Abstract
Objective:
To explore the long-term outcomes of patients with clinically isolated syndromes from the Barcelona cohort.
Methods:
We selected patients with a follow-up longer than 10 years to (1) estimate the risks of multiple sclerosis (MS) and disability accumulation according to the baseline number of T2 lesions and to compare treated versus untreated patients and early versus delayed treatment, and (2) to study baseline features of patients with aggressive MS (Expanded Disability Status Scale (EDSS) ⩾6.0 at 10 years).
Results:
In all, 401 patients were included (mean follow-up of 14.4 (standard deviation of 2.9) years). A higher number of T2 lesions was associated with an earlier MS diagnosis and an earlier risk of irreversible disability. Early treatment was associated with a decreased risk of EDSS of 3.0: adjusted hazard ratio = 0.4, 95% confidence interval = (0.2, 0.7). Patients with aggressive MS differed in their baseline brain magnetic resonance images: The median (interquartile range) number of T2 lesions and contrast-enhancing lesions (CEL) was 71 (28–95) versus 7 (1–19) and 3 (1–24) versus 0 (0–1), respectively. The cut-offs that better classified patients with aggressive MS were 20 for T2 lesions and 2 for CEL.
Conclusion:
Although MS natural history is changing, a high lesion load at onset is helpful to identify patients at risk of presenting an aggressive MS.
Keywords: Clinically isolated syndromes, multiple sclerosis, MRI, prognosis, disease-modifying treatment, prediction
Introduction
Multiple sclerosis (MS) is a chronic disease. Classical natural history studies have shown that more than 50% of patients will develop severe disability after 15–30 years.1–4 However, when considering inception cohorts of patients seen from their very first attack, the rates of disability accumulation seem to be milder. In this sense, the clinically isolated syndromes (CIS) cohort from the National Hospital for Neurology and Neurosurgery, Queen Square, showed that only 25% had reached an Expanded Disability Status Scale (EDSS) of 6.0 after 20 years of follow-up.5 Despite these figures, there is still a subgroup of MS patients with an aggressive disease who develop severe disability early in the disease course. Although there is no established definition of aggressive MS, the early identification of this population would be of utmost importance for establishing an accurate and personalized treatment strategy.6
The aim of this study was to explore the long-term outcomes of CIS patients from the Barcelona inception cohort. For this objective, we selected patients with a follow-up longer than 10 years to (1) estimate the risks of MS and disability accumulation according to baseline T2 lesion number and to compare treated versus untreated patients and early treatment versus delayed treatment, and (2) study the prevalence and baseline features of patients with an aggressive MS phenotype, defined as patients with an EDSS ⩾6.0 at 10 years.
Patients and methods
Study data and inclusion criteria
This is a retrospective analysis based on a prospective and open CIS cohort initiated in 1995. The cohort includes patients younger than 50 years who presented with a CIS suggestive of MS within 3 months of our first assessment. At baseline, we recorded the demographics, CIS topography and disability according to the EDSS score. The EDSS score and the occurrence of relapses were evaluated every 3–6 months or annually depending on each patient’s characteristics. For the purpose of this analysis, the database was locked on 15 February 2016. For the main analysis of the study, we included patients with a CIS before 15 February 2006 and with at least 10 years of follow-up. IgG oligoclonal bands (OB) were examined within the first 3 months of disease onset via agarose isoelectric focusing combined with immunoblotting.7 OB were considered positive when at least two discrete bands were demonstrated in cerebrospinal fluid (CSF) only or when the CSF had at least two more bands than serum.
Since 1996, disease-modifying treatment (DMT) was offered to patients presenting with at least two attacks in the previous 3 years according to the Catalonian Regulatory Agency guidelines. After 2001, patients presenting with a high-risk CIS (defined as the fulfilment of three to four Barkhof criteria) were also candidates for treatment. Age and sex, date of the CIS, CIS topography, steroid treatment, date of the second attack, EDSS measurements, date of DMT initiation and date of the most recent visit were prospectively collected. The number and location of baseline T2 lesions, the presence and number of contrast-enhancing lesions (CEL) and the number of new T2 lesions were recorded during follow-up. For the purposes of this study, the number of T2 lesions at baseline was divided into four categories: 0, 1–3, 4–9 and 10 or more. In addition, a normal brain magnetic resonance imaging (MRI) was defined as displaying zero T2 lesions.
Finally, the patients’ clinical and MRI data were entered and updated on a regular basis. Quality controls were performed including a review of the data using the primary sources of 10 randomly selected patients every month. This study received approval from the local ethics committee, and all patients signed a written informed consent form.
MRI protocol
The baseline brain MRI was performed 3–5 months after the CIS and repeated at 12 months and every 5 years thereafter. Baseline spinal cord MRIs were systematically performed since 2007 and therefore were not considered in this study. Brain MRI was performed using a 1.5 or 3.0 T magnet and included the following sequences: transverse proton density and T2-weighted conventional or fast spin-echo, transverse and sagittal T2 FLAIR, and un-enhanced and contrast-enhanced (0.1–2.0 mmol/kg; scan delay, 5–10 minutes) T1-weighted spin-echo. All sequences were obtained using a contiguous 3–5 mm slice thickness covering the entire brain. Having a normal brain MRI was not an exclusion criterion, since patients were included based solely on their clinical features if they were suggestive of MS (i.e. patients with optic neuritis and normal baseline brain MRIs are included).
