Abstract
Background:
Neurofilament light chain (NfL) is a promising marker of disease activity/treatment response in multiple sclerosis (MS), although its predictive value for long-term clinical outcomes remains unclear.
Objective:
We measured NfL from a phase 3 trial in relapsing-remitting MS and investigated its association with outcomes after 8 and 15 years.
Methods:
NfL concentrations were measured by single molecule array assay in cerebrospinal fluid (CSF) from MS patients (n = 235) in a 2-year randomized clinical trial (RCT) of intramuscular interferon β-1a, and in serum (n = 164) from the extension study.
Results:
Year 2 CSF and Year 3 serum NfL were associated with brain parenchymal fraction (BPF) change over 8 years (p < 0.0001, r = −0.46; p < 0.05. r = −0.36, respectively) and were predictive of reaching Expanded Disability Status Scale (EDSS) ⩾ 6.0 at Year 8 (odds ratio (OR) (upper vs lower tertile) = 3.4; 95% confidence interval (CI) = 1.2–9.9, p < 0.05; OR = 11.0, 95% CI = 2.0–114.6; p < 0.01, respectively). Serum NfL concentration (Year 4) was predictive of reaching EDSS score ⩾6.0 at 15 years (OR (upper vs lower tertile) = 4.9; 95% CI = 1.4–20.4; p < 0.05). NfL concentrations were complementary to 2-year BPF change in predicting long-term outcomes.
Conclusion:
Serum and CSF NfL concentrations were associated with long-term clinical outcomes in MS patients and are promising biomarkers for disease severity stratification supporting treatment decisions.
Keywords: Neurofilament, multiple sclerosis, MRI
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated disease with large heterogeneity in clinical presentation and course. The unpredictable course of MS on an individual level complicates treatment choices—in particular, deciding whether to escalate therapy over time or implement early aggressive induction therapy. For this reason, there is a clinical need for biomarkers that are able to identify underlying disease severity and predict long-term outcomes.1,2
Neurofilament light chain (NfL) has been proposed as a biomarker in MS.2–4 NfL is a scaffolding protein of the neuronal cytoskeleton5 and is released into the extracellular space following neuronal damage. Due to its exclusive expression in neurons, NfL offers excellent specificity for neuroaxonal damage.6,7 Concentrations of NfL have been reported to be increased in the cerebrospinal fluid (CSF) of patients with MS, with concentrations rising during relapse and associated with magnetic resonance imaging (MRI) lesion load and disability scores.8–11 The recently developed single molecule array NfL assay allows accurate quantification of NfL in serum. Higher serum NfL concentrations, as measured by this assay, are associated with increased clinical and MRI disease activity, increased risk of relapses, worsening Expanded Disability Status Scale (EDSS) scores, and more severe brain and spinal cord volume loss.11,12
The pivotal randomized clinical trial (RCT) of intramuscular (IM) interferon (IFN) β-1a (Avonex, Biogen, Cambridge, MA, USA) for relapsing-remitting MS (RRMS), conducted by the Multiple Sclerosis Collaborative Research Group (MSCRG), demonstrated that EDSS score worsening and relapse incidence were reduced as compared with placebo-treated patients.13 After 8 years of follow-up, fewer patients originally randomized to receive IM IFNβ-1a had reached an EDSS score of 6.0 compared with patients originally randomized to receive placebo.14 After 15 years, those patients who were still being treated with IM IFNβ-1a had significantly lower EDSS scores and better quality of life outcomes.15 The follow-up studies confirmed the predictive value of early brain atrophy, new lesions on MRI, and clinical disease activity observed during the 2-year study16,17
The aim of this study was to evaluate the potential utility of serum and CSF NfL concentrations as prognostic biomarkers of long-term disease outcomes in patients with MS, using samples and well-characterized clinical data from the phase 3 study of IM IFNβ-1a in RRMS13 and the subsequent 8- and 15-year long-term extension studies.14,15,17
Methods
Patients
This study used samples and data from the MSCRG trial and its long-term follow-up extensions studies (Figure 1). The MSCRG trial was a 2-year, randomized, double-blind, placebo-controlled, phase 3 RCT of IM IFNβ-1a. Details of the study methodology are published elsewhere.13 Briefly, 301 patients with RRMS were enrolled at four clinical sites in the United States. Clinical measures during the study included EDSS scoring and Multiple Sclerosis Functional Composite. Radiological assessments included T2 hyperintense and T1 hypointense lesion volume, and gadolinium-enhancing (Gd+) lesion number and volume. The number of new and enlarging T2 lesions at Year 2, brain parenchymal fraction (BPF), and BPF change over 2 years also were determined.18–20
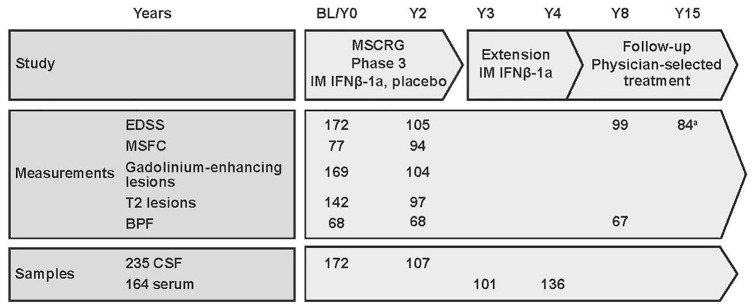
Figure 1.
