Abstract
Purpose
This study aimed to explore the potential role and mechanism of garlic-derived S-allylmercaptocysteine (SAMC), the major water-soluble fraction of garlic, in osteoarthritis (OA) both in vivo and in vitro.
Methods
The effect of SAMC in a surgical-induced OA model was examined by X-ray, staining, ELISA, and immunoblotting. Then the key role of Nrf2 by SAMC treatment in IL-1β stimulated chondrocytes in vitro was determined by gene-knockdown technique.
Results
SAMC could stabilize the extracellular matrix (ECM) by decreasing metalloproteinase (MMPs) expression to suppress type II collagen degradation in OA rats. The inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, were elevated in OA, which could be down-regulated by SAMC treatment. This effect was parallel with NF-κB signaling inhibition by SAMC. As oxidative stress has been shown to participate in the inflammatory pathways in OA conditions, the key regulator Nrf2 in redox-homeostasis was evaluated in SAMC-treated OA rats. Nrf2 and its down-stream gene NQO-1 were activated in the SAMC-treated group, accompanied by NAD(P)H oxidases 4 (NOX4) expression down-regulated. As a result, the toxic lipid peroxidation byproduct 4-hydroxynonenal (4HNE) was reduced in articular cartilage. In IL-1β-stimulated primary rat chondrocytes, which could mimic OA in vitro, SAMC could ameliorate collagen destruction, inhibit inflammation, and maintain redox-homeostasis. Interestingly, after Nrf2 gene knockdown by adenovirus, the protective effect of SAMC in IL-1β-stimulated chondrocytes disappeared.
Conclusion
Overall, our study demonstrated that SAMC targeted Nrf2 to protect OA both in vivo and in vitro, which would be a new pharmaceutical way for OA therapy.
Keywords: S-allylmercaptocysteine, Nrf2, osteoarthritis, oxidative stress, inflammation
Introduction
Osteoarthritis (OA) is a chronic and progressive joint disorder. It leads to severe chronic pain, loss of mobility, and disability, which affects millions of people worldwide.1 It is characterized by structural damage to one or more joints, other factors, like genetic, metabolic, environmental, or traumatic, also play a role in the development of OA.2
The treatments for OA include lifestyle changes to decrease weight-bearing, pharmacological interventions (corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs)), and surgical interventions (osteotomy or joint prosthesis). These therapeutic approaches may give relief to the patient, but the options are limited.3
Garlic-derived S-allylmercaptocysteine (SAMC) is the major water-soluble fraction of garlic, which has shown a potential role in anti-oxidative stress, anti-inflammation,4 anti-tumorigenesis,5 etc. Our previous studies have demonstrated the protective effect of SAMC in interleukin (IL)-1β-treated chondrocytes, which regulated inflammatory response by attenuating NF-κB-induced TNF-α secretion.6 TNF-α and IL-1β in chondrocytes have shown that they can accelerate cartilage degeneration in osteoarthritis via NADPH oxidase 4 (NOX4).7 NF-E2-related factor-2 (Nrf2) is an anti-oxidant transcriptor playing a pivotal role in cartilage integrity. The impairment of Nrf2 and the activation of NOX4 would cause redox imbalance.8 But the effect of SAMC in an animal OA model is unclear, and the deep mechanism involving inflammation and antioxidation in OA needs further exploration.
In this study, we focused on the therapeutic effects of SAMC in osteoarthritis rats, and evaluated the mechanism of SAMC in the OA model both in vivo and in vitro. Changes of matrix metalloprotease (MMPs) expression in extracellular matrix (ECM) synthesis and degradation, inflammatory cytokines, and oxidative status were examined to evaluate the role of SAMC in the OA model. Furthermore, we used gene-loss of function by adenovirus to determine the key effect of Nrf2 signaling in OA, and provide clues for clinical therapy of SAMC as a pharmaceutical target.
Materials and Methods
Animals
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of Shandong First Medical University, and was performed in accordance with the Guidelines for Animal Experimentation of Shandong First Medical University, China (NSFC: NO. 2019–314). Sprague-Dawley (SD) rats were housed under pathogen‑free conditions at room temperature (22‑25°C) and 50% humidity, with a 12‑hour light/dark cycle. Food and water were offered ad libitum.
Surgical Induction of OA
Male SD rats (10 weeks age) were random classified into four groups, including 1) Sham group: Sham surgery with saline intra-articular injection; 2) OA group: OA-induction surgery with saline intra-articular injection; 3) OA+SAMC 2 mM group: OA-induction surgery with SAMC (2 mM) intra-articular injection; and 4) OA+SAMC 5 mM group: OA-induction surgery with SAMC (5 mM) intra-articular injection (n=8 in each group).
Surgical [anterior cruciate ligament transection (ACLT) combined medial meniscectomy] approaches were used to induce the OA model in the left knee of rats.9 Briefly, rats were anesthetized by 1% pentobarbital sodium. The anterior attachment of the medial meniscus to the tibial plateau was transected to induce the instability of the left knee joint. Right knee joints were left intact in this study. In addition, sham operation was performed just by incision of the cutaneous and musculature of the knee in the present study. All surgical procedures were involved in aseptic techniques in animals. After surgery, 2 mM or 5 mM SAMC (purity of 99%) in 0.9% saline or saline alone were given by intra-articular injection (30 μL) in the SAMC group and sham group every day for 4 weeks until the rats were sacrificed.
X-Ray Imaging
The knee joints were examined with X-rays 4 weeks after operation to evaluate the severity of OA. After being anesthetized, the rats were placed on a digital X-ray machine (NKX-500, HuaRui Image Technologies Inc., China) and the ray images were obtained. The articular cartilage degeneration was assessed on the basis of the radiographic osteophytosis grading system: scores of 0, 1, 2, and 3 representing as normal, mild, moderate, and severe damage, respectively, based on joint space, cartilage surface calcification, and osteophyte formation.
Pathological Examination
After 4 weeks surgery, rats were euthanized, and the articular cartilage of knee harvested. Knee cartilage was fixed in 4% paraformaldehyde more than 48 hours and decalcified over 30 days, then dehydrated and paraffin-embedded. The samples were cut into 5 μm-thick sections, deparaffinized, and then the hematoxylin and eosin (H&E) and Safranin o-fast green (SO) staining were performed to assess the OA development. Morphological change was observed by using a light-microscope (Nikon Corporation, Japan). The cartilage degeneration was evaluated by the method recommended by the Osteoarthritis Research Society International (OARSI) scoring system with a total score of 0–24.10 In short, the depth and extent of cartilage injury of medial tibial plateau were divided into six and four grades, respectively. The score was obtained by multiplying the series, and the mean value was taken.