Definition of the outcomes
The 2017 McDonald criteria were applied to patients included after 2002 as lesion topography was not assessed individually before this date. For patients included from 1995 to 2002, the 2005 McDonald criteria were used.8,9 Clinically definite multiple sclerosis (CDMS) was established when new symptoms suggestive of a relapse occurred after an interval of at least 1 month.10 Disability was evaluated according to the EDSS at each visit during stability periods.11 The primary disability milestones were defined as reaching an EDSS score greater than or equal to 3.0 or 6.0 in two different evaluations. Finally, the follow-up duration was computed as the time elapsed between the date of the CIS and the date of the most recent visit.
Statistical analysis
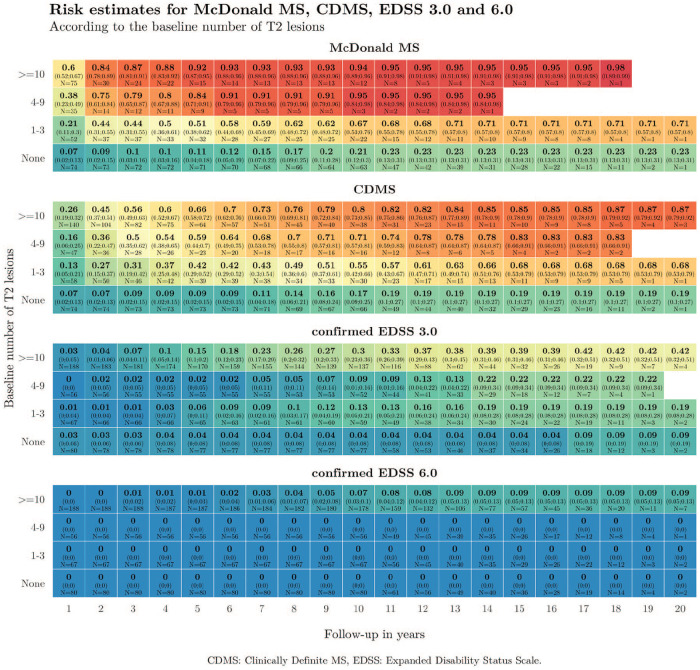
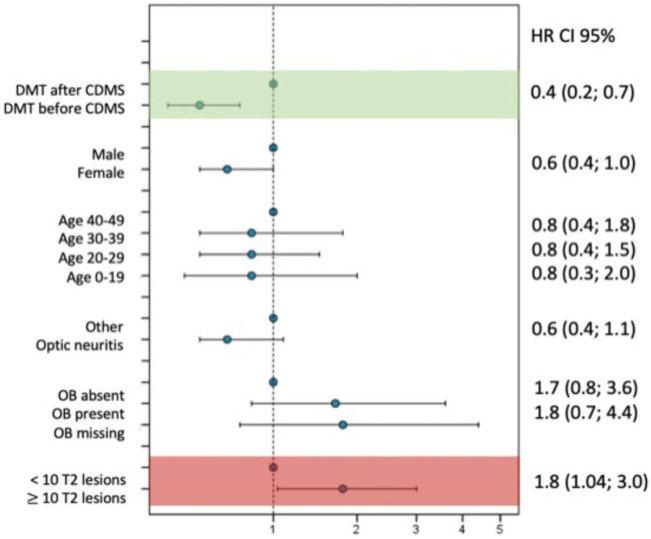
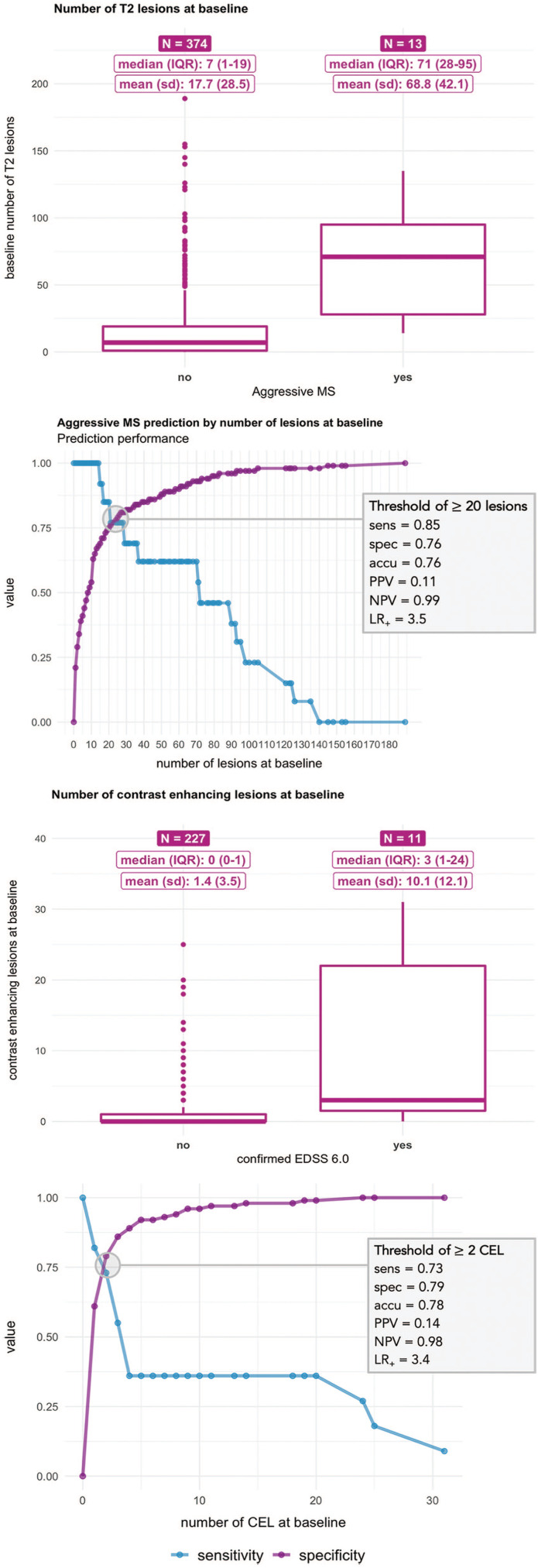
Kaplan–Meier risk estimates were obtained for the times to McDonald MS, CDMS and EDSS score of 3.0 and 6.0 according to the number of baseline T2 lesions. Instead of representing the resulting estimates using curves, we used heat maps to represent the proportion of patients achieving each of the four outcomes during follow-up. In the heat maps, columns represent follow-up time in years and rows indicate the number of baseline lesions. Thus, each cell of the map shows the proportion of patients (with its 95% confidence interval) fulfilling each outcome for each time point according to the number of baseline lesions. Heat maps offer the possibility of using colours as a scoring gradient that goes from blue (low risk) to red (high risk). The same range of colours has been used in the four heat maps to allow for an easier comparison of risks between outcomes (Figure 2). These estimates have been computed and represented until the last time point where an event or censoring happened. Thus, the heat map stops when the population under risk remained constant. Univariable and multivariable Cox proportional-hazards (PH) regressions were built for the progression to EDSS scores of 3.0 and 6.0. Covariates including age, sex, CIS topography, OB, baseline number of T2 lesions, MRI criteria and DMT status were considered. This last variable was first considered a time-dependent exposure and modelled later as an indicator of whether the DMT was initiated before or after the second attack. Possible interactions between age, sex, CIS topography, presence of OB, number of baseline T2 lesions and DMT were also evaluated (Figure 3). We defined the aggressive MS phenotype as reaching an EDSS ⩾6.0 at 10 years. We compared patients who presented this phenotype to those who did not in terms of sex, age at CIS, CIS topography, OB status, and number of T2 lesions and CEL at baseline (Figure 4). Next, we calculated the cut-off number of baseline T2 lesions and CEL that best predicted the development of an aggressive MS (Figure 4(b)). Both cut-offs were defined as those with the best balance between sensitivity and specificity rates. Finally, we combined both MRI variables to estimate the positive predictive values (PPV) of developing aggressive MS (Table 3).