Schematic diagram of the study and available data points and samples. Values indicate the number of available data points or samples at each time point. CSF samples were available from 235 patients: 172 at baseline and 107 at Year 2; 44 had CSF samples available from Year 0 to Year 2. Serum samples were available from 164 patients: 101 at Year 3 and 136 at Year 4; 73 had serum samples available from Year 3 to Year 4.
BL: baseline; BPF: brain parenchymal fraction; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; IFN: interferon; IM: intramuscular; MSCRG: Multiple Sclerosis Collaborative Research Group; MSFC: Multiple Sclerosis Functional Composite; Y: year.
aSelf-reported.
Patients who completed the RCT were eligible for the long-term follow-up studies, as described.14–17 Eight-year follow-up assessments included EDSS scores, relapse, treatment history, Multiple Sclerosis Functional Composite, and BPF. At 15 years of follow-up, patients completed a questionnaire that included a self-reported EDSS score, among other scales.15
Samples
CSF samples were collected at baseline and 2 years after enrolling into the study. Samples were stored at −70°C. A total of 235 patients from the original study had CSF samples available; of these, 172 had samples available at baseline (Year 0) and 107 had samples at Year 2 (Figure 1). Forty-four patients had samples from both Year 0 and Year 2.
Serum samples were available from 164 patients, of whom 101 and 136, respectively, had samples collected at Year 3 and Year 4. Serum samples from both time points were available for 73 patients. All serum samples were stored at −70ºC prior to being used for testing. No serum samples from Year 0 or Year 2 were available, and CSF sample collection at Year 3 or Year 4 was not part of the trial protocol.
Neurofilament assay
A single molecule array assay (Quanterix Corporation, Lexington, MA) was used to measure NfL levels as described.12 The mean intra-assay coefficients of variation were shown to be within 6% for both CSF and serum. Inter-assay coefficients of variations for both serum and CSF were within 12%.
Statistical analysis
Continuous variables were described by mean and standard deviation, except NfL that is described by median and interquartile ranges; categorical variables by counts and percentages. Correlations between longitudinal NfL concentrations and their associations with clinical and radiological assessments were calculated using Spearman’s rank correlation coefficients and analysis of variance (F test). The association of NfL concentration (separated into tertiles by NfL concentration) with cumulative incidence of progression to an EDSS score of 6.0 was conducted using Fisher’s exact test and Wald chi-square test based on Firth’s penalized univariate logistic regression. To explore the potential additive value of NfL concentration and brain atrophy, patients with both NfL concentrations and brain atrophy data were divided into four groups based on their CSF or serum NfL and BPF 2-year change values (below or above median for both serum NfL and BPF change). Mean NfL concentrations (mean of two time points when both measurements were available, and single value when only one measurement was available) were used for stratification to increase the number of measurements. Analyses were adjusted for age, and the results were similar between the analyses with and without adjustment for age. Therefore, we presented results not adjusted for age. All analyses were performed using R statistical software (R Foundation for Statistical Computing) and SAS version 9.4 (SAS Institute Inc.) on an HP-UX platform (Hewlett Packard). GraphPad Prism 7 software (GraphPad Software, Inc.) was used for graphical representation.
Results
Baseline characteristics
Demographic and clinical characteristics of patients with CSF (n = 235) and serum NfL assessments (n = 164) were similar to those in the overall RCT population (Table 1). Demographic and clinical characteristics by NfL tertile concentration are provided in Supplementary eTable 1.
Table 1.
