Abstract
Purpose
Steroids are known to inhibit osteogenic differentiation and subsequent bone formation in bone mesenchymal stem cells (BMSCs). However, little is known about the role of BMSC exosomes (Exos) and tRNA-derived small RNAs (tsRNAs) in steroid-induced osteonecrosis of the femoral head (SONFH). The objective of this study was to characterize the tsRNA expression profiles of plasma Exos collected from SONFH patients and healthy individuals using small RNA sequencing and further explore the effect of BMSC Exos carrying specific tsRNAs on osteogenic differentiation.
Materials and Methods
Based on insights from small RNA sequencing, five differentially expressed (DE) tsRNAs were selected for quantitative real-time polymerase chain reaction (qRT-PCR). The regulatory networks associated with interactions of the tsRNAs-mRNA-pathways were reconstructed. The osteogenesis and adipogenesis in BMSCs were detected via ALP and oil red O staining methods, respectively.
Results
A total of 345 DE small RNAs were screened, including 223 DE tsRNAs. The DE tsRNAs were enriched in Wnt signaling pathway and osteogenic differentiation. We identified five DE tsRNAs, among which tsRNA-10277 was significantly downregulated in plasma Exos of SONFH patients compared to that in healthy individuals. Dexamethasone-induced BMSCs were associated with an increased fraction of lipid droplets and decreased osteogenic differentiation, whereas BMSC Exos restored the osteogenic differentiation of that. After treatment of tsRNA-10277-loaded BMSC Exos, the lipid droplets and osteogenic differentiation ability were found to be decreased and enhanced in dexamethasone-induced BMSCs, respectively.
Conclusion
An altered tsRNA profile might be involved in the pathophysiology of SONFH. tsRNA-10277-loaded BMSC Exos enhanced osteogenic differentiation ability of dexamethasone-induced BMSCs. Our results provide novel insights into the osteogenic effect of BMSC Exos carrying specific tsRNAs on SONFH.
Keywords: steroid-induced femoral head necrosis, small RNA sequencing, tsRNA-10277, BMSC exosomes, osteogenic differentiation
Introduction
Osteonecrosis of the femoral head (ONFH) is a common and incurable orthopedic disorder that affects young and middle-aged individuals and is highly prevalent worldwide.1 The femoral heads of 80% of these patients could collapse within 1–3 years of diagnosis owing to the absence of effective treatment, and it is difficult to reverse the course of this disease.2,3 Steroid-induced osteonecrosis of the femoral head (SONFH) occurs following high doses or long-term use of steroid hormones and represents a serious complication associated with steroid use.4–6 The use of steroid hormones alone accounts for about 24.1% of all ONFH cases.7,8 However, the pathogenesis of SONFH is poorly understood, and the occurrence of fat embolism, intravascular coagulation, and retrograde embolization of bone marrow fat are believed to explain the occurrence and prognosis of this disease.9 Several studies have shown the high early failure rates after surgical treatment to arise from the poor prognosis of SONFH patients.10 Therefore, the key molecular mechanisms underlying SONFH need to be urgently explored.
Exosomes (Exos) are a class of bilayer membrane-bound nanovesicles released from different cells, that are 30–100 nm in diameter.11 Functionally, exosomes act as carriers of functional proteins, mRNAs, small RNAs, and lipids, and deliver signals to recipient cells mediated by their cargo.12 The content of exosomes may represent the condition of parental cells in pathology and physiology, including different stages of the disease or different diseases. These properties of exosomes make these suitable as a platform for disease diagnosis, prognosis, and treatment.13 Several studies have recently reported that exosomes released by mesenchymal stem cells (MSCs), either from bone marrow or adipose tissue, could promote osteoblast differentiation.14 For example, exosomes released by bone mesenchymal stem cells (BMSCs) have been shown to be involved in the therapeutic action of steroid-induced femoral head necrosis.15
The tsRNAs are generated through endonucleolytic cleavage of tRNAs. The widespread and conserved expression of these tsRNAs in several biological processes has attracted tremendous attention in the recent years.16 A massive body of evidences reveal that tsRNAs are involved in translational repression and play a regulatory role in diverse physiological and pathological phenomena.17 These tsRNAs also influence various functions of somatic cells such as cell proliferation, cancer progression, and the activity of endogenous retroelements.18,19 Moreover, recent findings have suggested that the striking differences in tsRNA expression patterns could be associated with the differentiation status of MSCs.20 However, tsRNA profiles in plasma exosomes of SONFH patients have not been characterized, and the role of tsRNAs in SONFH pathophysiology remains unclear.
In this backdrop, the present study was designed with the aim to identify the tsRNA expression profiles of plasma exosomes collected from SONFH patients (and healthy subjects, as a control) using small RNA sequencing. Additionally, we have established a cellular model of SONFH and utilized this to explore the effect of tsRNA-10277-loaded BMSC exosomes on osteogenic differentiation.
Materials and Methods
Study Participants
A total of 10 participants (five SONFH patients and five healthy subjects) were recruited from the First Affiliated Hospital of Fujian Medical University. The SONFH patients involved in this study exhibited the common clinical features of joint dysfunction, lower limb muscle atrophy, claudication, and hip pain. In addition, they had been subjected to either high-dose steroid impulsion treatments or long-term steroid intake (more than 16 mg/day for more than 1 week) before the appearance of these symptoms.21 The exclusion conditions included any significant medical history of severe chronic diseases, traumatic SONFH, and other hip diseases; and outside the diagnostic criteria for SONFH, intake of more than 400 mL alcohol per week.21 Healthy control subjects had no clinical manifestations of hip diseases, known severe chronic diseases, and no medical history of thromboembolic diseases. We collected the relevant clinical information from the medical records of the participants.