Immunohistochemistry
Immunohistochemistry examination was carried out as previously described.6 Briefly, sections were deparaffinized, rehydrated, and blocked with 10% normal goat serum. Then the primary antibody (anti-rat Col II antibody, anti-p65 antibody, Abcam) were incubated overnight, following biotin-labeled secondary antibodies for 1 hour at room temperature. A biotin-streptavidin detection system was used according to the manufacturer’s instruction. After rinsing, sections were counterstaining with hematoxylin and mounted.
Isolation, Culture of Rat Chondrocytes, and Adenovirus Infection
Chondrocytes isolation was conducted as previously described with modification.6 Primary rat chondrocytes were seeded in 6-well plates. Nrf2 loss-of-function approaches were achieved by infecting the cells with adenovirus of miNrf2 (Ad-miNrf2) and miScramble (Ad-Scramble)11 in serum free DMEM for 8 hours and then changed to DMEM supplemented with 10% FBS. After that, IL-1β (5 ng/mL) was used to induce OA model of chondrocyte,12 combined with or without SAMC (60 µM) for additional 24 hours. IL-1β (≥95%) was purchased from Sigma-Aldrich.
Enzyme-Linked Immune Sorbent Assay (ELISA) of Joint Cavity Fluid
Four weeks after surgery, rat joint cavity fluid was collected using a 1 mL syringe rinsing with 1 mL PBS, and centrifuged at 2,000 r/min for 10 minutes. The supernatant was collected for the following assay. C2C (eBioscience, US), C-terminal cross-linking telopeptide of type II collagen (CTX-II) (Westang, China), Cartilage oligomeric matrix protein (COMP) (cusabio, China), Tumor necrosis factor-α (TNF-α) (Invitrogen, US), IL-1β (MultiSciences Biotech, Shanghai, China), and Interleukin-6 (IL-6) (Neobioscience technology, China) concentrations were measured using ELISA Kit according to the manufactures’ instructions. Absorbance was read at 450 nm with a microplate reader (Tecan Trading Co., Ltd.).
Western Blot Analysis
Total protein of cartilage (10 mg), which was grinding in liquid nitrogen in a RNAase-free plastic bag or chondrocytes were lysed by RIPA lysis buffer containing protease and phosphatase inhibitor cocktails (Roche). Protein in the cytoplasm and nucleus were isolated by using Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China), according to the manufacturer’s instruction. The protein concentration was quantified by BCA assay (Beyotime). About 30 μL nuclear protein and 100 μL cytoplasmic protein could get in each group after extraction with the concentration between 0.5–1.0 μg/μL. Equal quantities of proteins were separated using 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred onto the polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). The membranes were blocked in with 5% skim milk for 2 hours, and then incubated with primary antibodies overnight at 4°C. After washing with TBST three times, the membranes were incubated with HRP-conjugated anti-rabbit or anti-mouse antibodies at room temperature for 1 hour. Finally, the signals of bands were detected by an enhanced chemiluminescence reagent (Millipore, Billerica, MA) using ChampChemi 500 system (Sage Creation Science, China).
The relative optical density of bands was quantified using Image Pro Plus, standardized by the density of PCNA or β-actin. The primary antibodies included those of anti-Collagen II (ab34712, Abcam), anti-MMP2 (ab181286), anti-MMP9 (ab28898, Abcam), anti-MMP13 (ab39012, Abcam), anti-TIMP1 (ab61224, Abcam), anti-COX2/Cyclooxygenase 2 (ab15191, Abcam), anti-iNOS (ab3523, Abcam), Nrf2 (sc-722, Santa Cruz), anti-NQO1 (ab2346, Abcam), anti-NOX4 (14,347-1-AP, proteintech), anti-4 Hydroxynonenal (ab46545, Abcam), anti-NF kappaB p65 (8242P, Cell signaling), anti-IκBα (#4812, Cell signaling), anti-PCNA (ab92552, Abcam), anti-alpha Tubulin (ab7291, Abcam), and β-actin (TA-09, ZSGB-BIO).
Quantitative Real Time (Q-PCR)
Total RNA from the articular cartilage (10 mg) which was grinding in liquid nitrogen in a RNAase-free plastic bag was extracted using Invitrogen TRIzol reagents (ThermoFisher), and reverse transcription reactions (SuperScript III First-Strand Synthesis System for RT-PCR, Invitrogen) were performed with 1.0 μg of RNA. PCR and quantitative real-time PCR (Q-PCR) were carried out using the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Laboratories, Inc.). Expression levels of target genes were normalized by concurrent measurement of glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA levels. Primers used for Q-PCR were as follows: Tnf (NM_012675): 5ʹ-CATTCCTGCTCGTGGCGGGG-3ʹ, 5ʹ-CGACGTGGGCTACGGGCTTG-3ʹ; IL-6 (NM_012589): 5ʹ-TGTTCTCAGGGAGATCTTGG-3ʹ, 5ʹ-TCCAGGTAGAAACGGAACTC-3ʹ; IL-1beta (NM_031512.2): 5ʹ- GACTTCACCATGGAACCCGT-3ʹ, 5ʹ-GGAGACTGCCCATTCTCGAC-3ʹ; Gapdh (NM_008084.2): 5ʹ-ATGTTCCAGTATGACTCCACTCACG-3ʹ, 5ʹ-GAAGACACCAGTAGACTCCACGACA-3ʹ.
Measurement of Oxidant-Antioxidant Biomarkers in Serum
Whole blood was collected from inferior vena cava in each animal under general anesthesia. The blood was centrifuged, and serum was obtained for redox balance determination, by measuring total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione (GSH), oxidized glutathione (GSSG), and malondialdehyde (MDA). All procedures were performed according to the manufacturer’s instruction of the kit (Nanjing Jiancheng Bioengineering Institute, China).
Statistical Analysis
Data are shown as mean±standard deviation (SD). Statistical differences were analyzed by paired double tailed t-test or one-way ANOVA followed by Bonferroni test for multiple comparisons using SPSS 21.0 statistical software (SPSS, Inc., Chicago, IL, USA). Differences were considered significant at P<0.05.