Figure 2.
Heat maps showing the proportion of patients reaching McDonald MS, CDMS, and EDSS of 3.0 and 6.0 according to the number of baseline T2 lesions.
Figure 3.
Multivariable Cox model results for reaching an EDSS of 3.0 in treated patients: initiation of DMT before or after CDMS.
EDSS: Expanded Disability Status Scale; DMT: disease-modifying treatment; CDMS: clinically definite multiple sclerosis; OB: oligoclonal bands; HR: hazard ratio; CI: confidence interval.
Figure 4.
Baseline MRI characteristics and cut-offs obtained at baseline for aggressive and non-aggressive MS.
IQR: interquartile range; SD: standard deviation; EDSS: Expanded Disability Status Scale, sens: sensibility, spec: specificity, acc: accuracy, PPV: positive predictive value, NPV: negative predictive value; LR+: positive likelihood ratio.
Table 3.
Positive predictive values for developing an aggressive MS according to the baseline number of T2 and contrast-enhancing lesions.
| T2 baseline lesions | CEL | PPV (95% CI) |
|---|---|---|
| Unknown | Unknown | 4.7% (2.1%, 7.7%) |
| ⩾10 | Unknown | 8.0% (7.2%, 8.9%) |
| ⩾10 | ⩾1 | 12.7% (8.6%, 16.9%) |
| ⩾10 | ⩾2 | 16.3% (10.2%, 22.7%) |
| ⩾10 | ⩾5 | 18.2% (4.8%, 33.3%) |
| ⩾10 | ⩾10 | 36.4% (12.5%, 66.7%) |
| ⩾20 | Unknown | 11.5% (8.6%, 13.9%) |
| ⩾20 | ⩾1 | 15.8% (9.8%, 22.4%) |
| ⩾20 | ⩾2 | 18.9% (10.6%, 28.1%) |
| ⩾20 | ⩾5 | 22.2% (6.3%, 41.7%) |
| ⩾20 | ⩾10 | 40.0% (14.3%, 71.4%) |
MS: multiple sclerosis; CEL: contrast-enhancing lesions; PPV: positive predictive value; 95% CI: 95% confidence interval.
A p-value of 0.05 was considered statistically significant. All analyses were performed using SPSS 22.0 (SPSS Inc. Chicago, IL, USA) and R 3.5.1 (R Foundation for Statistical Computing).
Results
Study population
From January 1995 to 15 February 2016, 1207 patients were enrolled in the prospective CIS cohort; 70 (5.8%) were ultimately excluded for various reasons: previous attack (n = 13), age above 50 (n = 4), exceeded entry window (n = 26) and alternative diagnoses (n = 27). These alternative diagnoses included acute disseminated encephalomyelitis (n = 1), neuromyelitis optica spectrum disorder (n = 5), chronic relapsing inflammatory optic neuritis associated with anti-MOG antibodies (n = 1), brain tumour (n = 5), ischemic stroke (n = 2), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL; n = 1), anterior ischemic optic neuropathy (n = 3), Leber’s hereditary optic neuropathy (n = 1), central nervous system vasculitis (n = 1), atypical brainstem lesions (n = 2), alcoholic polyneuropathy with vitamin B deficiency (n = 1), musculoskeletal disorders (n = 2), unspecified sensory symptoms (n = 1) and unspecified ophthalmological condition (n = 1).