Baseline characteristics.
| Variable | MSCRG cohort (N = 301) | Patients with CSF NfL (N = 235) | Patients with serum NfL (N = 164) |
|---|---|---|---|
| Placebo, n (%) | 143 (48) | 111 (47) | 84 (51) |
| IM IFNβ-1a, n (%) | 158 (52) | 124 (53) | 80 (49) |
| Age, mean (SD), years | 36.8 (7.4) | 36.7 (7.3) | 37.2 (7.5) |
| Female, n (%) | 221 (73) | 170 (72) | 120 (73) |
| Disease duration, mean (SD), years | 6.5 (5.8) | 6.6 (5.9) | 6.5 (6.0) |
| EDSS score, mean (SD) | 2.3 (0.8) | 2.4 (0.8) | 2.3 (0.8) |
| MSFC score, mean (SD) [n]a | 0.00 (0.71) [171] | 0.01 (0.70) [140] | 0.13 (0.56) [93] |
| Gd+ lesions, mean (SD) [n]a | 2.8 (6.1) [273] | 2.9 (6.3) [232] | 2.8 (6.9) [150] |
| Patients with Gd+ lesions at Year 0, na (%) | 145/273 (53) | 123/232 (53) | 77/150 (51) |
| T2 lesion volume, mean (SD) [n], mm3 | 12,993 (13,767) [271] | 12,770 (14,157) [232] | 12,148 (13,902) [151] |
| BPF, mean (SD) [n] | 0.830 (0.017) [137] | 0.830 (0.018) [114] | 0.831 (0.015) [82] |
MSCRG: Multiple Sclerosis Collaborative Research Group; CSF: cerebrospinal fluid; NfL: neurofilament light chain; IM: intramuscular; IFN: interferon; SD: standard deviation; EDSS: Expanded Disability Status Scale; MSFC: Multiple Sclerosis Functional Composite; Gd+: gadolinium-enhancing; BPF: brain parenchymal fraction.
Patients from the randomized clinical trial data are presented.
If different from the total n in the group.
CSF NfL concentrations during the RCT and serum NfL concentrations at Year 3 and Year 4
For 44 patients with available CSF samples at both time points, median (range) NfL concentrations were 1906 pg/mL (993–5669) at Year 0 and 1654 pg/mL (899–3110) at Year 2 with a positive correlation between the two measurements (r = 0.65; p < 0.001). No significant difference in CSF NfL change from Year 0 to Year 2 was observed when comparing patients in the placebo arm CSF NfL change (median % (interquartile range): −5.1% (−38.9% to 37.6%); n = 18) with the IM IFNβ-1a arms (−29.0% (−53.5% to 19.4%); n = 26; p = 0.68), respectively. The median (range) serum NfL concentrations at Year 3 and Year 4 were 34.5 pg/mL (24.6–55.9) and 34.8 pg/mL (25.3–54.4), respectively. A positive correlation between serum NfL at Year 3 and Year 4 was observed (r = 0.62; p < 0.00001; n = 73).
Association between CSF NfL concentrations and disease activity during 2 years of the RCT
Consistent with previously published data, CSF NfL concentrations at baseline were associated with baseline Gd+ lesion number (r = 0.46; p < 0.0001) and volume (r = 0.47; p < 0.001), as well as T2 lesion volume (r = 0.36; p < 0.0001; Supplementary eTable 2). Similarly, CSF NfL concentration at Year 2 was associated with several clinical and MRI parameters at Year 2 (Supplementary eTable 2). Baseline CSF NfL concentration also correlated with the number of new T2 lesions (r = 0.34; p < 0.01) and BPF change over 2 years (r = −0.27; p < 0.05; Supplementary eTable 2). Similar associations were observed between CSF NfL concentration at Year 2 and measures of disease activity that occurred over the 2-year study period (Supplementary eTable 2). The correlations were more pronounced in patients treated with IM IFNβ-1a versus placebo during the RCT (Supplementary eTable 2).
Association between CSF NfL concentrations and outcomes at 8 and 15 years
CSF NfL concentrations at Year 2 were associated with BPF change (Supplementary eTable 2, Figure 2(a)) and EDSS score change (Figure 2(b)) at 8-year follow-up (baseline to Year 8). The correlations between Year 2 NfL concentrations and 8-year changes in BPF and EDSS were significant for the entire group of patients (r = −0.46; p < 0.0001 and r = 0.27; p < 0.01, respectively) and for the IM IFNβ-1a cohort (r = −0.56; p < 0.001 and r = 0.32; p < 0.05, respectively), but the correlation was less strong or not significant for the placebo-treated cohort (r = −0.34; p < 0.05 and r = 0.14; not significant, respectively; Supplementary eTable 2).