Results
SAMC Ameliorates the Progression of OA in Rat Model
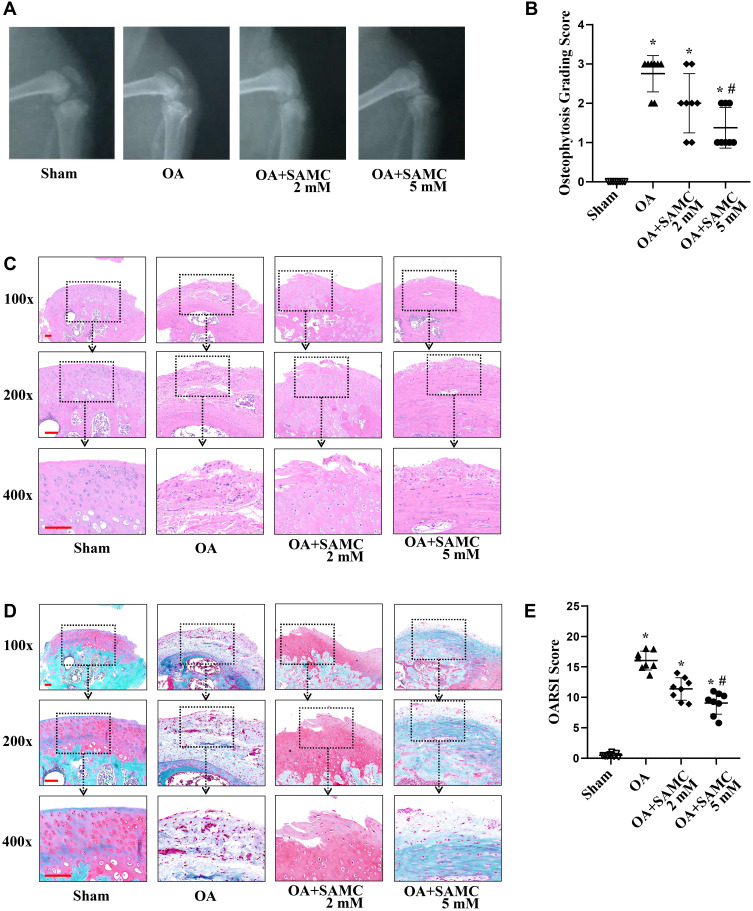
Destruction of cartilage structure contributes to the progression of OA. OA-induced degenerative changes, like cartilage loss, joint space narrowing, subchondral sclerosis, subchondral cysts, and bone spurs (osteophytes), can be recognized on X-ray imaging.13 At 4 weeks after OA-induction surgery, the knee joint space was significantly wider compared with the saline groups, while SAMC intra-articular injection attenuated this morphological change. The articular surface in the sham group was smoother than that in the OA group, while being better preserved in the SAMC group than the OA group (Figure 1A). The osteophytosis at the margins of the knee joint quantified by radiographic osteophytosis grading system (Figure 1B), the SAMC (2 mM) (2.00±0.76), and SAMC (5 mM) (1.38±0.52) groups were lower than that of the OA group (2.75±0.46), especially the SAMC 5 mM group showed a significant protection effect (P<0.05). These results indicated SAMC exhibited preventative effects on OA.
Figure 1.
SAMC repaired cartilage lesion in OA rats morphologically and pathologically. Following anterior cruciate ligament transection combined medial meniscectomy surgically induction of OA, micro X-Ray (A) was used to evaluate the severity of OA in the knee. (B) Radiographic osteophytosis was graded on a standard of a scale from 0–3 (0-normal, 1-mild, 2-moderate, 3-severe) based on the severity of osteophytosis at the margins of the knee joint. Histological sections (5 µm thick) of cartilage from the tibial plateau were stained with H&E (C) and Safranin-O/Fast Green (D). A smoother surface and less blue fibrosis was detected in the articular cartilage of the SAMC injection groups compared with the OA group. Scale bar: 100 μm. (E) OARSI histological grading score indicated that cartilage damage in the OA group was more severe than the sham group; but cartilage damage was attenuated in the SAMC (2 mM) and SAMC (5 mM) groups. Data were expressed as means±SD. *P<0.05, vs sham group; #P<0.05, vs OA group. n=8.
SAMC Protects OA-Induced Cartilage Pathological Damage in Rats
Histological changes to the cartilage surface were detected using H&E and SO staining. H&E examination showed that treatment with SAMC attenuated cartilage lesion formation (Figure 1C). SO staining was widely used to examine the ECM components in cartilage. The SO staining results showed OA-induction significantly decreased the ECM of cartilage more than that in the sham group; whereas the cartilage in the SAMC group had stronger proteoglycan staining and a more intact surface than that of the OA group (Figure 1D). OARSI scores were also determined in this study.10 Consistent with the results of SO staining, the OARSI scores of the OA group (3.13±0.44) were significantly more severe than those of the sham group (0.06±0.18) (P<0.01). In contrast, cartilage in the SAMC 2 mM (2.13±0.35) and SAMC 5 mM group (1.38±0.35) (P<0.05) exerts lower severity of damage than the OA group (Figure 1E).
SAMC Affects Collagen Degradation and MMPs/TIMP1 Ratio in OA Rats
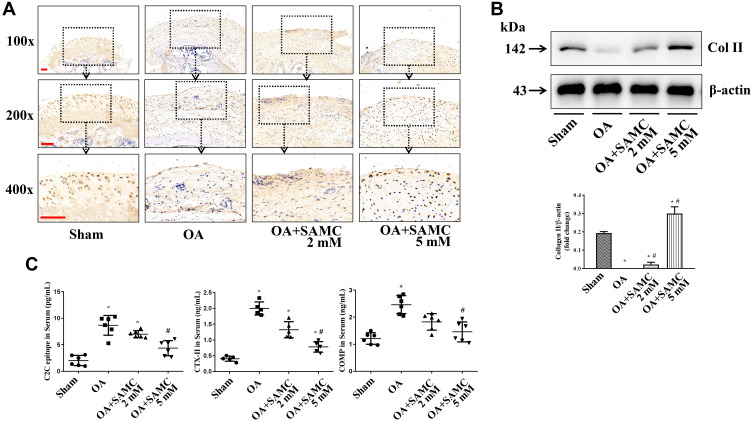
The most significant pathological manifestation of OA is the degeneration of articular cartilage. As the major components of cartilage tissue, type II collagen (Col II) expression was examined by immunohistochemistry and Western Blot. Histologically, the pericellular staining of Col II was locally pronounced in the mid and deep zone, with an even distribution in healthy cartilage. Col II staining was more weakened in the OA-induction than sham group, but stronger in the SAMC-treated groups (Figure 2A).
Figure 2.
SAMC increased Col II expression in articular cartilage, while reduced byproducts of Col II in the joint cavity fluid of OA rats. (A) Immunohistochemical staining was used for Col II detecting. Positive signals are indicated by brown staining, n=8. Scale bar: 100 μm. (B) Western Blot detection for Col II expression in articular cartilage, n=4. (C) Products of Col II, including C2C epitope, CTX-II, and COMP were examined by ELISA, n=8. The images obtained under the 10×, 20×, 40× objective lenses respectively. *P<0.05, vs Sham; #P<0.05, vs OA.