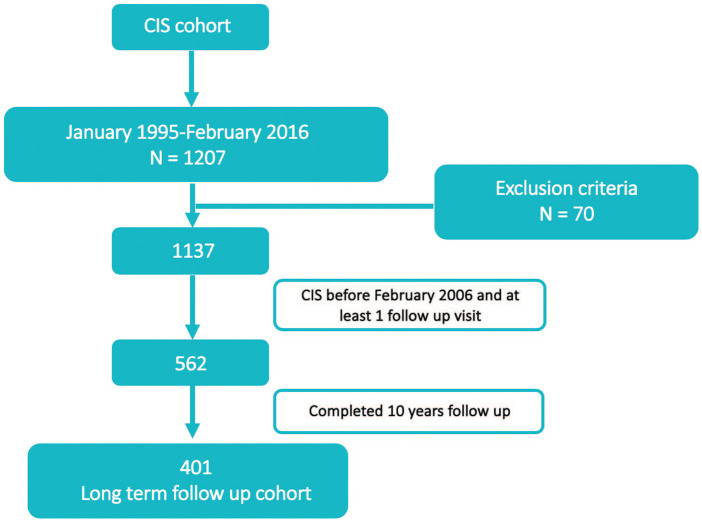
According to the database lock date, we identified 562 cases with a CIS before 15 February 2006 and at least one follow-up visit (Figure 1). Of these, 401 (71.3%) patients had a minimum follow-up of 10 years (Figure 1). Compared to these, patients with a shorter follow-up (n = 161, 28.6%) were similar in age but were more likely to have a normal baseline MRI and negative OB (Supplementary Table 1).
Figure 1.
Flowchart: patient’s disposition.
CIS: clinically isolated syndrome.
Ultimately, 401 patients were included in this analysis. Of these, one died during follow-up due to an acute myeloid leukaemia with a complex karyotype not related to MS treatment.
Table 1 shows the baseline characteristics of this long-term cohort: as expected, two out of three were females, the mean age at onset was 30 years and one-third presented with an optic neuritis. The mean clinical follow-up duration was 14.4 (standard deviation (SD) 2.9) years. Of the total cohort, 334 patients underwent a CSF tap, of whom 221 (66.2%) had positive OB. The baseline brain MRI was normal in 20.5% of the patients, almost half of the patients displayed 10 or more T2 lesions, and one-third showed at least one CEL. Out of the entire cohort, 190 (47.4%) patients received no DMT; 55 (13.7%) initiated a DMT prior to their second attack and 156 (38.9%) after their second attack.
Table 1.
Baseline characteristics of patients with at least 10 years of follow-up.
| Baseline clinical and demographic characteristics | n = 401 |
|---|---|
| Females: n (%) | 287 (71.6) |
| Mean (SD) age in years | 30.0 (8.1) |
| CIS topography: n (%) | |
| ON | 142 (35.4) |
| BS | 112 (27.9) |
| SC | 110 (27.4) |
| Other | 37 (9.2) |
| +OB: n (%) | 221/334 (66.2) |
| Baseline number of T2 lesions on brain and SC MRI: n (%) | |
| 0 | 80/391 (20.5) |
| 1–3 | 67/391 (17.1) |
| 4–9 | 56/391 (14.3) |
| ⩾10 | 188/391 (48.1) |
| CEL: n (%) | 97/239 (40.6) |
| Median (IQR) time of follow-up in years | 14.0 (11.9–16.7) |
SD: standard deviation; CIS: clinically isolated syndrome; ON: optic neuritis; BS: brainstem; SC: spinal cord; OB: oligoclonal bands; MRI: magnetic resonance imaging, CEL: contrast-enhancing lesions; IQR: interquartile range.
The median time from CIS onset to drug prescription was 23.5 months (interquartile range (IQR): 8.3–63.2).
Long-term outcomes: CDMS, McDonald MS, and EDSS of 3.0 and 6.0
Figure 2 shows the proportion of patients reaching each of the four outcomes according to the number of baseline T2 lesions. A higher number of T2 lesions was associated with an earlier second attack and an earlier McDonald MS diagnosis. In addition, the risk of irreversible disability was especially worrisome in patients with at least 10 baseline lesions: 30% of them reached an EDSS of 3.0, and 7% needed a cane before 10 years. These numbers rose to 39% and 9% within the first 15 years of the disease, respectively.
Long-term outcomes: treated versus not treated patients
Patients who were treated had clearly a more aggressive phenotype (Supplementary Table 2). It is worth highlighting that covariates were not balanced across treated and untreated groups. Thus, time-dependent variables could not control for this unbalance, and no further analyses comparing treated and untreated patients were performed.
Long-term outcomes in treated patients: DMT before or after the second attack
Table 2 compares the baseline characteristics of patients who received an early treatment (median time: 4 months) and patients starting treatment after the second attack (median time: 36 months). Although patients who received an early treatment had a higher number of T2 and CEL lesions at baseline, univariable and multivariable analyses showed that treatment before the second attack was associated with a decreased risk (adjusted hazard ratio (aHR) = 0.4 (0.2, 0.7)) of reaching an EDSS score of 3.0 (Figure 3). When analysing EDSS of 6.0 as the outcome, the hazards proportionality assumption was violated (p-value of 0.2 in the log-rank test), and therefore no further research on this outcome was performed.