Figure 2.
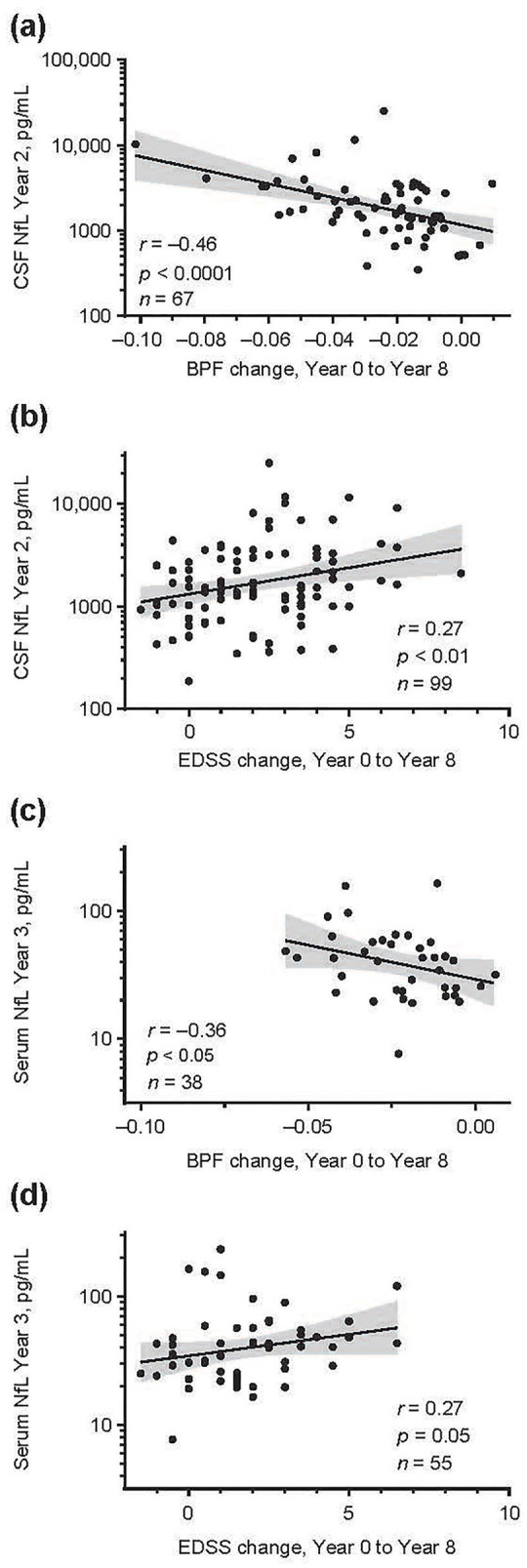
Correlation between CSF concentration at Year 2 and serum NfL concentration at Year 3 with 8-year outcomes. (a) CSF NfL and BPF change. (b) CSF NfL and EDSS change. (c) Serum NfL and BPF change. (d) Serum NfL and EDSS change. Scatterplots (log base 2) were used to depict the association between NfL and clinical and radiological parameters; r and p values were computed to characterize the association between NfL levels and clinical and radiological assessments.
BPF: brain parenchymal fraction; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; NfL: neurofilament light; r: Spearman’s rank correlation coefficient.
For patients with CSF NfL concentrations in the upper tertile at Year 2, the risk of reaching an EDSS score of 6.0 at 8 years was three times higher compared with patients with NfL concentrations in the lowest tertile (odds ratio (OR) = 3.4; 95% confidence interval (CI) = 1.2–9.9; p < 0.05; Figure 3(a)). This increase in risk did not persist at 15 years of follow-up (OR = 1.43; 95% CI = 0.49–4.28; p = 0.5; Figure 3(b)).
Figure 3.