Immunoblotting results revealed that Col II expression decreased in cartilage of OA rats, after SAMC treatment, Col II was up-regulated dose-dependently in cartilage tissues (Figure 2B). Matrix degeneration mainly results in the losses of proteoglycans and Col II.14 We then examined the Col II degradation products C2C epitope and CTX-II, and COMP expression using ELISA, and the results showed an increased expression in joint cavity fluid of OA rats, but down-regulated by SAMC treatment (Figure 2C).
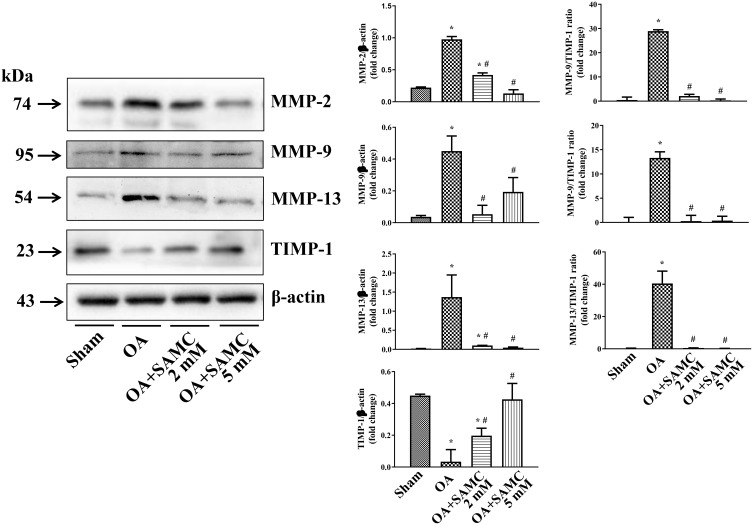
Proteolytic cleavage of Col II involves several proteases including MMPs15 and cathepsins (Cats), and its balance was regulated by the tissue inhibitors of metalloproteinases (TIMPs), which are the endogenous inhibitors of the MMPs.16 The expression of MMP-2, MMP-9, and MMP-13 were elevated in OA rats, accompanied with the decreasing of TIMP-1; but the SAMC treatment reversed these changes (Figure 3).
Figure 3.
SAMC affected collagen degradation and MMPs/TIMP1 ratio in OA rats. The expression of MMP-2, MMP-9, and MMP-13 in articular cartilage were tested by Western Blot; and the inhibitors of MMPs, TIMP-1 was showed an opposite change, n=4. *P<0.05, vs Sham; #P<0.05, vs OA.
SAMC Attenuates the Inflammatory Response in OA Rats
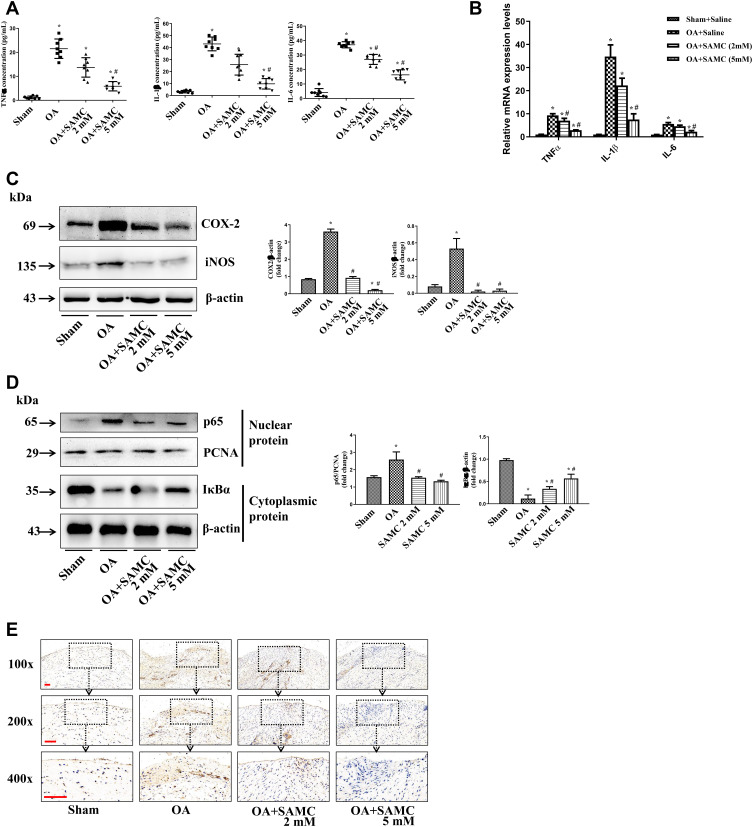
Various proinflammatory cytokines contribute to the pathogenesis of OA.17 Pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6 are elevated in OA rats, especially IL-1β, which has been documented as the most important role in the OA process.18 After SAMC treatment, the inflammatory response attenuated in a dose-dependent manner both in protein and mRNA levels (Figure 4A and B).
Figure 4.
SAMC influenced the inflammatory cytokines expression and NF-κB activity in OA rat. (A) Pro-inflammatory cytokines, TNF-α, IL-1β, IL-6 have elevated in OA rats, while SAMC treatment down-regulated these cytokines in joint cavity fluid, n=8. (B) Q-PCR detection of TNF-α, IL-1β, IL-6 mRNA level, n=4. (C) In articular cartilage, the elevated level of COX-2 and iNOS were down-regulated by the treatment of SAMC, n=4. (D) The nuclear NF-κB p65 in articular cartilage protein increased in OA rats, accompanied with IκBα degradation increased in cytoplasmic protein, n=4. SAMC treatment reversed the NF-κB activation by preventing p65 translocated to nucleus exanimated by immunohistochemistry, n=4, Scale bar: 100 μm (E). *P<0.05, vs Sham; #P<0.05, vs OA. n=8.
COX-2 and iNOS are two inducible enzymatic pathways that produce mediators (prostaglandins and nitric oxide) to cause inflammation and tissue damage in OA.19 The elevated level of COX-2 and iNOS in OA cartilage were down-regulated by the treatment of SAMC (Figure 4C).
Due to our and other previous studies, NF-κB exerts a significant role in osteoarthritis.20 Immunoblot (Figure 4D) and immunohistochemistry (Figure 4E) results revealed that the nuclear NF-κB p65 in articular cartilage protein increased in OA rats, accompanied with IκBα degradation increased in cytoplasmic protein. SAMC treatment reversed the NF-κB activation.