Sample Collection and RNA Isolation from Plasma Exosomes
Plasma samples of 3 healthy subjects and 3 SONFH patients were obtained from the First Affiliated Hospital of Fujian Medical University. The procedure for sample collection was approved by the local Ethics Committee and written informed consent were obtained from all the participants. The exosomes were precipitated from the collected plasma samples using the ExoQuick Plasma Prep and Exosome Precipitation Kit (Cat# EXOQ5A-1, Systembio, USA). The isolated exosomes were resuspended in PBS and used immediately or stored at –80°C till further use.
Identification of Plasma Exosomes
Exosomes were fixed using a 2% paraformaldehyde solution, processed into ultrathin section. Then, the exosome sections were examined directly using a transmission electron microscope (Hitachi H-7500 TEM, Tokyo, Japan), and the data were recorded in an AMT 2k charge-coupled-device camera. A nanoparticle tracking analysis (NTA) system was used to construct the three-dimensional map of particle size, solid shape, and relative intensity of the exosomes.
RNA Extraction, Small RNA Sequencing Library Preparation, and Sequencing
Total RNA from plasma exosomes was extracted using the TRIzol Plus RNA Purification Kit (Cat# 12183555, Thermo Fisher Scientific, Waltham, MA, USA). Then, the purified RNA was sent to Yingbiotech (Shanghai, China) for the construction of small RNA libraries. Briefly, the RNA was ligated with adaptors, and complementary DNA strands were created during PCR amplification. A small RNA fragments with length of approximately 15–40 nt were used for quality control. We further quantified and validated the purified libraries. Subsequently, RNA sequencing was performed on a HiSeq 2500 sequencing system (Illumina, San Diego, California, USA).
Bioinformatics Analysis of Small RNA Sequencing Data
The raw sequences were filtered to exclude short (<15 nt) and low-quality reads. Then, all the clean small RNA reads were matched to the miRBase database (http://www.mirbase.org/), PIWI-interacting RNA (piRNA) database, NCBI, genomic tRNA database (http://gtrnadb.ucsc.edu/), tRFdb (http://genome.bioch.virginia.edu/trfdb/), and MINTBase in turn to identify known miRNAs and tRFs.
Following the target prediction of differentially expressed (DE) tRFs, the GO and KEGG databases were used to classify the functions and pathways of all DE tRFs. Then, an interaction network of the candidate tRFs/mRNA/pathway was constructed using Cytoscape 2.8.3 for underlying mechannism analysis.
Verification of tsRNAs Expression by Real-Time Quantitative PCR (RT-qPCR)
Total RNA extracted from plasma exosomes of SONFH patients and healthy subjects were used to synthesize the complementary DNA strands using the RevertAid First Strand cDNA Synthesis Kit (Cat#K1622, Thermofisher, Waltham, MA, USA), and RT-qPCR was performed with the SYBR Premix Ex Taq (Takara Bio, China) using a StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). U6 was chosen as the internal control for tsRNAs quantification in plasma exosomes. The relative expression levels were calculated using the 2-∆∆Ct method. The primers used for RT and RT-qPCR are shown in Table 1.
Table 1.
The Primers and Sequence
| Name | Primer Sequence (5ʹ-3ʹ) |
|---|---|
| RT hsa-miR-150-5p | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACCACTGGT |
| RT hsa-miR-452-5p | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTCAGTTT |
| U6-F | CGATACAGAGAAGATTAGCATGGC |
| U6-R | AACGCTTCACGAATTTGCGT |
| hsa-miR-150-5pF | CGCAGTCTCCCAACCCTTG |
| hsa-miR-452-5pF | CGCAGAACTGTTTGCAGAGG |
| all-R | AGTGCGTGTCGTGGAGTCG |
| RT tsRNA-04590 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTGGCTCC |
| RT tsRNA-10277 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCAGAGT |
| RT tsRNA-23731 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCACGCG |
| RT tsRNA-19733 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGCAGACG |
| RT tsRNA-10522 | GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAAAGCGA |
| tsRNA-04590F | CCTGTCACGCGGGAGACC |
| tsRNA-10277F | GGCCGTGATCGTATAGTGGTTAG |
| tsRNA-23731F | GGGGGTATAGCTCAGTGGTAGAG |
| tsRNA-19733F | CTGGGTTCCATAGTGTAGTGGTTA |
| tsRNA-10522F | GGCTCGTTGGTCTAGGGGTAT |
| all-R | AGTGCGTGTCGTGGAGTCG |
Isolation of BMSC Exosomes
Exosomes from the cell supernatants were isolated as described above. Briefly, rat BMSCs (Procell, Wuhan, China) were cultured in DMEM-F12 medium containing 10% fetal bovine serum (Gibco, 10099–14) and 1% penicillin–streptomycin (Gibco), and incubated at 37°C and 5% CO2 until 80% confluence was reached. The supernatant was collected after 48 h for isolation of exosomes. The isolated exosomes were resuspended in PBS.
Characterization of BMSCs by Immunofluorescence
BMSCs were fixed during the logarithmic phase by treating with 4% paraformaldehyde for 10 min. Then, the cells were permeabilized using 0.1% triton X-100 and blocked by PBS containing 2% bovine serum albumin and 0.1% Tween 20. Subsequently, the BMSCs were incubated with primary antibodies against rat CD44 (BD Biosciences, San Jose, CA, USA; 1:50) and CD45 overnight at 4°C. After removing the primary antibodies, the cells were incubated with Cy3-conjugated secondary antibodies for 1 h at room temperature. Then, the cells were washed thrice using 1% TBST, stained with DAPI (Beyotime Institute of Biotechnology, Shanghai, China), and visualized under a fluorescent microscope (TE2000, Nikon, Japan).
