We recently examined the reproducibility of MYC and BCL-2 immunohistochemical (IHC) scoring and the impact of high expression of MYC and BCL-2 (double expresser status, DE) on survival and progression in a large retrospective cohort of aggressive B-cell lymphoma patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) or R-CHOP-like regimens.1 We found that IHC scoring for MYC and BCL-2 was highly reproducible when cut-off values of ≥70% for MYC and ≥50% for BCL-2 were used. This threshold also predicted the presence of gene rearrangements identifying MYC translocations in 88% of cases. Patients with dual MYC expression of ≥70% and BCL-2 expression of ≥50% showed a significantly inferior clinical course and, therefore, represent candidates for novel treatment modalities.1 We have now validated these findings in an independent cohort of 461 patients enrolled in prospective clinical trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL).2,3
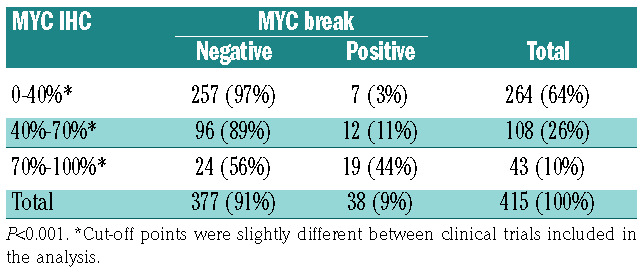
In these trials, patients underwent R-CHOP-14 if >60 years of age and R-CHOEP/R-MegaCHOEP if ≤60 years of age. In the MegaCHOEP trial reported by Schmitz et al.,4 no significant differences in outcome between RCHOEP- 14 and R-MegaCHOEP had been observed, but to date, no randomized trial has been conducted to answer if R-CHOEP in younger patients is superior in comparison with R-CHOP. In a subgroup analysis for young low-risk patients from the MInT trial reported by Pfreundschuh et al.,5 no difference in outcome was observed between R-CHOEP-21 and R-CHOP-21. In elderly patients, the Cunningham trial6 revealed that the outcome of R-CHOP-14 is not better than that of R-CHOP-21. In the German cohort of 428 patients with MYC and BCL-2 IHC scoring available, 104 cases (24%) were MYC–/BCL-2– (double negative, DN), 283 (66%) were MYC–/BCL-2+ (BCL2only), 8 (2%) were MYC+/BCL-2– (MYConly), and 33 were MYC+/BCL-2+ using the above-mentioned cut-off values, meaning that 8% of DLBCL were assigned a DE status. Results from both MYC IHC scoring and MYC fluorescence in situ hybridization (FISH) were available from samples of 415 patients. In this analysis, 19 of 43 (44%) samples with high MYC expression (70/71-100%) harbored a MYC translocation (Table 1). The lower number of cases noted in our report with both high MYC expression and MYC breakage in comparison with the Ambrosio paper1 are not easily explained. Most probably, this is due to a difference in the genetic constitution of the two different patient populations that were examined or to the analysis strategy: in the German cohort, the analysis was made on TMA while in the paper of Ambrosio et al.,1 full sections were analyzed.7 According to the results of molecular cell of origin (COO) analysis, we identified 50% of patients with an ABC subtype within the DE cohort using a MYC cut-off point of 70% and 68% using a cut-off point of 40%. The sample sizes, however, are too small to conclude that the groups differ from the proportion of the ABC subtype. It has to be stressed, however, that the DE status does not identify a homogeneous biological group of tumors and, especially, that the DE status in ABCDLBCL arises through very different mechanisms.