SAMC Regulates the Redox Balance of OA
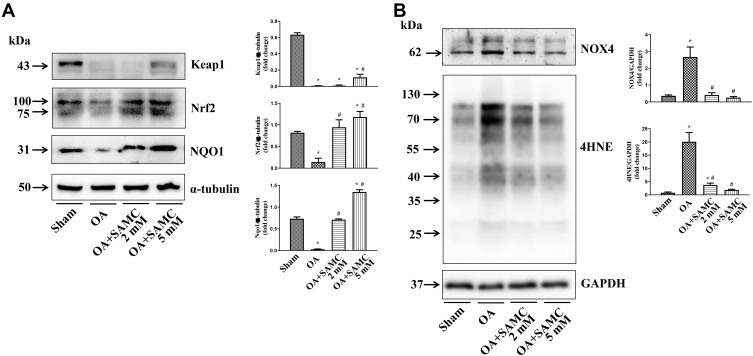
The NF-κB signaling pathway, which is involved in inflammatory disease, followed by inflammatory mediators expression, could be inhibited by Nrf2.21 Nrf2 is an anti-oxidative stress transcriptor, which can prevent reactive oxygen species (ROS)-caused cartilage collagen degradation.22 In response to articular cartilage injury, Nrf2 and its regulator Kelch-like ECH-associated protein-1 (Keap1) showed diminished expression; while SAMC at low dosage (2 mM) could reverse the change to cope with the inflammation change. But the high dosage (5 mM) could not further enhance the Nrf2 activation due to some unknown reasons in this study. Also, SAMC upregulated some Nrf2-related downstream antioxidant enzymes such as NAD(P)H quinone oxidoreductase-1 (NQO1) in OA rats (Figure 5A).
Figure 5.
SAMC inhibited oxidative stress in OA cartilage. (A) SAMC activated the expression of anti-oxidative regulation system Keap1-Nrf2, and its down-stream enzyme NQO1. (B) The oxidative sensor NOX4, and lipid peroxidative byproducts 4HNE, were down-regulated by SAMC in OA. *P<0.05, vs Sham; #P<0.05, vs OA. n=4.
During oxidative stress, chondrocyte lipid peroxidation causes collagen fragmentation, this mechanical fatigue failure could initiate osteoarthritis. ROS, as an initiator of oxidative stress, is regulated by NOX4, the NAD(P)H oxidases (NOXs) family member.23 The OA model exhibited enhanced NOX4 expression compared with the sham group. Consequently, a common byproduct of lipid peroxidation 4-hydroxynonenal (4HNE) showed the same change in articular cartilage of OA rats. However, both of these changes have been attenuated by SAMC treatment (Figure 5B).
In addition, redox balance was determined by measuring T-AOC, SOD, CAT, GSH-Px, GSH, and GSSG.24 As shown in Table 1, the T-AOC, SOD, CAT, GSH-Px, and GSH were down-regulated in the joint cavity fluid of OA rats, however, the SAMC treatment reverses these changes. While the level of GSSG, the oxidized GSH, and MDA were up-regulated in OA rats, the SAMC treatment alleviated the oxidative stress status in a dose-dependent manner.
Table 1.
Serum Oxidative and Anti-Oxidative Biomarkers in OA Rat (Mean±S.D., n=8)
| Group | Sham | OA | OA + SAMC 2 mM |
OA + SAMC 5 mM |
|---|---|---|---|---|
| T-AOC (U/mg prot) | 3.43±0.29 | 1.07±0.22* | 1.94±0.31* | 2.85±0.45,*, # |
| SOD (U/mg prot) | 47.42±11.26 | 24.04±12.27* | 36.40±9.43* | 42.19±7.53*, # |
| CAT (U/mg prot) | 27.54±9.33 | 22.30±10.38* | 23.71±11.05* | 25.38±12.67* |
| GSH-Px (U/mg prot) | 38.40±12.33 | 16.21±10.38* | 26.75±10.05 | 33.74±12.05* |
| GSH (U/mg prot) | 7.97±1.58 | 4.08±1.21* | 5.57±1.99* | 6.78±1.37*, # |
| GSSG (U/mg prot) | 3.08±0.74 | 6.05±0.57* | 4.40±0.74* | 3.26±0.98*, # |
| Ratio of GSSG/GSH | 0.39±0.47 | 1.48±0.47* | 0.79±0.37* | 0.48±0.72*, # |
| MDA (nmol/mg prot) | 4.77±0.25 | 6.05±0.78* | 5.58±0.13* | 5.01±0.27*, # |
Notes: *P<0.05, vs sham group; #P<0.05, vs OA group.
Abbreviations: T-AOC, total antioxidant capacity; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; GSH, glutathione; GSSG, oxidized glutathione; MDA, malondialdehyde.
SAMC Cannot Attenuate Oxidative Stress in Nrf2-Deficiency OA Chondrocytes
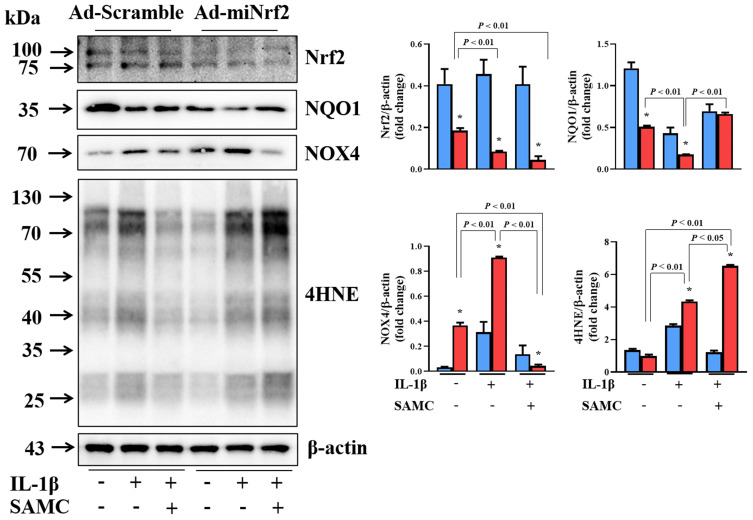
In order to clarify the key role of Nrf2 in SAMC-regulated OA protection, we used IL-1β-induced OA chondrocytes as an in vitro model for further exploration. IL-1β stimulated the Nrf2 and its downstream gene NQO1 activation against the cellular oxidative stress, and SAMC can enhance the anti-oxidative system. Then we used adenovirus to knockdown Nrf2 gene expression. Loss of Nrf2 function resulted in aggravated oxidative status, which can be detected by increased expression of NOX4, and more lipid peroxidation byproducts (4HNE) production. SAMC can inhibit oxidative stress in IL-1β-induced OA chondrocytes, but this effect diminished in gene-loss function of Nrf2 (Figure 6).
Figure 6.
SAMC could not rescue IL-1β-Induced oxidative stress in Nrf2-deficiency OA chondrocytes. Primary rat chondrocytes were infected with Ad-scramble orAd-miNrf2. IL-1β-induced OA chondrocytes were treated with or without SAMC to observe Nrf2, NQO1, NOX4 and 4HNE expression. *P<0.05, vs Ad-scramble. Experiments were performed in triplicate.