Steroid Induction and Oil Red O Staining
The rat BMSCs were divided into three groups: control group (healthy BMSCs), SONFH model group (BMSCs treated with 10−7 mol/L dexamethasone) and Exos group (SONFH BMSCs treated with healthy BMSCs exosomes). After induction for 18 days, cells were washed once with PBS, fixed by treatment with 4% paraformaldehyde for 30 min at room temperature, and then stained with oil red O solution (Jiancheng Biotechnology, Nanjing, China) for 60 min at room temperature. A microscope was used to observe the stained cells.
Exosome Treatment, Steroid Induction, and ALP Staining
The rat BMSCs were divided into 3 groups, namely control group (healthy BMSCs), SONFH model group, and Exos group, and each group repeated for three times. BMSCs were seeded into 24-well plates at a density 1×104 cells/cm2 until 70% confluence was reached. Then, the old complete medium was removed, and fresh medium containing 0.1 μmol/L dexamethasone (Cat# RASMX-90021, Oricell, Cyagen Biosciences) was added to the plates. The BMSC exosomes group was also treated with 5 µg of healthy BMSC exosomes. All groups were cultured for 3 days prior to ALP staining. The cells were washed thrice with PBS and fixed by treatment with 4% Paraformaldehyde Fix Solution (Cat# E672002, Sangon Biotech, Shanghai, China) for 1 min. Then, the cells were incubated with ALP for 20 min at 25°C in the dark and observed under a light microscope.
Cell Transfection and Treatment of tsRNA-10277-Loaded BMSC Exosomes
The tsRNA-10277 mimics (5ʹ-GGCCGTGATCGTATAGTGGTTAGTACTCTGC-3ʹ and 5ʹ-GCAGAGTACTAACCACTATACGATCACGGCC-3ʹ) and negative control mimics (NC; 5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ and 5ʹ-ACGUGACACGUUCGGAGAATT-3ʹ) were purchased from Shanghai GenePharma Co., Ltd. After transfecting the tsRNA-10277 mimics or NC into BMSCs using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc. Waltham, MA, USA) and a 48h incubation, the BMSCs supernatant were collected for exosome isolation. The levels of tsRNA-10277 expression in BMSC exosomes were detected by RT-qPCR. The effect of exosomes derived from BMSCs by transfection of the tsRNA-10277 mimics or NC mimics on adipogenesis and osteogenic ability was assessed by staining with oil red O and ALP staining, respectively.
Statistical Analysis
Data from independent experiments performed in triplicates are shown as mean ± SD. The p-values were calculated using the Graph Pad Prism 8 software. Student’s t-test was used for comparisons for two groups. A value of p<0.05 was considered statistically significant.
Results
Participant Demographics
As shown in Table 2, a total of 10 participants were enrolled in the study, composed of five SONFH patients and five healthy subjects. The SONFH group had significant differences in age compared to the control group; however, no difference in body mass index was noted. Moreover, the SONFH patients were mainly in clinical stage IV.
Table 2.
Baseline Data of Participants
| Variables | Healthy Subjects | SONFH Patients | p-value |
|---|---|---|---|
| (n=5) | (n=5) | ||
| Age (years) | 25.8±1.60 | 50.2±3.82 | <0.0001a |
| Gender (male,%) | 5 (100) | 3 (60.0) | |
| BMI (kg/m2) | 23.6±1.54 | 23.1±3.15 | 0.7829 a |
| Clinical stages | |||
| Stage I | NA | NA | |
| Stage II | NA | NA | |
| Stage III | NA | 1 (20%) | |
| Stage IV | NA | 4 (80%) | |
| Hip lesions | NA | ||
| Unilateral | NA | 2 (40%) | |
| Bilateral | NA | 3 (60%) |
Notes: Continuous variables are shown as “mean ± SD”; categorical variables are exhibited as “number (%)”. aIndependent samples t test.
Abbreviations: BMI, body mass index; SONFH, steroid-induced osteonecrosis of the femoral head.
Characterization of Isolated Plasma Exosomes by TEM
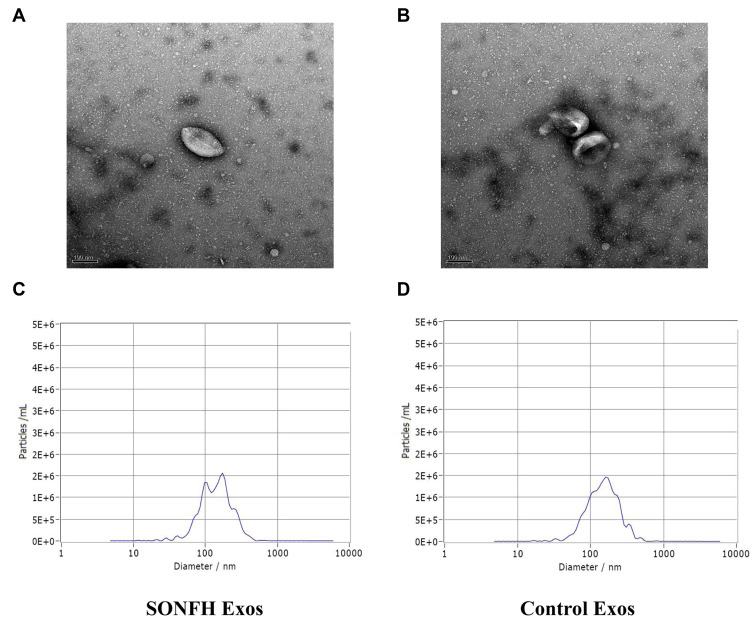
The plasma exosomes collected from SONFH patients and healthy subjects were distinguished by TEM and NTA analysis. These experiments revealed that the obtained particles were covered with an intact membranous structure and were 100–150 nm in diameter (Figure 1A–D). Collectively, this data suggests that the collected particles were plasma exosomes.
Figure 1.
Characterization of plasma exosomes. (A and B) Transmission electron microscopy (TEM) image showed the morphology of plasma exosomes in steroid-induced osteonecrosis of the femoral head (SONFH) patients and healthy control subjects. (C and D) The nanoparticle tracking analysis (NTA) was used to estimate particle size distribution of plasma exosomes in SONFH patients and healthy control subjects.