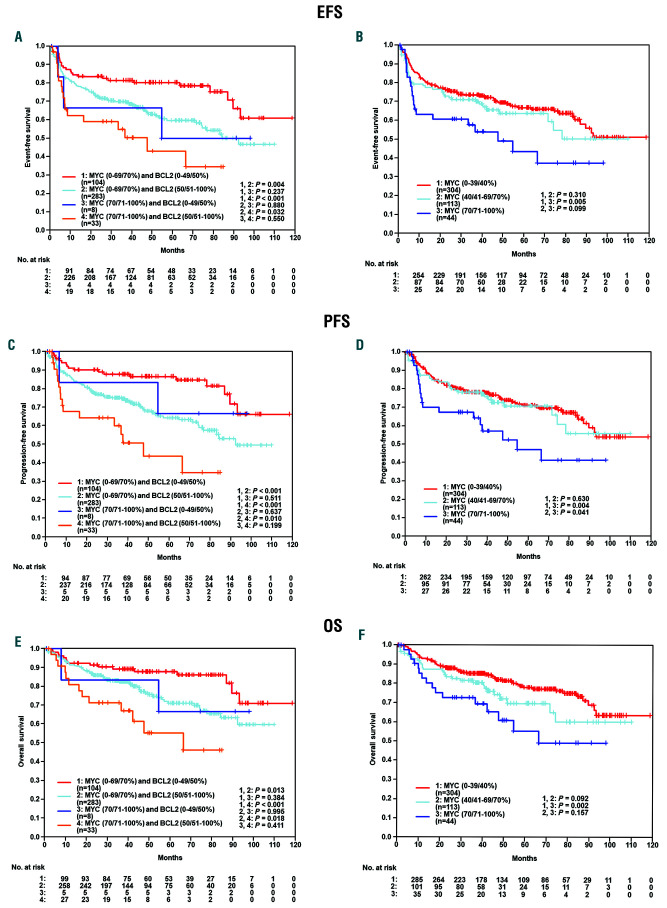
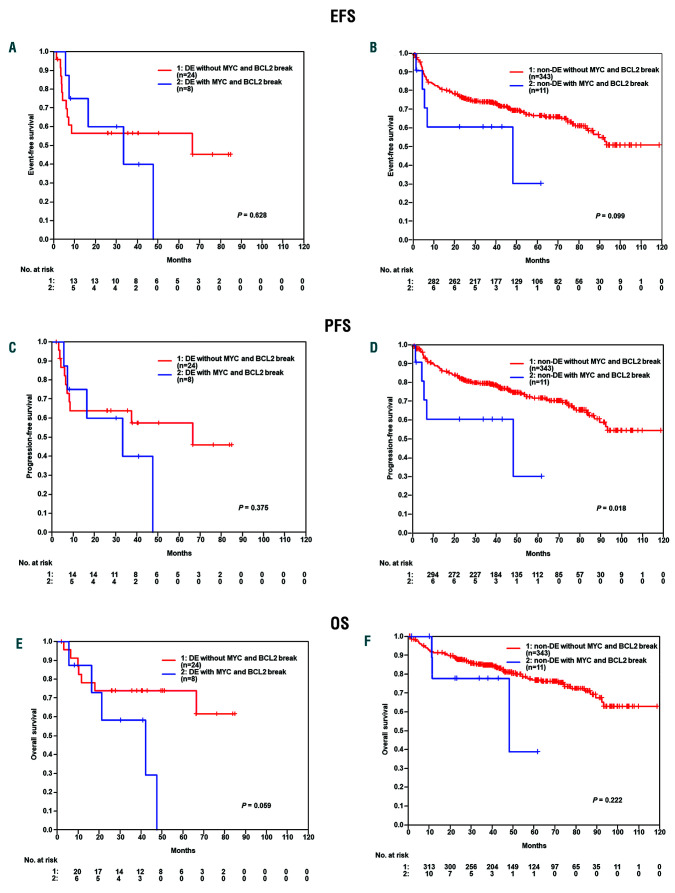
In the German cohort, the DE subgroup had a significant inferior clinical course, while the DN subset had a superior outcome and the MYC–/BCL-2+ subset had an intermediate prognosis. The differences were statistically significant for event-free survival (EFS), progression-free survival (PFS), and overall survival (OS) (EFS: DN vs. DE, P<0.001; DN vs. BCL2only P=0.004; BCL2only vs. DE P=0.032) (Figure 1A-C). These results could be confirmed in a multivariate analysis (Hazard ratios [HR] for DE vs. other: EFS: 2.1 95%CI:1.2-3.5, P=0.005; PFS: 2.5 95%CI:1.5-4.3, P=0.001; OS: 2.7 95%CI:1.5-4.8, P=0.001) adjusted for the factors of the International Prognostic Index (IPI) (age > 60 years, lactate dehydrogenase [LDH]>N, Eastern Cooperative Oncology Group [ECOG]>1, stage III/IV, extralymphatic involvement >1). In multivariate analyses adjusted for the International Prognostic Index (IPI) factors (age > 60 years, LDH>N, ECOG>1, stage III/IV and more than one site of extralymphatic involvement) both MYC (70/71-100% vs. other) and BCL2 (50/51-100% vs. other) expression were significant risk factors in EFS (MYC: HR1.9, 95%CI: 1.2-3.1, P=0.007 and BCL2: HR1.8, 95%CI: 1.2-2.7, P=0.006), PFS (MYC: HR2.1, 95%CI: 1.3-3.5, P=0.004 and BCL2: HR2.4, 95%CI: 1.5-3.8, P<0.001) and OS (MYC: HR2.3, 95%CI: 1.3-4.0, P=0.004 and BCL2: HR2.0, 95%CI: 1.2-3.3 and P=0.009). When cases were stratified according to MYC protein expression only, patients with MYC ≥70%, again, experienced inferior outcome in EFS (P=0.005), PFS (P=0.004), and OS (P=0.002) in comparison with patients with low MYC expression (≤40%) (Figure 1D-F), while no difference in prognosis was seen between patients whose tumors had MYC expression ≤40% and >40-70%. Within the DE group, the occurrence of a genetic double hit for MYC and BCL-2 (n=8 of 32, 25%) failed to confer a significant prognostic difference in EFS (P=0.628), PFS (P=0.375), and OS (P=0.059) between patients with DH positive and DH negative tumors (Figure 2A-C). Within the non- DE group, we observed a genetic double hit for MYC and BCL-2 in only 11 of 354 (3%) patients with no relevant survival differences between patients with DH positive and DH negative tumors (Figure 2D-F). However, due to the low number of events, these results have to be interpreted with caution.