SAMC Cannot Relieve Chondrocytes Matrix Destruction in Nrf2 Deficient OA Chondrocytes
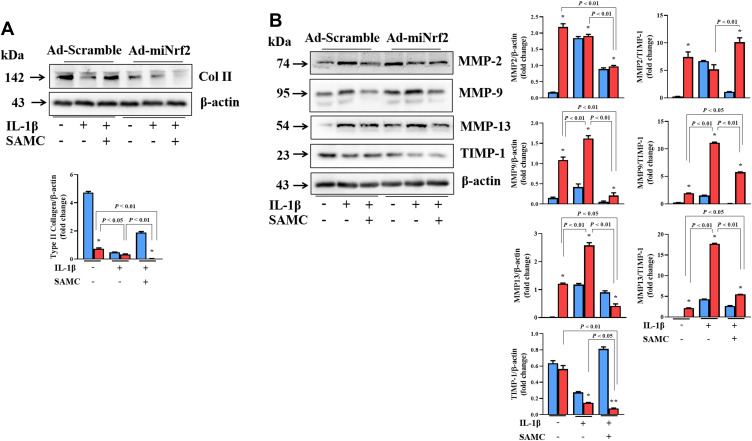
Col II showed a decreased IL-1β-induced OA model in chondrocytes, and SAMC treatment can reverse collagen destruction by up-regulating Col II expression. However, Nrf2 knockdown exaggerated the collagen destruction in IL-1β-induced OA chondrocytes, and this change cannot be attenuated by SAMC treatment (Figure 7A). In accordance with the Col II destruction, the MMP-2, MMP-9, and MMP-13 up-regulated in IL-1β-induced OA chondrocytes, with the negative regulator TIMP-1 decreased. Data analysis showed the MMPs/TIMP-1 ratio increased in the OA model, but SAMC treatment reversed the MMP/TIMP-1 ratio; interestingly, Nrf2 knockdown blocked the protection role of SAMC by increased MMP/TIMP-1 ratio, which functioning in collagen destruction (Figure 7B).
Figure 7.
Nrf2 deficiency influences the protecting effect of SAMC in chondrocytes matrix destruction in OA chondrocytes. Expression of miNrf2 on the effect of SAMC on Col II (A) expression and the MMPs (MMP-2, MMP-9, MMP-13) and TIMP-1 (B) expression in IL-1β-induced OA chondrocytes; also, MMPs/TIMP-1 ratios was calculated. *P<0.05, vs Ad-scramble. Experiments were performed in triplicate.
SAMC Plays a Protective Role in OA Chondrocytes Through NF-κB Regulated Inflammatory Pathway
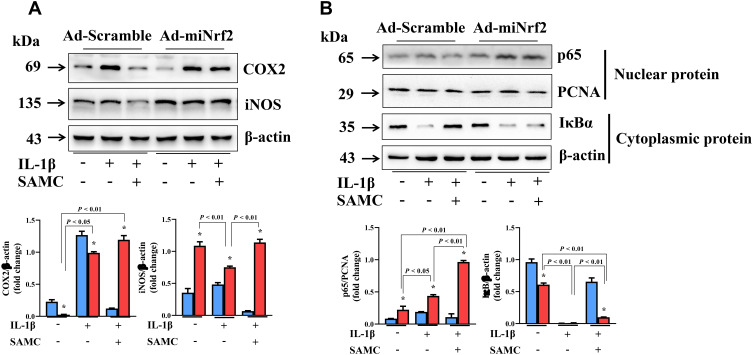
In order to determine the signaling pathway regulated by SAMC, we also examined inflammation response in IL-1β-induced chondrocytes. COX2 and iNOS expression were up-regulated after IL-1β stimulation, but attenuated by SAMC treatment; but the protective effect was inhibited in Nrf2-deficient OA chondrocytes (Figure 8A). Also, NF-κB activation can be inhibited by SAMC, but Nrf2 knockdown diminished the role of SAMC in IL-1β-induced OA chondrocytes (Figure 8B).
Figure 8.
SAMC targets Nrf2 to mediate NF-κB regulated inflammatory pathway in OA chondrocytes. (A) COX2 and iNOS expression were up-regulated after IL-1β stimulation, but attenuated by SAMC treatment; but the protective effect was inhibited in Nrf2-deficient OA chondrocytes. (B) Western Blot analysis of p65 in nuclear and IκBα in cytoplasm protein level. *P<0.05, vs Ad-scramble. Experiments were performed in triplicate.
Discussion
OA is a joint degeneration disease with cartilage degradation and abnormal bone growth. This pathologic process is accompanied with increased expression of proinflammatory cytokines, chemokines, and degradative enzymes. Furthermore, oxidative stress leads to hyper-peroxidation which exacerbates the degradative process. Whereas medicine for OA treatment is limited. In this study, we establish the therapeutic effect of SAMC in rat OA model, and explore the key role of NOX4/Nrf2 signaling pathway in OA progression both in vivo and in vitro.
Rats are one of the most studied animals in total meniscectomies, which cause destabilization of the joint leading to rapid degeneration and severe case of osteoarthritis.9 In this study, we established the OA model by ACLT combined medial meniscectomy of knee joint, to explore the effect of SAMC in OA rats after 4 weeks post-surgery. Radiography has been used to observe the joint surfaces of OA rats, which showed narrowing joint space and articular surface as indistinct because of bone structure degeneration.25 But the lesions were repaired at a certain degree in the SAMC-treated group.
H&E was used to observe the morphological changes in the articular cartilage of the knee. While SO staining showed better results in observation of the morphology of chondrocytes, the structure of cartilage layers, tide line, and the changes of subchondral bone.26 H&E and SO staining revealed cartilage and bony destruction in the articular surface in OA rats; however, the severity of articular destruction was attenuated in the SAMC group.
In OA, Col II cleavage firstly initiates around articular superficial chondrocytes; with progression, matrix degradation happens in the middle and deeper zones.27 Histologically, Col II was expressed extensively in the control group, with obvious pericellular staining. After OA-induction for 4 weeks, Col II showed a uniform distribution with less pericellular staining in the fibrillated area, superficial, and upper mid zone. After SAMC treatment, the immunoreactivity of Col II is significantly more pronounced in articular chondrocytes than the OA group, which is in concordance with the cartilage repair. The protein expression of chondrocytes also confirmed the Col II expression attenuation by SAMC treatment in a dose-dependent manner. These results supported that SAMC helps to stabilize cartilage matrix integrity.