Identification of Small RNAs and Differentially Expressed tsRNAs in SONFH Patients and Healthy Subjects
We preformed small RNA sequencing to obtain a profile of small RNAs in the SONFH and healthy groups. After quality control of the 6 sequencing libraries used, each library received an average of 23.21 million raw reads, out of which about 19.85 (85.10%) clean reads with a length >15 nt were retained (Table 3). There were several small RNA biotypes in the SONFH and heathy groups, including miRNAs, piRNAs, and tsRNAs (Figure 2A and B). Among the total clean reads, miRNAs were the most abundant small RNAs in exosomes, accounting for about 48%; whereas only 4.09% and 3.37% reads were mapped to piRNAs and tsRNAs, respectively (Table 4). We further performed a comparative analysis of DE small RNAs between the SONFH and healthy groups. |Log2Fold Change|>1 and p<0.05 were set as the filter conditions. A total of 345 DE small RNAs were screened out, including 112 DE miRNAs, 223 DE tsRNAs, and 10 DE piRNAs. Among the DE tsRNAs, 137 were upregulated and 86 downregulated in the SONFH group compared to the control group (Figure 2C). The heat map generated by hierarchical cluster analysis showed similar spectral clustering and samples in each group (Figure 2D).
Table 3.
Summary of Cleaning Data Produced by Small RNA Sequencing
| Sample | Total Reads | Clean Reads (%) | Total Base | Clean Base (%) | GC (%) |
|---|---|---|---|---|---|
| SONFH-1 | 25,862,144 | 21,191,046 (81.94%) | 3,879,279,186 | 554,964,161 (14.31%) | 51 |
| SONFH-2 | 17,567,864 | 14,560,008 (82.88%) | 2,635,148,982 | 363,651,380 (13.8%) | 49 |
| SONFH-3 | 28,765,255 | 27,183,453 (94.5%) | 4,314,742,480 | 709,831,082 (16.45%) | 50 |
| Control-1 | 20,529,070 | 18,072,999 (88.04%) | 3,079,324,088 | 457,634,856 (14.86%) | 49 |
| Control-2 | 25,425,246 | 21,279,324 (83.69%) | 3,813,745,364 | 536,466,416 (14.07%) | 51 |
| Control-3 | 21,126,154 | 16,812,559 (79.58%) | 3,168,887,826 | 428,744,417 (13.53%) | 52 |
| Average | 23,212,622 | 19,849,898 (85.10%) | 3,481,854,654 | 508,548,718 (14.61%) | 50 |
Figure 2.
Differential expression (DE) analysis of tRNA-derived small RNAs (tsRNAs) in SONFH patients. (A and B) Types of small non-coding RNAs identified in plasma exosomes from SONFH patients and healthy subjects. (C) Volcano plot showing the DE tsRNA between SONFH patients and healthy subjects. Red points denote the upregulated tsRNAs and blue points indicate the downregulated ones in SONFH patients compared to healthy controls across all 6 samples. (D) Heat map of DE tsRNAs across all 6 samples. A1, A2, and A3 represent the SONFH group; B1, B2, and B3 were from the healthy group.
Table 4.
Clean Reads Mapped to Different Small RNAs
| Sample | Total Clean Reads | miRNA Mapped | piRNA Mapped | tsRNA Mapped |
|---|---|---|---|---|
| SONFH-1 | 21,191,046 | 9,205,095 | 1,050,837 | 877,798 |
| SONFH-2 | 14,560,008 | 7,522,215 | 631,162 | 440,788 |
| SONFH-3 | 27,183,453 | 13,321,466 | 689,186 | 705,982 |
| Control-1 | 18,072,999 | 9,563,503 | 856,929 | 695,868 |
| Control-2 | 21,279,324 | 11,009,926 | 851,045 | 701,508 |
| Control-3 | 16,812,559 | 6,840,654 | 787,675 | 586,384 |
| Total | 119,099,389 | 57,462,859 (48.25%) | 4,866,834 (4.09%) | 4,008,328 (3.37%) |
Target Gene Prediction and GO and KEGG Enrichment Analysis of DE tsRNA Target Genes
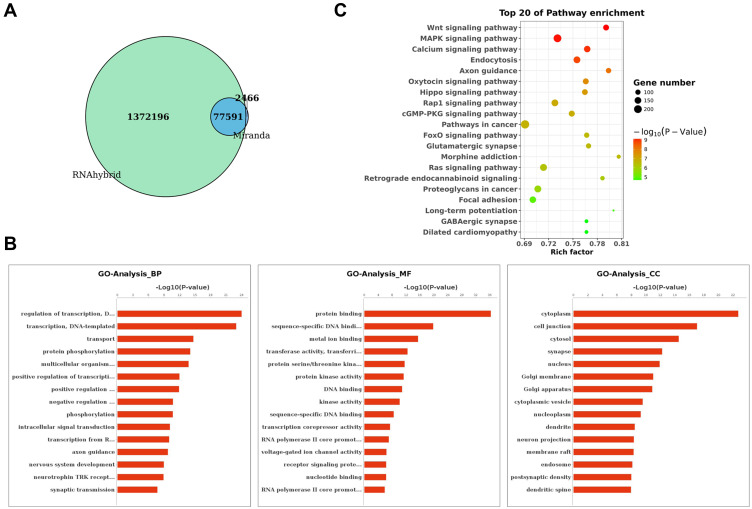
MiRanda and RNAhybrid algorithms were used to identify a total of 77591 target mRNAs for the DE tsRNAs between SONFH and healthy subjects (Figure 3A). To understand the potential functions and mechanisms of the DE tsRNAs involved in SONFH, we performed GO and KEGG pathway analysis. As shown in Figure 3B, the results revealed that tsRNAs mainly functioned in the biological process of “regulation of transcription, DNA-templated” (GO:0006355), “transcription, DNA-templated” (GO:0006351), and “transport” (GO:0006810). These tsRNAs were found to be involved in the cellular component of “cytoplasm” (GO:0005737), “cell junction” (GO:0030054), and “cytosol” (GO:0005829), and were implicated in the molecular function of “protein binding” (GO:0005515), “sequence-specific DNA binding transcription factor activity” (GO:0003700), and “metal ion binding” (GO:0046872). The KEGG analysis suggested that the predicted target genes were mainly involved in the Wnt, MAPK, and calcium signaling pathways (Figure 3C). These enriched pathways might have potential significance in the progression of SONFH.