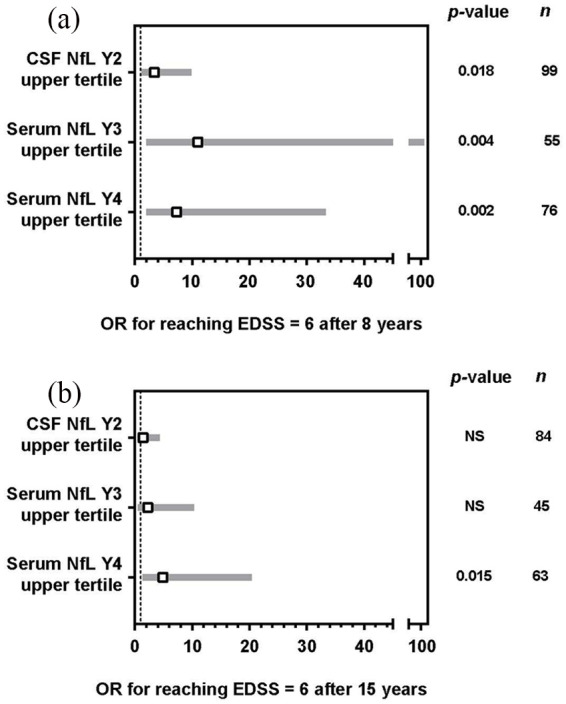
ORs for reaching an EDSS score of 6.0. (a) After 8 years of follow-up. (b) After 15 years of follow-up. For the purpose of illustration, the range of NfL assessments was broken down into tertiles; n indicates the total for each category. Hypothesis testing of the association of NfL levels with cumulative incidence of progression to EDSS score ⩾6.0 was conducted using Fisher’s exact test, as well as Wald chi-square test based on Firth’s penalized univariate logistic regression. OR are shown relative to the lowest tertile.
EDSS: Expanded Disability Status Scale; NfL: neurofilament light chain; NS: not significant; OR: odds ratio.
Association between serum NfL concentrations and outcomes at Year 8 and Year 15
Serum NfL concentrations at Year 3 were associated with BPF change at 8-year follow-up (r = −0.36; p < 0.05; Supplementary eTable 3, Figure 2(c)). In addition, serum NfL concentrations at Year 3 and Year 4 were associated with change in EDSS score at Year 8 (r = 0.27; p < 0.05 and r = 0.26; p < 0.05, respectively). The concentration of serum NfL at Year 4 was also associated with EDSS score change at Year 15 (r = 0.3; p < 0.05; Supplementary eTable 3, Figure 2(d)).
The risk of reaching an EDSS score of 6.0 after 8 years of follow-up was significantly increased in patients in the upper serum NfL tertile compared with the lowest tertile (Year 3, OR = 11.0; 95% CI = 2.0–114.6; p < 0.01; Year 4, OR = 7.3; 95% CI = 2.0–33.3; p < 0.01; Figure 3(a)). Similar results were observed for the EDSS outcome at Year 15, but only associations with serum NfL at Year 4 reached statistical significance (OR = 4.9; 95% CI = 1.4–20.4; p < 0.05; Figure 3(b)).
Likelihood of reaching an EDSS score ⩾6 at Year 8 when stratifying by early BPF change and NfL concentration
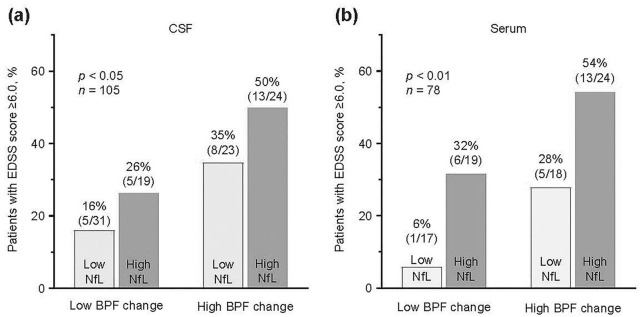
Patients with CSF NfL concentrations (mean concentration between Year 0 and Year 2) and BPF change (baseline to Year 2) below the median had a lower likelihood of reaching an EDSS score ⩾6 (16%; n = 31) by Year 8 compared with patients with both NfL levels and BPF change above the median (50%; p < 0.05; n = 24; Figure 4(a)).
Figure 4.
Proportion of patients reaching EDSS score ⩾6.0 at Year 8 stratified by BPF change and NfL levels. (a) CSF: the proportion of patients reaching an EDSS ⩾6.0 at Year 8 was stratified by BPF change from Year 0 to Year 2 and mean (Year 0 and Year 2) CSF NfL levels (below or above median). (b) Serum: the proportion of patients reaching an EDSS ⩾6.0 at Year 8 was stratified by BPF change from Year 0 to Year 2 and mean (Year 3 and Year 4) serum NfL levels (below or above median). Hypothesis testing of the association of NfL levels with cumulative incidence of progression to an EDSS score ⩾6.0 was conducted using Fisher’s exact test. Mean NfL levels represent the mean of two time points when both measurements were available, and a single value when only one measurement was available. Light gray bars indicate NfL below median; dark gray bars indicate NfL above median.