The products of matrix degeneration of proteoglycans and type II collagen serve as biomarkers of OA progression.28 The levels of collagen II epitope C2C, CTX-II, and COMP in joint cavity fluid were examined in this study, to reflect the metabolism of type II collagen fibers. The OA-induction increased these biomarkers. According to the pathological and protein change in articular, SAMC reduces these products production.
MMPs are a family of neutral Zn2+ metalloproteinases, which have a key role in the joint destruction via the degradation of extracellular matrix components, including proteolytic cleavage of type II collagen and aggrecan. In humans, roles for MMP-2, MMP-9, MMP-13, MMP14, and MMP-16 have been characterized in some of the bone pathologies and skeletal syndromes.29 Collagenases MMP‑1 and MMP‑13 can degrade collagen type II in articular cartilage.30 The biological activities of MMPs are strictly controlled by TIMP‑1. The imbalance between MMPs and TIMPs is critical in the OA progression.16 Our study revealed that the MMP-2, MMP-9, and MMP-13 levels were elevated, while TIMP-1 level was down-regulated in rat OA cartilage; SAMC reversed these changes. By examination of the MMPs/TIMP-1 ratio, we can see the changes paralleled to the expression of MMP-2, MMP-9, and MMP-13 in cartilage.
It has been demonstrated that MMPs are synthesized by the chondrocytes and synovial cells in response to cytokines and growth factors.31 The inflammatory cytokines IL‑1β, TNF‑α, and IL‑6 play key roles in cartilage matrix destruction in OA pathogenesis.17 It has been demonstrated that IL‑1β can dysregulate the MMPs/TIMPs ratio, then destroy the matrix in articular cartilage with OA progression. OA-induction elevated TNF‑α, IL‑1β, and IL‑6 expression both in mRNA level in cartilage and protein level in joint cavity fluid, especially IL‑1β showed a much more significant change. SAMC can reduce these cytokines changes in a dose-dependent manner, which may indicate that SAMC established the joint stability by down-regulating inflammatory cytokines, then prevented over-production of MMPs.
IL-1β could induce the expression of two inducible enzymatic pathways (COX-2 and iNOS) involved in OA pathological change. Previous studies showed that inhibition of COX-2 and iNOS could attenuate the inflammatory response participating in the osteoarthritic cartilage destruction.19 In this study, we found that SAMC down-regulated the COX-2 and iNOS expression. NF-κB is presented in the cytoplasm in an inactivated status by IκB; under OA-induction, NF-κB is activated and translocated into the nucleus to initiate the expression of inflammatory mediators. NF-κB p65 inhibition could reduce the expression of genes of COX-2, iNOS, and MMP-9 in IL-1β-treated chondrocytes.32 Thus, we examined the NF-κB signaling pathway in SAMC treated OA-induction in the rat. The level of NF-κB p65 expression in nuclear protein was elevated with the IκB expression reduced in cytoplasmic protein; SAMC treatment can reverse the changes dose-dependently. The immunohistochemistry of p65 showed the same trend, which demonstrated that the NF-κB activation in OA and SAMC is a potent inhibitor of NF-κB.
The inflammatory cascade in OA cartilage has been indicated via NOX4. The inflammatory cytokines TNF-α, IL-1β, and IL-6 contribute to oxidative stress and degradation of the cartilage matrix. NOX4 is highly expressed in human OA articular cartilage, and has been identified to produce superoxide for the overexpression of metalloproteinases that directly contributes to bone resorption.7 In this study, we observed that the OA-induced NOX4 elevation has been diminished dose-dependently by SAMC. The excessive superoxide caused lipid peroxidation. The oxidative lipid metabolites, especially 4HNE, aggravate OA by upregulating inflammation.33 Our study demonstrated the antioxidant effects of SAMC in significantly decreasing 4HNE expression in articular cartilage.
Nrf2 is the key regulator of cellular redox homeostasis and inflammatory pathway, and has been shown to play a key role in OA pathophysiology.34 Physiologically, Nrf2 binds to Keap1 in an inactive status. After dissociating from Keap1, Nrf2 enters the nucleus and binds to antioxidant-responsive elements, resulting in the expression of several downstream genes, including cytoprotective and antioxidant genes, such as NQO1, heme oxygenase 1 (HO-1), GSH, thioredoxin, and SOD2; Phase II detoxification enzymes, such as glutathioneS-transferase (GST), and aldo-ketoreductases. Our data revealed that SAMC activated Nrf2 and NQO1 which may endure oxidative stress in OA-induction, thus protected articular cartilage damage from inflammatory response. Meanwhile, oxidative-redox status was determined by measuring T-AOC, SOD, CAT, GSH-Px, GSH, and GSSG; and lipid-oxidative byproducts MDA, all these data showed the antioxidant effect of SAMC.
To further validate the key role of Nrf2 in the protective effect of SAMC, we used adenovirus to knockdown Nrf2 expression in the IL-1β-induced rat chondrocytes osteoarthritis model. Under the same SAMC (60 mM) treatment condition, Nrf2 gene loss of function cannot rescue oxidative stress induced by inflammatory mediators; and NOX4 expression was further activated to form more lipid peroxidation metabolites. With the deteriorated oxidation, the MMPs/TIMP-1 ratio was elevated much more significantly, especially the MMP-9 and MMP-13/TIMP-1 ratios in Nrf2 gene knockdown OA-chondrocytes; and the Col II destruction worsened in the same trend. Even though SAMC exhibited an obvious ECM protective effect in IL-1β-treated chondrocytes, this effect was almost held back in Nrf2 knockdown chondrocytes. Finally, with the Nrf2 loss of function, COX2 and iNOS expression could not be inhibited by SAMC treatment in OA chondrocytes, indicating an aggravated inflammation response under IL-1β stimulation. Additionally, the NF-κB signaling pathway activated persistently after Nrf2 knockdown. Taken together, these studies indicated that the activation of Nrf2 is involved in the SAMC inhibition of IL-1β-induced expression of the inflammatory response in chondrocytes.
Conclusion
This study showed the protective role of SAMC both in vivo and in vitro OA model, and its effect is by decreasing production of inflammatory cytokines (TNF-α, IL-1β, and IL-6) and lipid peroxidation to reduce MMPs production, thus preventing the cartilage destruction in the OA process to a certain degree. The mechanism of SAMC may target Nrf2 through the NOX4/NF-κB signaling pathway. In conclusion, SAMC may serve as a novel natural agent for the treatment of OA, and provide a new clue for the therapeutic option.
Acknowledgments
SAMC was kindly provided by Professor Zhongxi Zhao of School of Pharmaceutical Sciences, Shandong University.