Table 2.
Baseline characteristics of patients according to early versus delayed treatment.
| Baseline characteristics | DMT after CDMS (n = 156) | DMT before CDMS (n = 55) | p-value |
|---|---|---|---|
| Females: n (%) | 112 (71.8) | 39 (70.9) | 1 |
| Mean (SD) age in years | 28.3 (7.3) | 29.1 (7.7) | 0.553 |
| CIS topography: n (%) | |||
| ON | 47 (30.1) | 14 (25.5) | 0.320 |
| BS | 50 (32.1) | 16 (29.1) | |
| SC | 47 (30.1) | 16 (29.1) | |
| Other | 12 (7.7) | 9 (16.4) | |
| +OB: n (%) | 115/137 (83.9) | 32/38 (84.2) | 1.0 |
| Baseline T2 brain lesions: n (%) | |||
| 0 | 2/154 (1.3) | 0/52 (0) | 0.053 |
| 1–3 | 22/154 (14.3) | 2/52 (3.9) | |
| 4–9 | 29/154 (18.8) | 6/52 (11.5) | |
| ⩾10 | 101/154 (65.6) | 44/52 (84.6) | |
| CEL: n (%) | 50/110 (45.4) | 31/47 (66.0) | 0.029 |
| Median(IQR) time to start treatment in months | 36 (16–72) | 4 (1–6) | <0.001 |
| Median (IQR) time of follow-up in years | 14.6 (12.8–16.8) | 12.0 (11–12.9) | <0.001 |
| Outcomes | DMT after CDMS (n = 156) | DMT before CDMS (n = 55) | p-value |
| CDMS | 156 (100.0) | 40 (72.7) | < 0.001 |
| 2005 McDonald MS | 156 (100.0) | 43 (78.2) | < 0.001 |
| 2017 McDonald MS | 66/66 (100.0) | 43/46 (93.5) | 0.067 |
| Confirmed EDSS of 3.0 | 74 (47.4) | 11 (20.0) | < 0.001 |
| Confirmed EDSS of 6.0 | 11 (7.1) | 4 (7.3) | 1.0 |
DMT: disease-modifying treatment; CDMS: clinically definite multiple sclerosis; SD: standard deviation; CIS: clinically isolated syndrome; ON: optic nerve; BS: brainstem; SC: spinal cord; OB: oligoclonal bands; CEL: contrast-enhancing lesions; MS: multiple sclerosis; EDSS: Expanded Disability Status Scale. IQR: Interquartile range.
All tests are Fisher’s exact and chi-square tests, except for age (Student’s t-test) and follow-up time (Mann–Whitney test).
Aggressive MS: EDSS of 6.0 at 10 years
We compared the baseline characteristics of patients who presented with this aggressive phenotype to those who did not. There were no differences in sex (76.9% of aggressive MS vs 72.7% of non-aggressive MS, p = 1) and age at CIS (mean (SD) of 27.3 (5.5) vs 30.1 (8.2) years, p = 0.1). Positive OB were more common in patients with aggressive MS (90.0% vs 65.4%, p = 0.174), although these differences were not statistically significant. On the contrary, treatment was clearly more frequent on the aggressive cohort (92.3% vs 50.9%, p = 0.003). In terms of the MRI characteristics, all patients with aggressive MS presented 10 or more T2 lesions at baseline (100.0% vs 45.9%, p = 0.02) and more frequently presented at least one CEL (92.3% vs 50.9%, p = 0.003). Furthermore, the median (IQR) number of baseline T2 lesions was 71 (28–95) compared to 7 (1–19), and the median number of CEL was 3 (1–24) compared to 0 (0–1) (p < 0.0001 for both tests) (Figure 4).
In addition, presenting at least 20 T2 lesions at baseline was the cut-off that best discriminated patients with aggressive MS from those who did not present this phenotype, with a sensitivity of 0.85 and a specificity of 0.76. Performing the same analysis for baseline CEL, the best cut-off was at least two lesions, with a sensitivity of 0.73 and a specificity of 0.79 (Figure 4). Table 3 shows the PPVs for developing aggressive MS for several combinations of baseline T2 lesions and CEL. If MRI information was not considered, the risk of having aggressive MS for patients in our cohort was 4.7% (Table 3). For patients having more than 20 T2 lesions and more than 2 CEL, this probability increased to 19.0% (10.4%, 28.1%). The probability further increased to 40% for patients with more than 20 T2 lesions and more than 10 CEL (14.3%, 71.4%).
Discussion
Our study confirmed that when the baseline MRI is abnormal, a high proportion of patients with CIS presented a second attack in the long term. Moreover, almost all patients developed McDonald MS. The heat maps show that a higher number of lesions is associated with an earlier outcome. Less than 5% of our patients developed an aggressive MS (reaching an EDSS status of 6.0 at 10 years). It is worth highlighting that in patients with an aggressive phenotype, baseline characteristics such as sex, age and OB were not helpful in identifying them as patients at risk. Nevertheless, a higher proportion of OB, a lower proportion of optic neuritis and a higher proportion of spinal cord CIS were observed. Comparing patients with an aggressive phenotype to those with a more standard evolution, the former mainly differed in their baseline brain MRIs: the median number of baseline T2 lesion was 71 (28–95) compared to 7 (1–19), and the median for CEL was 3 (1–24) compared to 0 (0–1). The cut-offs that better classified patients with aggressive MS were 20 for T2 lesions and 2 for CEL. It is important to note that both the number and activity of the lesions seem to contribute to better predict patients with an aggressive MS phenotype. Also, we would like to highlight that there is no consensus on the definition of aggressive MS, and specific guidelines for its treatment are also lacking.