Table 1.
Results from both MYC immunohistochemical (IHC) scoring and MYC fluorescence in situ hybridization.
In essence, these results are in agreement with our previous findings indicating that high (≥70%) MYC expression identifies a subset of DLBCL with adverse clinical outcome independent of the presence of a double hit of MYC and BCL-2.
Increasing evidence suggests that the sole identification of the double hit (MYC and BCL-2) status may not be the optimal tool to identify patients in need of alternative therapies and in many studies, a proportion of DE patients nevertheless experience long-term survival. Two recent papers shed light on this seeming discrepancy.8,9 In the first paper, the authors defined a clinically and biologically distinct subgroup of aggressive lymphomas with inferior prognosis among GCB-DLBCL. This tumor subgroup was characterized by a gene expression signature derived from HGBL-DH/TH lymphomas (DHIT signature). 8 Using this signature, however, only 50% of the cases stratified into the subgroup actually had dual rearrangements of MYC and BCL-2 genes, and some DE cases were not assigned into the DHIT signature positive group. Gene set enrichment analysis demonstrated (over-) expression of MYC and E2F target genes, and of genes associated with oxidative phosphorylation and MTORC1 signaling in the DHIT-positive tumors, implying a pivotal role for MYC protein expression irrespective of the DH status. Unfortunately, the study did not document the precise percentage of MYC protein expression; it also did not correlate MYC protein expression to MYC gene rearrangements. The second paper identified 9% of DLBCL (83 of 928) as “molecular high grade (MHG)” Bcell lymphomas using gene expression analysis.9 Most MHG (75 of 83) were GCB-like, and again, only half of them were MYC rearranged or double-hit lymphomas. The MHG subset treated with R-CHOP had a significantly poorer outcome than MHG negative DLBCL. Furthermore, in vivo experiments demonstrated that MYC-expressing lymphoma cells were obviously addicted to its oncogenic effect and, therefore, were critically relying on MYC expression regardless of MYC gene rearrangements.10
Figure 1.