Figure 3.
Target gene prediction and functional enrichment analyses of DE tsRNAs. (A) Venn diagram showing the overlap in predicted target genes for DE tsRNAs between SONFH and healthy subjects using Miranda and RNAhybrid algorithms. (B) Gene Ontology (GO) classification for predicted target genes of DE tsRNAs in SONFH patients. The x-axis shows the enrichment factor including gene numbers and -log10 (p-value) and the y-axis represents the top 20 GO enrichment terms. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis for the predicted target genes of DE tsRNAs in SONFH patients compared to healthy subjects. The horizontal axis refers to the number of genes and the vertical axis refers to the KEGG pathway terms. Node color: p value.
Validation of tsRNAs Expression by Real Time-qPCR
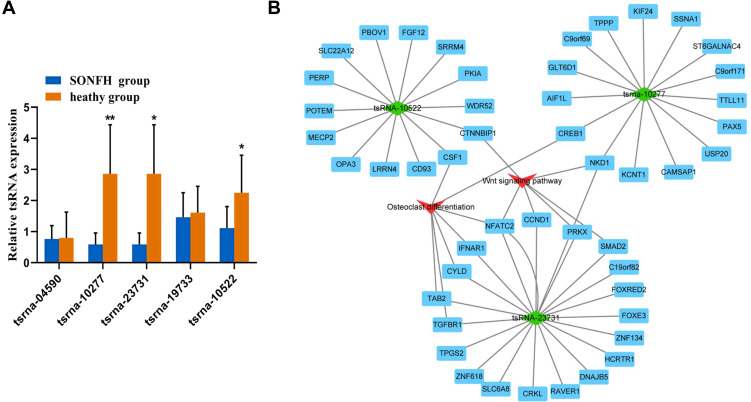
To further validate the small RNA sequencing results, 5 of the identified DE tsRNAs (4 downregulated (tsRNA-10277, tsRNA-23731, tsRNA-19733, and tsRNA-10522) and 1 upregulated (tsRNA-04590)) with relatively high fold change value and abundance were selected as candidate tsRNAs. This choice was informed by the involvement of these tsRNAs and their target mRNAs in the Wnt signaling pathway and osteogenic differentiation, as indicated by bioinformatics-based analysis. Moreover, studies have shown that the Wnt signaling pathway is itself associated with osteogenic differentiation.22,23 Our results revealed differences in the expression levels of tsRNA-10277, tsRNA-23731, and tsRNA-10522 between SONFH patients and healthy subjects (Figure 4A).
Figure 4.
Validation of tsRNA expression and construction of DE tsRNAs/mRNA pathway interaction network using Cytoscape software. (A) Relative expression levels of the 5 candidate tsRNAs were validated by real time-qPCR. The values were expressed as mean ± SD (n=3). *p<0.05 and **p<0.01 are shown for SONFH group vs healthy heathy group. (B) Integrated tsRNAs/mRNA pathway analysis. The red triangle refers to Wnt signal pathway and osteogenic differentiation, and the green rhombus represents DE tsRNAs. Blue rectangles represent mRNAs.
tsRNA/mRNA Pathway Interaction Network Analysis
We used the Cytoscape software to depict an integrated mRNA/tsRNA pathway interaction network for uncovering the potential mechanism of SONFH progression (Figure 4B). The 3 tsRNAs (tsRNA-10277, tsRNA-23731, and tsRNA-10522) targeted various genes, with the latter being involved in the Wnt signaling pathway and osteogenic differentiation. The target genes NKD1 and CREB1 of tsRNA-10277 were enriched in Wnt signaling pathway and osteogenic differentiation, respectively. The target genes CTNNBIP11 and CSF1 of tsRNA-10522 were also implicated in Wnt signaling pathway and osteogenic differentiation, respectively. The target genes (NFATC2, TAB2, TGFBR1, CYLD, and IFNAR1) of tsRNA-23731 were enriched only in osteogenic differentiation. Additionally, tsRNA-23731 was selected as a future research focus owing to its high fold change value and various target genes that were involved in Wnt signaling pathway and osteogenic differentiation.
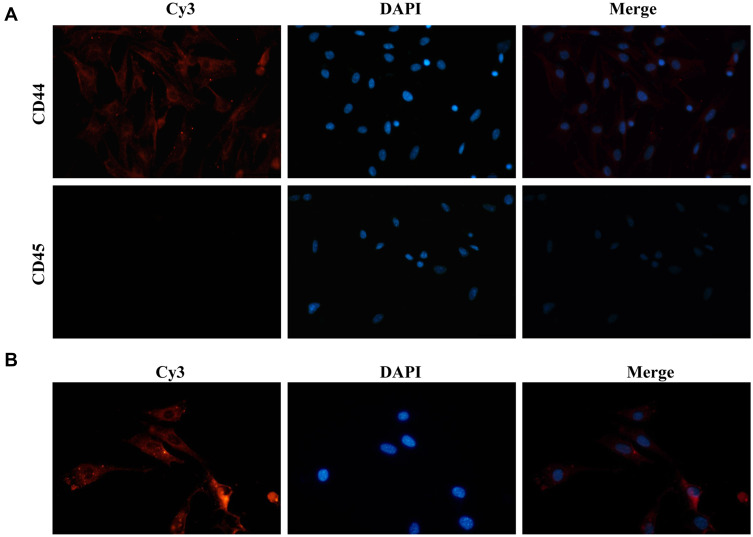
Identification of BMSCs and BMSC-Exos Internalization in Cells
To explore the functional role of BMSC-Exos carrying tsRNAs, we first identified the BMSCs. To this end, CD44 and CD45 were selected as BMSC markers. According to the immunofluorescence results, BMSCs expressed CD44 (but not CD45), indicating that these BMSCs were successfully separated (Figure 5A). Furthermore, we observed that Cy3-labeled BMSC-Exos were internalized in the cellular model of SONFH, indicating that BMSC-Exos could be taken up by BMSCs (Figure 5B).