A high proportion of our patients received DMT during their follow-up, but for the majority of them, there was a long time between the first clinical event and treatment initiation. These data reflect our clinical practice two decades ago when the concept of early treatment was in its infancy. Treated patients had a more aggressive disease phenotype than untreated patients, and for this reason we were unable to match both populations. In our study, after excluding non-treated patients, it appeared that treatment before the second attack could decrease the risk of disability accumulation, highlighting the importance of early treatment. As a rule, patients were treated with first-line treatments. This study is in line with our previous work analysing our whole cohort of 1058 CIS patients followed up for a mean of 6.5 years, in which we showed that MRI features (number and topography of T2 lesions) were the main prognostic factor at disease onset and that initiation of a DMT before a second attack could reduce the risk of disability accumulation.12 Focusing on patients with more than 10 T2 lesions at baseline, compared to the London cohort, we found similar rates of patients presenting a second attack (80% vs 85% at 10 years).13,14 Although a small proportion of our patients started DMT before their second attack (n = 55), compared to the largely untreated London CIS cohort, these similar rates of conversion probably reflect the fact that first-line DMTs (mainly interferons and glatiramer acetate in our cohort) only delay the occurrence of second attack in the short to medium term. Conversely, in patients with at least 10 baseline T2 lesions, we found lower rates of disability accumulation than the London cohort (30% vs 75% for EDSS of 3.0 and 7% vs 35% for EDSS of 6.0 at 10 years). Our rates of disability are closer to other treated cohorts: The Betaferon® / Betaseron® in Newly Emerging Multiple Sclerosis for Initial Treatment (BENEFIT) cohort reported 30% of patients with an EDSS of 3.0 and 6% with an EDSS of 6.0 after an 11-year follow-up.15 This is also in line with the expression/genomics, proteomics, imaging, and clinical (EPIC) cohort, a prospective single-centre cohort including actively managed CIS and early MS patients which showed 4.7% of patients reaching an EDSS of 6.0 after 10 years.16 Taking all together, and considering other important factors such as the Will Rogers phenomenon derived from diagnostic criteria modifications and improvement in general health among others, our data seem to confirm that natural history is changing in the treated era. Others have addressed the impact of first-line treatments on long-term disability in relapsing MS patients.17,18 This protective effect is modest in patients treated with first-line drugs, with an estimated prevention of 1.0 EDSS point increase for every 11.6 years of interferon-beta/glatiramer acetate exposure.19
Limitations of our study include the following aspects: MRI criteria to define MS have changed overtime, and therefore not all patients had the required information to apply the 2017 McDonald criteria. To overcome this limitation, a combination of 2005 and 2017 criteria has been used. Another concern relates to the lack of spinal cord imaging in this analysis. Unfortunately, this limitation reflects our practice at a time where spinal cord MRI was only performed in patients presenting with a myelitis. The treatments that were available at that time were mainly interferons and glatiramer acetate. The use of other treatments was restricted to exceptional cases. Again, only an intention-to-treat analysis has been performed taking into consideration the date of initiation of DMT. However, time on treatment or changes among preparations have not been evaluated. We also acknowledge that this is a culturally and genetically homogeneous cohort, as non-Caucasian people were an exception in our setting. We are also aware that the conclusions referring to patients with aggressive MS are based on a limited number of patients. This could have limited the possibility of finding statistically significant differences in other variables such as OB. However, this limitation also mirrors the exceptionality of this end point.
The data from the long-term Barcelona cohort confirm that patients with a normal baseline MRI have a low risk of developing MS after 10 or 15 years of follow-up. Conversely, patients with an abnormal MRI have a very high risk of developing further attacks or McDonald MS. In line with other cohort studies, we showed that early treatment seems to prevent disability accrual. Our long-term study confirms that MS natural history has changed and that aggressive MS, defined as reaching an EDSS of 6.0 at 10 years, is infrequent in the treatment era. A high lesion load (more than 20 T2 lesions and more than 2 CEL) at onset was helpful to identify patients at risk of an aggressive MS.20
Supplemental Material
Supplemental material, MSJ877810_supplementary_table_1 for The long-term outcomes of CIS patients in the Barcelona inception cohort: Looking back to recognize aggressive MS by Mar Tintore, Georgina Arrambide, Susana Otero-Romero, Pere Carbonell-Mirabent, Jordi Río, Carmen Tur, Manuel Comabella, Carlos Nos, María Jesús Arévalo, Elisenda Anglada, Rebeca Menendez, Luciana Midaglia, Ingrid Galán, Angela Vidal-Jordana, Joaquin Castilló, Patricia Mulero, Ana Zabalza, Breogan Rodríguez-Acevedo, Marta Rodriguez, Carmen Espejo, Joao Sequeira, Raquel Mitjana, Andrea de Barros, Deborah Pareto, Cristina Auger, Santiago Pérez-Hoyos, Jaume Sastre-Garriga, Alex Rovira and Xavier Montalban in Multiple Sclerosis Journal
Supplemental material, MSJ877810_supplementary_table_2 for The long-term outcomes of CIS patients in the Barcelona inception cohort: Looking back to recognize aggressive MS by Mar Tintore, Georgina Arrambide, Susana Otero-Romero, Pere Carbonell-Mirabent, Jordi Río, Carmen Tur, Manuel Comabella, Carlos Nos, María Jesús Arévalo, Elisenda Anglada, Rebeca Menendez, Luciana Midaglia, Ingrid Galán, Angela Vidal-Jordana, Joaquin Castilló, Patricia Mulero, Ana Zabalza, Breogan Rodríguez-Acevedo, Marta Rodriguez, Carmen Espejo, Joao Sequeira, Raquel Mitjana, Andrea de Barros, Deborah Pareto, Cristina Auger, Santiago Pérez-Hoyos, Jaume Sastre-Garriga, Alex Rovira and Xavier Montalban in Multiple Sclerosis Journal
Acknowledgments