BPF: brain parenchymal fraction; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; NfL: neurofilament light.
Similarly, patients with both serum NfL concentration and BPF change below the median had a lower likelihood of reaching an EDSS score ⩾6 (6%; n = 17) by Year 8 compared with patients with both serum NfL concentrations and BPF change above the median (54%; p < 0.01; n = 24; Figure 4(b)).
Discussion
Here we provide evidence in a large cohort of RRMS patients that CSF and serum NfL concentrations are associated with long-term clinical and MRI outcomes. The design of the MSCRG study, available data from the long-term follow-up studies, and well-preserved samples allowed the evaluation of CSF and serum NfL concentration in well-characterized patients with long-term clinical and radiological data. Our results also show that NfL can be assessed in CSF and serum samples stored for more than 20 years, in line with previous experience on the stability of this protein.8,21
Both CSF and serum NfL concentrations were associated with short- and long-term clinical disability and brain atrophy. At baseline and after 2 years of follow-up, CSF NfL concentrations correlated with measures of MRI disease activity (Gd+ lesions), and MS disease burden (T2 lesion volume), corroborating earlier findings.10,22–25 The value of CSF and serum NfL measurements to predicting longer-term prognosis was comparable to MRI predictors such as early brain atrophy and breakthrough disease activity.16,17
Our findings add to earlier studies that investigated the prognostic value of CSF NfL in smaller cohorts. In a study of 95 patients with MS over 14 years of follow-up, higher CSF NfL concentration was associated with faster conversion to secondary progressive MS.26,27 In a study of 41 patients with clinically isolated syndrome or RRMS, clinical and MRI disease activity were best predicted by CSF NfL concentration after 2 years of follow-up.28 Recently, a study by Chitnis et al.29 based on the data from 122 patients reported an association of averaged serum NfL concentrations during the first few years after clinical onset of MS with 10-year MRI brain lesion load and atrophy. To our knowledge, ours is the first study that demonstrates associations of serum NfL with not only long-term brain atrophy but also with other clinically meaningful long-term outcomes in MS, such as EDSS. In addition, this study demonstrated the prognostic ability of a single NfL measurement.
Correlations between CSF NfL concentration and measures of disease activity and progression were more robust in patients treated with IFNβ-1a than placebo. We hypothesize that the presence of disease activity on treatment, and consequent elevation in NfL levels, would be more meaningful than on placebo as demonstrated in the previous publication in the same cohort.17 These data suggest that increased serum NfL levels in patients receiving disease-modifying therapies could indicate suboptimal response to therapy and the potential need of a therapy change.
In this study, combining NfL concentrations with short-term brain atrophy produced a more robust prediction for long-term progression of EDSS score than NfL concentrations alone. This finding suggests that serum NfL concentrations might complement MRI measurements, such as brain atrophy rates, in predicting long-term outcome. Further studies using prospective data in a validation cohort could confirm the improved predictability of such a combined model.
The invasive nature of lumbar punctures limits the value of CSF NfL in routine clinical settings. Previous studies have demonstrated associations between serum and CSF NfL concentrations, as well as serum NfL association with clinical and radiological measures of disease activity in RRMS.11,12 In this study, serum NfL at Year 3 correlated with brain atrophy and EDSS change over 8 years and was associated with reaching EDSS 6.0 by Year 8. The inconsistency in the strength of these associations in samples derived at different time points likely reflects the heterogeneity in the natural history of the patients recruited and the relatively small number of subjects in each analysis. For example, patients are likely to have changed treatments over the follow-up period, which may have altered their longer-term outcomes. Future work in prospective cohorts should correct for factors that may influence natural disease course such as treatment changes.
Limitations of this study include its retrospective design, the variable number of samples available at various time points, availability of serum samples at Year 3 and Year 4 only, and the lack of complete follow-up at 8 and 15 years.