Figure 5.
BMSCs identification and BMSCs Exos internalization. (A) Immunofluorescence assays showing expression levels of BMSCs surface markers (magnification, ×100); BMSCs, bone marrow-derived mesenchymal stem cells. (B) Uptake of Cy3-labeled BMSC-Exos by SONFH BMSCs.
Effect of BMSC Exos on Osteogenesis
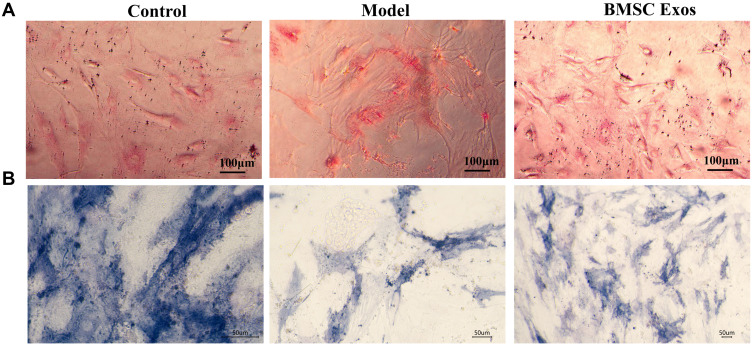
BMSC Exos has been previously shown to regulate osteoblast differentiation. Adipogenic generation decreases the osteogenic ability of cells.15 To explore the effect of BMSC Exos on osteogenic ability in the SONFH cellular model, oil red O and ALP staining were performed. Increased lipid droplets were observed in the SONFH model cells compared to that in control cells, while BMSC Exos decreased the lipid droplets compared to SONFH model cells (Figure 6A). ALP staining showed a decrease in the number of BMSCs stained blue/purple in the SONFH group compared to the control. This effect could be reversed by treatment with BMSC Exos (Figure 6B). Therefore, BMSC Exos significantly enhanced osteogenic differentiation in steroid-induced BMSCs.
Figure 6.
BMSC Exos regulated osteogenic differentiation of steroid-induced BMSCs. (A) Oil red O staining showed significant increase in lipid droplets in BMSCs from the SONFH model compared to the control group (Scale bars: 100um). (B) ALP staining revealed lower numbers of BMSCs stained blue/purple in the SONFH cellular model than that in the control group, while treatment with BMSC Exos reversed this effect (Scale bars: 50um).
Effect of tsRNA-10277-Loaded BMSC Exos on Adipogenesis and Osteogenesis
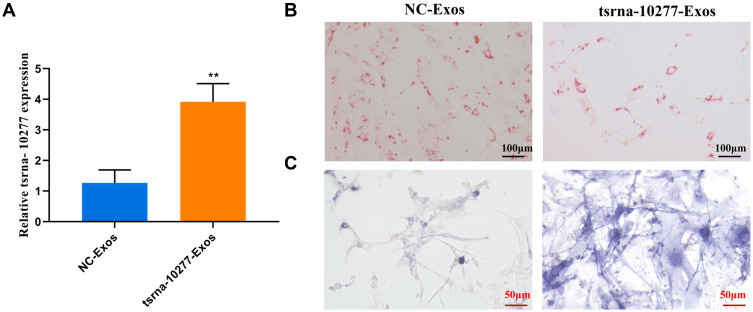
We focused on the role of tsRNA-10277, since it was found to be associated with significant statistical difference and relatively higher fold change value compared to other tsRNAs. First, we confirmed that the tsRNA-10277 mimics significantly increased tsRNA-10277 expression in BMSC Exos (Figure 7A). The tsRNA-10277-loaded BMSC Exos led to decreased fraction of lipid droplets in the SONFH cellular model, compared to that in the NC cells (Figure 7B). After incubation with tsRNA-10277-loaded BMSC Exos, the number of BMSCs stained blue/purple increased in the SONFH model compared to the NC Exos treatment (Figure 7C). Therefore, tsRNA-10277-loaded BMSC Exos regulated adipogenesis and osteogenesis of steroid-induced BMSCs.
Figure 7.
Effect of tsRNA-10277-loaded BMSC Exos on osteogenic differentiation of steroid-induced BMSCs. (A) Real time-qPCR showing the expression level of tsRNA-10277 in tsRNA-10277-loaded BMSC Exos (tsRNA-10277 Exos) and NC-loaded BMSC Exos (NC Exos). Data shown represents mean ± SD (n=3). **p<0.01 is shown for tsRNA-10277 Exos group vs NC Exos group. (B) Oil red O staining showed that the presence of tsRNA-10277 Exos led to significant decrease in lipid droplets in the SONFH model compared to that in the presence of NC Exos (Scale bars: 100um). (C) ALP staining revealed higher numbers of BMSCs stained blue/purple in tsRNA-10277 Exos group than that in the NC Exos group (Scale bars: 50um).
