986). [PubMed] [Google Scholar]
- 54.Imtiaz M. S., von der Weid P. Y., van Helden D. F., Synchronization of Ca2+ oscillations: A coupled oscillator-based mechanism in smooth muscle. FEBS J. 277, 278–285 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Mroue R., Inman J., Mott J., Budunova I., Bissell M. J., Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland. Dev. Biol. 399, 15–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talhouk R. S.et al., Developmental expression patterns and regulation of connexins in the mouse mammary gland: Expression of connexin30 in lactogenesis. Cell Tissue Res. 319, 49–59 (2005). [DOI] [PubMed] [Google Scholar]
- 57.Stewart M. K. G.et al., The severity of mammary gland developmental defects is linked to the overall functional status of Cx43 as revealed by genetically modified mice. Biochem. J. 449, 401–413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratz P. H., Inhibitor κB kinase: Another node in the cell signaling network regulating smooth muscle contraction. Circ. Res. 113, 484–486 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Ying Z.et al., Inhibitor κB kinase 2 is a myosin light chain kinase in vascular smooth muscle. Circ. Res. 113, 562–570 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artamonov M. V.et al., RSK2 contributes to myogenic vasoconstriction of resistance arteries by activating smooth muscle myosin and the Na+/H+ exchanger. Sci. Signal 11, eaar3924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somlyo A. V.et al., Myosin light chain kinase knockout. J. Muscle Res. Cell Motil. 25, 241–242 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Hill-Eubanks D. C., Werner M. E., Heppner T. J., Nelson M. T., Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 3, a004549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakano H., Furuya K., Furuya S., Yamagishi S., Involvement of P2-purinergic receptors in intracellular Ca2+ responses and the contraction of mammary myoepithelial cells. Pflugers Arch. 435, 1–8 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Brodskiy P. A., Zartman J. J., Calcium as a signal integrator in developing epithelial tissues. Phys. Biol. 15, 051001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y.et al., The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park C. Y., Shcheglovitov A., Dolmetsch R., The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 330, 101–105 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Villette V.et al., Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 179, 1590–1608.e23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Blasio B. F., Iversen J. G., Røttingen J. A., Intercellular calcium signalling in cultured renal epithelia: A theoretical study of synchronization mode and pacemaker activity. Eur. Biophys. J. 33, 657–670 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Gherghiceanu M., Popescu L. M., Interstitial Cajal-like cells (ICLC) in human resting mammary gland stroma. Transmission electron microscope (TEM) identification. J. Cell. Mol. Med. 9, 893–910 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Condon N. D., FMD cell contraction script. GitHub. https://github.com/NickCondon/FMD_Cell_Contraction_Script. Deposited 4 June 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the article and SI Appendix. Scripts are available on GitHub, https://github.com/NickCondon/FMD_Cell_Contraction_Script (70).