Event-free survival (EFS), progression-free survival (PFS) and overall survival (OS) of patients stratified according to Myc and BCL-2 expression. (A-C) The double expressor (DE) subgroup had a significant inferior clinical course, while the double negative (DN) subset had a superior outcome and the MYC-/BCL- 2+ subset had an intermediate prognosis, with the differences being statistically significant for EFS, PFS and OS. (D-F) The cases were stratified according to MYC protein expression only, patients with MYC >70% experienced inferior outcome in EFS (P=0.005), PFS (P=0.004) and OS (P=0.002) in comparison to patients with low MYC expression (<40%). No difference in prognosis was seen between patients whose tumors had MYC expression <40% and 40-70%.
Figure 2.
Comparison of double hit in double expressor and non-double expressor (DE). (A-C) Within the DE group, the occurrence of a genetic double hit for MYC and BCL-2 (n=8 of 32, 25%) failed to confer a significant prognostic difference in EFS (P=0.628), PFS (P=0.375) and OS (P=0.059) between patients with DH positive and DH negative tumors. (D-F) Within the non DE group, the occurrence of a genetic double hit for MYC and BCL-2 (n=11 of 354, 3%) failed to confer a significant prognostic difference in EFS (P=0.099) and OS (P=0.222) between patients with DH positive and DH negative tumors.
Although genomic testing has entered clinical practice, sophisticated tests like those reported are not yet widely available in all laboratories. Therefore, gene expression signatures identifying high-risk subgroups are currently difficult to apply in the clinic. Our findings describe a more readily available tool to identify patients at risk with a high MYC protein expression cut-off circumventing problems related to interobserver variability.11 Our findings are corroborated in a recent paper by Pedersen et al.12 who demonstrated that stratification by MYC expression has prognostic impact in MYC translocated DLBCL.12
In summary, we have confirmed that the prognosis of DLBCL is inversely correlated with MYC protein expression levels, and, by using diagnostic thresholds of high reproducibility, we were able to identify a subset of patients with adverse outcome in need of alternative therapeutic strategies.
Supplementary Material
References
- 1.Ambrosio MR, Lazzi S, Lo Bello G, et al. MYC protein expression scoring and its impact on the prognosis of aggressive B-cell lymphoma patients. Haematologica. 2019;104(1):e25-e28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staiger AM, Altenbuchinger M, Ziepert, et al. A novel lymphomaassociated macrophage interaction signature (LAMIS) provides robust risk prognostication in diffuse large B-cell lymphoma clinical trial cohorts of the DSHNHL. Leukemia. 2019;34(2):543-552. [DOI] [PubMed] [Google Scholar]
- 3.Mellert K, Martin M, Lennerz JK, et al. The impact of SOCS1 mutations in diffuse large B-cell lymphoma. Br J Haematol. 2019;187(5):627-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250-1259 [DOI] [PubMed] [Google Scholar]
- 5.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379-391. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;25;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 7.Hupp M, Williams S, Dunnette B, et al. Comparison of evaluation techniques, including digital image analysis, for MYC protein expression by immunohistochemical stain in aggressive B-cell lymphomas. Hum Pathol. 2019;83:124-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennishi D, Jiang A, Boyle M, et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(3):190-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sha C, Barrans S, Cucco F, et al. Molecular high-grade B-cell lymphoma: defining a poor-risk group that requires different approaches to therapy. J Clin Oncol. 2019;37(3):202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Gupta SK, Han W, et al. Targeting MYC activity in double-hit lymphoma with MYC and BCL2 and/or BCL6 rearrangements with epigenetic bromodomain inhibitors. J Hematol Oncol. 2019;12(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahmoud AZ, George TI, Czuchlewski DR, et al. Scoring of MYC protein expression in diffuse large B-cell lymphomas: concordance rate among hematopathologists. Mod Pathol. 2015;28(4):545-551. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen MØ, Gang AO, Clasen-Linde E, et al. Stratification by MYC expression has prognostic impact in MYC translocated B-cell lymphoma identifies a subgroup of patients with poor outcome. Eur J Haematol. 2019;102(5):395-406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.