Discussion
SONFH is a disease of mesenchymal or bone cells and is a common incurable orthopedic disorder that leads to femoral head collapse and may even need total hip replacement for therapy.24,25 Exos act as carriers of proteins, mRNAs, and small non-coding RNAs that target cells and perform intercellular communication.26 However, the tsRNA expression profile of plasma exosomes from SONFH patients and healthy subjects has not been reported. The present results represent the first experimental evidence of altered tsRNA expression patterns in plasma exosomes of SONFH patients and healthy subjects. A total of 223 DE tsRNAs (137 upregulated and 86 downregulated) were identified in the SONFH patients, compared to the healthy subjects. The target genes of the identified DE tsRNAs were predicted to be involved in Wnt signaling pathway, and might participate in SONFH progression. Moreover, various target genes of the identified tsRNAs (tsRNA-10277, tsRNA-23731, and tsRNA-10522) were enriched in Wnt signaling pathway and osteogenic differentiation.
MSCs have the ability of multiple potential differentiation and can differentiate into bone cells during specific conditions, which could be used to treat SONFH.27 Interestingly, communication between osteoclasts and osteoblasts may occur through small membrane-enclosed vesicular particles named as EVs.28 Early studies have revealed that exosomes derived from MSCs have protective effects on injury or diseased tissues, and are known to promote angiogenesis in ONFH.29 LncRNA MALAT1 derived BMSC Exos could potentially enhance osteoblast activity in osteoporotic mice by mediating the miR-34c/SATB2 axis.30 The present study, in overall agreement with these reports, shows that tsRNA-10277-loaded BMSC Exos also regulates adipogenesis and osteogenesis of steroid-induced BMSCs, thereby implicating BMSC Exos in SONFH progression.
In our study, tsRNA-10277, tsRNA-10522, and tsRNA-23731 were identified to target CREB1, CSF-1, and NFATC2, respectively. The cAMP-responsive element-binding protein 1 (CREB1) has been shown to participate in osteogenic differentiation of rat periosteum-derived stem cells.31 The colony-stimulating factor-1 (CSF-1) is critical for the differentiation of bone marrow precursor cells into bone-resorbing osteoclasts.32 The osteoblast-derived receptor activator of NF-κB and CSF-1 synergistically affect osteoclast formation.33 CREB-binding protein and BMP-2 markedly increase the expression of osteoclastogenic CSF-1.33 A key regulatory gene from the Wnt/β-catenin pathway, NFATC2, is upregulated on the modified surfaces of human alveolar bone-derived osteoprogenitor cells, and this has been correlated with a higher expression of osteogenic markers.34 Therefore, tsRNA-10277, tsRNA-10522, and tsRNA-23731 might be involved in SONFH pathophysiology via modulation of their respective target genes related to osteogenic differentiation.
Intriguingly, the Wnt/β-catenin pathway, MAPK signaling pathway, and calcium signaling pathway have been shown to participate in osteogenic differentiation.22,23 Wnt signaling is considered to be one of the major pathways regulating bone formation, and inhibition of these Wnt modulators could represent a promising modality for osteoporosis treatment.35 Abnormal Wnt signaling results in defects of the human skeleton, and high Wnt/β-catenin signaling promotes osteoblast differentiation.36 Here, we found that target genes of tsRNA-10277 from plasma Exos isolated from SONFH and healthy subjects were enriched in Wnt signaling pathway and osteogenic differentiation, indicating that tsRNA-10277 might play a role in SONFH progression via Wnt signaling pathway and osteogenic differentiation.
There are several limitations in this study that should be explained. Although we have studied the tsRNA expression profiles of plasma exosomes from SONFH patients and healthy subjects, the sample size is too small to consider exosomal tsRNA as a promising biomarker for SONFH diagnosis. Moreover, the study design is not enriched. We should further study the mechanistic basis for the involvement of tsRNA-10277-loaded BMSC Exos in the SONFH cellular model.
In conclusion, we have identified, for the first time, altered tsRNAs of plasma exosomes from patients diagnosed with SONFH. A total of 223 tsRNAs were found to be differentially expressed, among which tsRNA-10277 was significantly downregulated in plasma exosomes of SONFH patients compared to the control group. TsRNA-10277 was implicated in Wnt signaling pathway and osteogenic differentiation. Additionally, tsRNA-10277-loaded BMSC Exos were found to influence adipogenesis and osteogenesis of dexamethasone-induced BMSCs. Our results provide novel insights into the osteogenic effect of tsRNA-10277-loaded BMSC Exos on SONFH.
Funding Statement
This work was supported by the Fujian Provincial Department of Finance Special Allocation (2018B022, BPB-LJH2015-2), the Youth Backbone Talent Training Program of Fujian Provincial Health System (2017-ZQN-48), the 2018 “13th Five-Year Plan” project of the Fujian Provincial Department of Education (FJJKCG18-021), and the Startup Fund for Scientific Research of Fujian Medical University (2018QH1062).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the First Affiliated Hospital of Fujian Medical University. Written informed consent for research purposes were obtained from all patients who participated in the study.
Disclosure
The authors declare that they have no conflicts of interest.