We thank Josep Graells, Rosalia Horno, Miquel Angel Robles, Clara Oriol and Milagros Fraga for their continuous support. We also wish to thank our multiple sclerosis patients for their continuous enthusiasm in participating in clinical research studies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.T. has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis and Teva Pharmaceuticals. M.T. is co-editor of Multiple Sclerosis Journal – Experimental, Translational and Clinical. G.A. has received compensation for consulting services or participation in advisory boards from Biogen-Idec, Sanofi and Merck; research support from Novartis; travel expenses for scientific meetings from Novartis, Roche and Stendhal; and speaking honoraria from Sanofi and Merck. S.O.-R. has received compensation for consulting services from Biogen-Idec and Genzyme, and research support from Novartis. P.C. has received travel expenses from Biogen. P.C.’s yearly salary is supported by a grant from Biogen to Fundacio privada Cemcat towards statistical analysis. J.R. has received speaking honoraria and personal compensation for participating on Advisory Boards from Almirall, Bayer Schering Healthcare, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Teva and Sanofi-Aventis. C.T. has received a post-doctoral research ECTRIMS fellowship (2015). C.T. has received honoraria and support for travelling from Novartis, Teva Pharmaceuticals, Ismar Healthcare and F. Hoffmann-La Roche, Ltd. M.C. has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merk Serono, Biogen-Idec, Teva Pharmaceuticals, Sanofi-Aventis and Novartis. C.N. has received funding for travel from Biogen-Idec and F. Hoffmann-La Roche, Ltd., and speaker honoraria from Novartis. A.V.-J. receives support for contracts Juan Rodes (JR16/00024) from Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Spain, and has received speaking honoraria and travel expenses from Novartis, Roche, Teva, Biogen and Genzyme-Sanofi. D.P. has received speaking honoraria from Novartis and Biogen. C.A. has received speaking honoraria from Novartis, Biogen and Stendhal. J.S.-G. has received compensation for participating on Advisory Boards, speaking honoraria and travel expenses for scientific meetings, consulting services or research support from Celgene, Novartis, Biogen, Teva, Merck, Almirall and Genzyme. A.R. serves on scientific advisory boards for Biogen-Idec, Novartis, Genzyme, Bayer and OLEA Medical, and on the editorial board of the American Journal of Neuroradiology; he has received speaker honoraria from Bayer, Genzyme, Sanofi-Aventis, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd, OLEA Medical, Novartis and Biogen-Idec and has research agreements with Siemens AG. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past with Actelion, Almirall, Bayer, Biogen, Celgene, Genzyme, Hoffmann-La Roche, Novartis, Oryzon Genomics, Sanofi-Genzyme and Teva Pharmaceutical. M.J.A., L.M., I.G., C.E., J.C., PM., B.R.-A., S.P.-H., R.M., M.R., E.A., R.M., A.Z. and A.d.B. report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been funded by European Regional Development Fund and co-funded by Instituto Carlos III through the projects PI15/00170, granted to M.T. and PI14/01439 granted to X.M. It has also received support by a grant from Genzyme foundation (GENZYME-2015-01) granted to M.T. and from the ‘Red Española de Esclerosis Múltiple (REEM)’, which is sponsored by FIS, the Instituto de Salud Carlos III, the Ministry of Economy and Competitiveness in Spain, and the ‘Ajuts per donar Suport als Grups de Recerca de Catalunya’, which is sponsored by the ‘Agència de Gestió d’Ajuts Universitaris i de Recerca’ (AGAUR) of the Generalitat de Catalunya in Spain. This work is independent of all the funding bodies, which have played no part in any of its stages (design, data analysis or manuscript writing).
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mar Tintore, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Georgina Arrambide, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Susana Otero-Romero, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain/ Department Preventive Medicine and Epidemiology, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Pere Carbonell-Mirabent, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Jordi Río, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Carmen Tur, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain/Queen Square MS Centre, Department of Neuroinflammation, UCL Queen Square Institute of Neurology, University College London, London, UK/Luton and Dunstable University Hospital, University College London, London, UK.
Manuel Comabella, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Carlos Nos, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
María Jesús Arévalo, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Elisenda Anglada, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Rebeca Menendez, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Luciana Midaglia, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Ingrid Galán, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Angela Vidal-Jordana, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Joaquin Castilló, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Patricia Mulero, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Ana Zabalza, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Breogan Rodríguez-Acevedo, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Marta Rodriguez, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Carmen Espejo, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Joao Sequeira, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Raquel Mitjana, Section of Neuroradiology and Magnetic Resonance Unit, Department of Radiology (IDI), Vall d’Hebron Institut de Recerca, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Andrea de Barros, Section of Neuroradiology and Magnetic Resonance Unit, Department of Radiology (IDI), Vall d’Hebron Institut de Recerca, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Deborah Pareto, Section of Neuroradiology and Magnetic Resonance Unit, Department of Radiology (IDI), Vall d’Hebron Institut de Recerca, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Cristina Auger, Section of Neuroradiology and Magnetic Resonance Unit, Department of Radiology (IDI), Vall d’Hebron Institut de Recerca, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Santiago Pérez-Hoyos, Unitat d’Estadística i Bioinformatica, Vall d’Hebron Institut de Recerca (VHIR), Universitat Autònoma de Barcelona, Barcelona, Spain.