Conclusion
The prognosis of individual patients with MS is a challenge. Despite decades of research, there are no accurate quantitative metrics to predict long-term outcome in patients with MS. Notably, brain atrophy measures have been shown to be predictive in patient populations, but to date, these metrics have not been widely applied in the clinical practice setting.16,17 In addition, reliable prognostic body fluid biomarkers, especially blood-based ones, are lacking.30 This inability to predict disease course is problematic because neurologists now have a wide range of treatment options, ranging from less effective but very safe treatments to higher-efficacy drugs with potentially serious side effects. Choosing the most appropriate treatment for individual patients would be informed by accurate quantitative prognostic markers that could be used early in the disease, when treatment is likely most effective. Of great importance is establishing a comprehensive database of normative NfL values to generate age-related reference values and to quantify the impact of comorbidities on NfL as a prerequisite to establish sNfL as a standard biomarker in clinical practice. Furthermore, commutability of values across the different assay protocols and platforms needs to be established. Based on results from this study, NfL appears to be an attractive candidate biomarker for disease severity stratification in MS, and warrants further evaluation in larger well-characterized cohorts of patients.
Supplemental Material
Supplemental material, MSJ885613_supplemental_material for Neurofilament light levels are associated with long-term outcomes in multiple sclerosis by Jens Kuhle, Tatiana Plavina, Christian Barro, Giulio Disanto, Dipen Sangurdekar, Carol M Singh, Carl de Moor, Bob Engle, Bernd C Kieseier, Elizabeth Fisher, Ludwig Kappos, Richard A Rudick and Jaya Goyal in Multiple Sclerosis Journal
Acknowledgments
J.K. and T.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author Contributions: J.K., T.P., L.K., R.A.R., and J.G. contributed to the study concept, design, and drafting of the manuscript. All authors contributed to the acquisition, analysis, or interpretation of data. J.K. and T.P. contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.K.’s institution (University Hospital Basel) received and used exclusively for research support consulting fees from Biogen, Novartis, Protagen AG, Roche, and Teva; speaker fees from Biogen, Genzyme, Novartis, Roche, and the Swiss Multiple Sclerosis Society; travel expenses from Merck Serono, Novartis, and Roche; grants from Bayer AG, Biogen, the ECTRIMS Research Fellowship Programme, Genzyme, Merck, Novartis, Roche, the Swiss Multiple Sclerosis Society, the Swiss National Research Foundation (320030_160221), and the University of Basel. T.P., D.S., C.M.S., C.d.M., B.E., B.C.K., E.F., and R.A.R. are employees of and hold stock/stock options in Biogen. C.B. has received travel support by Teva and Novartis. G.D. has nothing to disclose. L.K.’s institution (University Hospital Basel) received in the last 3 years and used exclusively for research support at the Department: steering committee, advisory board, and consultancy fees from Actelion, Alkermes, Almirall, Bayer, Biogen, Celgene/Receptos, df-mp, EXCEMED, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi Pharma, Novartis, Roche, Sanofi-Aventis, Santhera, Teva, Vianex, and royalties for Neurostatus-UHB products. J.G. was employed at Biogen at the time of the analysis and holds stock/stock options in Biogen.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This RCT was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (grant number RO1-26321), National Multiple Sclerosis Society (grant number RG3099), and Biogen; the follow-up studies were supported by Biogen; the study was supported by the Swiss National Science Foundation grant number 320030_160221. The research of the MS Center in Basel has been supported by grants from Bayer, Biogen, Novartis, the Swiss MS Society, the Swiss National Research Foundation, the European Union, and Roche Research Foundations.
Role of the Funder/Sponsor: Biogen employees participated in collection, management, analysis, and interpretation of the data; participated in preparation, review, and approval of the manuscript; and participated in the decision to submit the manuscript for publication.
ORCID iD: Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Jens Kuhle, Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Tatiana Plavina, Biogen, Cambridge, MA, USA.
Christian Barro, Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Giulio Disanto, Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland/Neurocenter of Southern Switzerland, Ospedale Civico, Lugano, Switzerland.
Dipen Sangurdekar, Biogen, Cambridge, MA, USA.
Carol M Singh, Biogen, Cambridge, MA, USA.
Carl de Moor, Biogen, Cambridge, MA, USA.
Bob Engle, Biogen, Cambridge, MA, USA.
Bernd C Kieseier, Biogen, Cambridge, MA, USA.
Elizabeth Fisher, Biogen, Cambridge, MA, USA.
Ludwig Kappos, Neurologic Clinic and Policlinic, Departments of Medicine, Biomedicine and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Richard A Rudick, Biogen, Cambridge, MA, USA.
Jaya Goyal, Biogen, Cambridge, MA, USA.