References
- 1.Choi H-R, Steinberg ME, Y. Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8(3):210–220. doi: 10.1007/s12178-015-9278-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu W, Liu B, Wang B, Zhao D. Early diagnosis and treatment of steroid-induced osteonecrosis of the femoral head. Int Orthop. 2019;43(5):1083–1087. doi: 10.1007/s00264-018-4011-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao H, Guan H, Lai Y, Qin L, Wang X. Review of various treatment options and potential therapies for osteonecrosis of the femoral head. J Orthop Transl. 2016;4:57–70. doi: 10.1016/j.jot.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han N, Yan Z, Guo CA, et al. Effects of p-glycoprotein on steroid-induced osteonecrosis of the femoral head. Calcif Tissue Int. 2010;87(3):246–253. doi: 10.1007/s00223-010-9385-9 [DOI] [PubMed] [Google Scholar]
- 5.Kuribayashi M, Fujioka M, Takahashi KA. Combination analysis of three polymorphisms for predicting the risk for steroid-induced osteonecrosis of the femoral head. J Orthop Sci. 2008;13(4):297–303. doi: 10.1007/s00776-008-1244-4 [DOI] [PubMed] [Google Scholar]
- 6.Zhao -J-J, Wu Z-F, Wang L, Feng D-H, Cheng L. MicroRNA-145 mediates steroid-induced necrosis of the femoral head by targeting the OPG/RANK/RANKL signaling pathway. PLoS One. 2016;11(7):e0159805. doi: 10.1371/journal.pone.0159805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan H-F, Christina VR, Guo C-A, Chu Y-W, Liu R-H, Yan Z-Q. Involvement of MicroRNA-210 demethylation in steroid-associated osteonecrosis of the femoral head. Sci Rep. 2016;6:20046. doi: 10.1038/srep20046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31(4):893–898. doi: 10.1016/j.arth.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 9.Guan X-Y, Han D. Role of hypercoagulability in steroid-induced femoral head necrosis in rabbits. J Orthop Sci. 2010;15(3):365–370. doi: 10.1007/s00776-010-1452-6 [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Liu L, Dang X, Ma S, Zhang M, Wang K. The effect of core decompression on local expression of BMP-2, PPAR-γ and bone regeneration in the steroid-induced femoral head osteonecrosis. BMC Musculoskelet Disord. 2012;13(1):142. doi: 10.1186/1471-2474-13-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H-G, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184(1):28–41. doi: 10.1016/j.ajpath.2013.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Weston WW, Ganey T, Temple HT. The relationship between exosomes and cancer: implications for diagnostics and therapeutics. BioDrugs. 2019;33(2):137–158. doi: 10.1007/s40259-019-00338-5. [DOI] [PubMed] [Google Scholar]
- 14.Masaoutis C, Theocharis S. The role of exosomes in bone remodeling: implications for bone physiology and disease. Dis Markers. 2019;2019:9417914. doi: 10.1155/2019/9417914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang S, Li Y, Chen P. Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Des Devel Ther. 2019;13:45–55. doi: 10.2147/DDDT.S178698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebetsberger J, Polacek N. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 2013;10(12):1798–1806. doi: 10.4161/rna.27177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu P, Yu J, Zhou P. Role of tRNA-derived fragments in cancer: novel diagnostic and therapeutic targets tRFs in cancer. Am J Cancer Res. 2020;10(2):393–402.eCollection 2020. [PMC free article] [PubMed] [Google Scholar]
- 18.Goodarzi H, Liu X, Nguyen HB, Zhang S, Fish L, Tavazoie S. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015;161(4):790–802. doi: 10.1016/j.cell.2015.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maute RL, Schneider C, Sumazin P, et al. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(4):1404–1409. doi: 10.1073/pnas.1206761110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglio SR, Rooijers K, Koppers-Lalic D. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6(1):127. doi: 10.1186/s13287-015-0116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Jin T, Cao Y. Association between genetic polymorphisms of MMP8 and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China. Medicine. 2016;95(37):e4794. doi: 10.1097/MD.0000000000004794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X-D, Liu Z-Y, Chang B. Panax Notoginseng saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK signaling pathways. Cellu Physiol Biochem. 2011;28(2):367–376. doi: 10.1159/000331753 [DOI] [PubMed] [Google Scholar]
- 23.Kim JH, Liu X, Wang J. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 2013;5(1):13–31. doi: 10.1177/1759720X12466608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86(6):1153–1160. doi: 10.2106/00004623-200406000-00006 [DOI] [PubMed] [Google Scholar]
- 25.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2003;417:84–92. [DOI] [PubMed] [Google Scholar]
- 26.Potaczek DP, Michel S, Sharma V. Different FCER1A polymorphisms influence IgE levels in asthmatics and non-asthmatics. Pediatr Allergy Immunol. 2013;24(5):441–449. doi: 10.1111/pai.12083 [DOI] [PubMed] [Google Scholar]
- 27.Liao W, Ning Y, Xu H-J. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci. 2019;133(18):1955–1975. doi: 10.1042/CS20181064 [DOI] [PubMed] [Google Scholar]
- 28.Yuan F-L, Wu Q-Y, Miao Z-N. Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front Physiol. 2018;9:628. doi: 10.3389/fphys.2018.00628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Li Q, Niu X. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int J Biol Sci. 2017;13(2):232–244. doi: 10.7150/ijbs.16951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging. 2019;11(20):8777–8791. doi: 10.18632/aging.102264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Xu J, Ruan YC. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida H, Hayashi S-I, Kunisada T. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345(6274):442–444. doi: 10.1038/345442a0 [DOI] [PubMed] [Google Scholar]
- 33.Ghosh-Choudhury N, Singha PK, Woodruff K. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J Biol Chem. 2006;281(29):20160–20170. doi: 10.1074/jbc.M511071200 [DOI] [PubMed] [Google Scholar]
- 34.Chakravorty N, Hamlet S, Jaiprakash A, et al. Pro-osteogenic topographical cues promote early activation of osteoprogenitor differentiation via enhanced TGFβ, Wnt, and Notch signaling. Clin Oral Implants Res. 2014;25(4):475–486. doi: 10.1111/clr.12178. Epub 2013 Apr 21. [DOI] [PubMed] [Google Scholar]
- 35.Boudin E, Fijalkowski I, Piters E, Van Hul W. The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Semin Arthritis Rheum. 2013;43(2):220–240. doi: 10.1016/j.semarthrit.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 36.Regard JB, Zhong Z, Williams BO, Yang Y. Wnt signaling in bone development and disease: making stronger bone with Wnts. Cold Spring Harb Perspect Biol. 2012;4(12):a007997. doi: 10.1101/cshperspect.a007997. [DOI] [PMC free article] [PubMed] [Google Scholar]