Jaume Sastre-Garriga, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Alex Rovira, Section of Neuroradiology and Magnetic Resonance Unit, Department of Radiology (IDI), Vall d’Hebron Institut de Recerca, Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Xavier Montalban, Centre d’Esclerosi Múltiple de Catalunya (Cemcat), Servei de Neurologia/Neuroimmunologia, Vall d’Hebron Institut de Recerca (VHIR), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain/Division of Neurology, University of Toronto, St Michael’s Hospital, Toronto, ON, Canada.
References
- 1. Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000; 343(20): 1430–1438. [DOI] [PubMed] [Google Scholar]
- 2. Pittock SJ, Mayr WT, McClelland RL, et al. Change in MS-related disability in a population-based cohort: A 10-year follow-up study. Neurology 2004; 62(1): 51–59. [DOI] [PubMed] [Google Scholar]
- 3. Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006; 66(2): 172–177. [DOI] [PubMed] [Google Scholar]
- 4. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: A geographically based study. I. Clinical Course and Disability. Brain 1989; 112(Pt1): 133–146. [DOI] [PubMed] [Google Scholar]
- 5. Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008; 131(Pt3): 808–817. [DOI] [PubMed] [Google Scholar]
- 6. Rush CA, MacLean HJ, Freedman MS. Aggressive multiple sclerosis: Proposed definition and treatment algorithm. Nat Rev Neurol 2015; 11(7): 379–389. [DOI] [PubMed] [Google Scholar]
- 7. Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch Neurol 2005; 62(6): 865–870. [DOI] [PubMed] [Google Scholar]
- 8. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58(6): 840–846. [DOI] [PubMed] [Google Scholar]
- 9. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol 1983; 13(3): 227–231. [DOI] [PubMed] [Google Scholar]
- 11. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 12. Tintore M, Rovira A, Rio J, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 2015; 138(Pt7): 1863-1874. [DOI] [PubMed] [Google Scholar]
- 13. Brex PA, Ciccarelli O, O’Riordan JI, et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med 2002; 346(3): 158–164. [DOI] [PubMed] [Google Scholar]
- 14. O’Riordan JI, Thompson AJ, Kingsley DP, et al. The prognostic value of brain MRI in clinically isolated syndromes of the CNS. Brain 1998; 121(Pt3): 495–503. [DOI] [PubMed] [Google Scholar]
- 15. Kappos L, Edan G, Freedman MS, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology 2016; 87(10): 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cree BA, Gourraud PA, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80(4): 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tedeholm H, Skoog B, Lisovskaja V, et al. The outcome spectrum of multiple sclerosis: Disability, mortality, and a cluster of predictors from onset. J Neurol 2015; 262(5): 1148–1163. [DOI] [PubMed] [Google Scholar]
- 18. Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of disability worsening in clinically isolated syndrome. Ann Clin Transl Neurol 2015; 2(5): 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol 2016; 80(1): 89–100. [DOI] [PubMed] [Google Scholar]
- 20. Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis – insights from real-world observational studies. Nat Rev Neurol 2017; 13(2): 105–118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ877810_supplementary_table_1 for The long-term outcomes of CIS patients in the Barcelona inception cohort: Looking back to recognize aggressive MS by Mar Tintore, Georgina Arrambide, Susana Otero-Romero, Pere Carbonell-Mirabent, Jordi Río, Carmen Tur, Manuel Comabella, Carlos Nos, María Jesús Arévalo, Elisenda Anglada, Rebeca Menendez, Luciana Midaglia, Ingrid Galán, Angela Vidal-Jordana, Joaquin Castilló, Patricia Mulero, Ana Zabalza, Breogan Rodríguez-Acevedo, Marta Rodriguez, Carmen Espejo, Joao Sequeira, Raquel Mitjana, Andrea de Barros, Deborah Pareto, Cristina Auger, Santiago Pérez-Hoyos, Jaume Sastre-Garriga, Alex Rovira and Xavier Montalban in Multiple Sclerosis Journal
Supplemental material, MSJ877810_supplementary_table_2 for The long-term outcomes of CIS patients in the Barcelona inception cohort: Looking back to recognize aggressive MS by Mar Tintore, Georgina Arrambide, Susana Otero-Romero, Pere Carbonell-Mirabent, Jordi Río, Carmen Tur, Manuel Comabella, Carlos Nos, María Jesús Arévalo, Elisenda Anglada, Rebeca Menendez, Luciana Midaglia, Ingrid Galán, Angela Vidal-Jordana, Joaquin Castilló, Patricia Mulero, Ana Zabalza, Breogan Rodríguez-Acevedo, Marta Rodriguez, Carmen Espejo, Joao Sequeira, Raquel Mitjana, Andrea de Barros, Deborah Pareto, Cristina Auger, Santiago Pérez-Hoyos, Jaume Sastre-Garriga, Alex Rovira and Xavier Montalban in Multiple Sclerosis Journal