Funding Statement
This work was supported by National Natural Science Foundation of China (81800353), China Postdoctoral Science Foundation (2019M662369).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Reynard LN, Barter MJ. Osteoarthritis year in review 2019: genetics, genomics and epigenetics. Osteoarthritis Cartilage. 2020;28(3):275–284. doi: 10.1016/j.joca.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 2.Maly MR, Marriott KA, Chopp-Hurley JN. Osteoarthritis year in review 2019: rehabilitation and outcomes. Osteoarthritis Cartilage. 2020;28(3):249–266. doi: 10.1016/j.joca.2019.11.008 [DOI] [PubMed] [Google Scholar]
- 3.Barnett R. Osteoarthritis. Lancet. 2018;391(10134):1985. doi: 10.1016/S0140-6736(18)31064-X [DOI] [PubMed] [Google Scholar]
- 4.Zhu X, Jiang X, Li A, et al. S-allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients. 2017;9(2):166. doi: 10.3390/nu9020166 [DOI] [Google Scholar]
- 5.Li S, Yang G, Zhu X, et al. Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol Rep. 2017;38(3):1637–1644. doi: 10.3892/or.2017.5849 [DOI] [PubMed] [Google Scholar]
- 6.Yang G, Li S, Li B, et al. Protective effects of garlic-derived S-Allylmercaptocysteine on IL-1beta-stimulated chondrocytes by regulation of MMPs/TIMP-1 ratio and type II collagen expression via suppression of NF-kappaB pathway. Biomed Res Int. 2017;2017:8686207. doi: 10.1155/2017/8686207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao CR, Wang SN, Zhu SY, et al. Advanced oxidation protein products increase TNF-alpha and IL-1beta expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020;28:101306. doi: 10.1016/j.redox.2019.101306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecker L, Logsdon NJ, Kurundkar D, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231r247r. doi: 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra CI, Soler C. Animal models of osteoarthritis in small mammals. Vet Clin North Am Exot Anim Pract. 2019;22(2):211–221. doi: 10.1016/j.cvex.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Gerwin N, Bendele AM, Glasson S, et al. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–S34. doi: 10.1016/j.joca.2010.05.030 [DOI] [PubMed] [Google Scholar]
- 11.Li S, Wang W, Niu T, et al. Nrf2 deficiency exaggerates doxorubicin-induced cardiotoxicity and cardiac dysfunction. Oxid Med Cell Longev. 2014;2014:748524. doi: 10.1155/2014/748524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J. 2016;209:40–49. doi: 10.1016/j.tvjl.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 13.Kijowski R, Demehri S, Roemer F, et al. Osteoarthritis year in review 2019: imaging. Osteoarthritis Cartilage. 2019;27(3):401–411. [DOI] [PubMed] [Google Scholar]
- 14.Kumahashi N, Sward P, Larsson S, et al. Type II collagen C2C epitope in human synovial fluid and serum after knee injury–associations with molecular and structural markers of injury. Osteoarthritis Cartilage. 2015;23(9):1506–1512. doi: 10.1016/j.joca.2015.04.022 [DOI] [PubMed] [Google Scholar]
- 15.Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. doi: 10.1016/j.lfs.2019.116786 [DOI] [PubMed] [Google Scholar]
- 16.Ko JH, Kang YM, Yang JH, et al. Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy. Knee. 2019;26(2):317–329. doi: 10.1016/j.knee.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 17.Han PF, Wei L, Duan ZQ, et al. Contribution of IL-1beta, 6 and TNF-alpha to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int Immunopharmacol. 2018;65:212–220. [DOI] [PubMed] [Google Scholar]
- 18.Vincent TL. IL-1 in osteoarthritis: time for a critical review of the literature. F1000Res. 2019;8(F1000 Faculty Rev):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Z, Wang Y, Piao T, et al. Echinocystic acid inhibits IL-1beta-Induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation. 2016;39(2):543–549. doi: 10.1007/s10753-015-0278-y [DOI] [PubMed] [Google Scholar]
- 20.Choi MC, Jo J, Park J, et al. NF-kappaB signaling pathways in osteoarthritic cartilage destruction. Cells-Basel. 2019;8(7):734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed SM, Luo L, Namani A, et al. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Zuo R, Wang Y, Li J, et al. Rapamycin induced autophagy inhibits inflammation-mediated endplate degeneration by enhancing Nrf2/Keap1 signaling of cartilage endplate stem cells. Stem Cells. 2019;37(6):828–840. doi: 10.1002/stem.2999 [DOI] [PubMed] [Google Scholar]
- 23.Drevet S, Gavazzi G, Grange L, et al. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp Gerontol. 2018;111:107–117. doi: 10.1016/j.exger.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 24.Hui W, Young DA, Rowan AD, et al. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75(2):449–458. doi: 10.1136/annrheumdis-2014-206295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marenzana M, Hagen CK, Das NBP, et al. Visualization of small lesions in rat cartilage by means of laboratory-based x-ray phase contrast imaging. Phys Med Biol. 2012;57(24):8173–8184. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Wang Q, Yang M, et al. Histomorphology and innate immunity during the progression of osteoarthritis: does synovitis affect cartilage degradation? J Cell Physiol. 2018;233(2):1342–1358. [DOI] [PubMed] [Google Scholar]
- 27.Firner S, Zaucke F, Michael J, et al. Extracellular distribution of collagen II and perifibrillar adapter proteins in healthy and osteoarthritic human knee joint cartilage. J Histochem Cytochem. 2017;65(10):593–606. doi: 10.1369/0022155417729154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao HQ, Zhang JF, He QQ, et al. Cartilage oligomeric matrix protein, C-terminal cross-linking telopeptide of type II collagen, and matrix metalloproteinase-3 as biomarkers for knee and hip osteoarthritis (OA) diagnosis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2019;27(5):726–736. doi: 10.1016/j.joca.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 29.Paiva K, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci. 2017;148:203–303. [DOI] [PubMed] [Google Scholar]
- 30.Jackson MT, Moradi B, Smith MM, et al. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis Rheumatol. 2014;66(6):1525–1536. doi: 10.1002/art.38401 [DOI] [PubMed] [Google Scholar]
- 31.Nissinen L, Kahari VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840(8):2571–2580. doi: 10.1016/j.bbagen.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 32.Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage. 2006;14(4):367–376. [DOI] [PubMed] [Google Scholar]
- 33.Abusarah J, Bentz M, Benabdoune H, et al. An overview of the role of lipid peroxidation-derived 4-hydroxynonenal in osteoarthritis. Inflamm Res. 2017;66(8):637–651. doi: 10.1007/s00011-017-1044-4 [DOI] [PubMed] [Google Scholar]
- 34.Khan NM, Haseeb A, Ansari MY, et al. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301. doi: 10.1016/j.freeradbiomed.2017.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]