References
- 1. Comabella M, Montalban X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol 2014; 13: 113–126. [DOI] [PubMed] [Google Scholar]
- 2. Teunissen CE, Malekzadeh A, Leurs C, et al. Body fluid biomarkers for multiple sclerosis—the long road to clinical application. Nat Rev Neurol 2015; 11(10): 585–596. [DOI] [PubMed] [Google Scholar]
- 3. Deisenhammer F, Egg R, Giovannoni G, et al. EFNS guidelines on disease-specific CSF investigations. Eur J Neurol 2009; 16(6): 760–770. [DOI] [PubMed] [Google Scholar]
- 4. Varhaug KN, Barro C, Bjørnevik K, et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2018; 5(1): e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teunissen CE, Khalil M. Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 2012; 18: 552–556. [DOI] [PubMed] [Google Scholar]
- 6. Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 2004; 6(8): 699–706. [DOI] [PubMed] [Google Scholar]
- 7. Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res 2015; 360(3): 609–620. [DOI] [PubMed] [Google Scholar]
- 8. Kuhle J, Plattner K, Bestwick JP, et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler 2013; 19(12): 1597–1603. [DOI] [PubMed] [Google Scholar]
- 9. Lycke JN, Karlsson JE, Andersen O, et al. Neurofilament protein in cerebrospinal fluid: A potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 1998; 64(3): 402–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teunissen CE, Iacobaeus E, Khademi M, et al. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009; 72(15): 1322–1329. [DOI] [PubMed] [Google Scholar]
- 11. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141(8): 2382–2391. [DOI] [PubMed] [Google Scholar]
- 12. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol 1996; 39: 285–294. [DOI] [PubMed] [Google Scholar]
- 14. Rudick RA, Cutter GR, Baier M, et al. Estimating long-term effects of disease-modifying drug therapy in multiple sclerosis patients. Mult Scler 2005; 11(6): 626–634. [DOI] [PubMed] [Google Scholar]
- 15. Bermel RA, Weinstock-Guttman B, Bourdette D, et al. Intramuscular interferon beta-1a therapy in patients with relapsing-remitting multiple sclerosis: A 15-year follow-up study. Mult Scler 2010; 16(5): 588–596. [DOI] [PubMed] [Google Scholar]
- 16. Fisher E, Rudick RA, Simon JH, et al. Eight-year follow-up study of brain atrophy in patients with MS. Neurology 2002; 59(9): 1412–1420. [DOI] [PubMed] [Google Scholar]
- 17. Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 2013; 73(1): 95–103. [DOI] [PubMed] [Google Scholar]
- 18. Rudick RA, Fisher E, Lee JC, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Neurology 1999; 53(8): 1698–1704. [DOI] [PubMed] [Google Scholar]
- 19. Simon JH, Lull J, Jacobs LD, et al. A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon beta-1a. Neurology 2000; 55(2): 185–192. [DOI] [PubMed] [Google Scholar]
- 20. Simon JH, Jacobs LD, Campion MK, et al. A longitudinal study of brain atrophy in relapsing multiple sclerosis. Neurology 1999; 53: 139–148. [DOI] [PubMed] [Google Scholar]
- 21. Gaiottino J, Norgren N, Dobson R, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013; 8(9): e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villar LM, Picón C, Costa-Frossard L, et al. Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis. Eur J Neurol 2015; 22(8): 1169–1175. [DOI] [PubMed] [Google Scholar]
- 23. Kuhle J, Disanto G, Lorscheider J, et al. Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology 2015; 84(16): 1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arrambide G, Espejo C, Eixarch H, et al. Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 2016; 87: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Novakova L, Zetterberg H, Sundström P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89(22): 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norgren N, Sundström P, Svenningsson A, et al. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology 2004; 63(9): 1586–1590. [DOI] [PubMed] [Google Scholar]
- 27. Salzer J, Svenningsson A, Sundström P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 2010; 16(3): 287–292. [DOI] [PubMed] [Google Scholar]
- 28. Håkansson I, Tisell A, Cassel P, et al. Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol 2017; 24(5): 703–712. [DOI] [PubMed] [Google Scholar]
- 29. Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 2018; 5(12): 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cantó E, Tintoré M, Villar LM, et al. Chitinase 3-like 1: Prognostic biomarker in clinically isolated syndromes. Brain 2015; 138(Pt 4): 918–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ885613_supplemental_material for Neurofilament light levels are associated with long-term outcomes in multiple sclerosis by Jens Kuhle, Tatiana Plavina, Christian Barro, Giulio Disanto, Dipen Sangurdekar, Carol M Singh, Carl de Moor, Bob Engle, Bernd C Kieseier, Elizabeth Fisher, Ludwig Kappos, Richard A Rudick and Jaya Goyal in Multiple Sclerosis Journal