Monoclonal gammopathy of undetermined significance (MGUS) is a common benign precursor condition of multiple myeloma (MM) and related disorders.1,2 MGUS is considered asymptomatic but has been shown to be associated with peripheral neuropathy (PN).3 However, the literature is unclear regarding the prevalence, clinical implications, and even the existence of MGUS-associated PN.4 We therefore conducted a large population-based study of MGUS and PN. We found PN to be truly associated with MGUS and under-recognized in clinical practice. Furthermore, PN was associated with a 2.9-fold risk of a light-chain amyloidosis (AL).
We included individuals with MGUS diagnosed in Sweden between 1986-2013, as has been described previously. 5 Four controls that were alive and free of lymphoproliferative disease were matched to each case on the day of MGUS diagnosis by sex, year of birth, and county of residence. Data from Swedish national registries were cross-linked to participants using a unique identification number.
The primary endpoint was PN as recorded in the Swedish Patient Registry using International Classification of Diseases (ICD) codes by Swedish physicians recording their clinical diagnoses. However, underlying symptoms or diagnostic testing leading to PN diagnosis were not available. Acute inflammatory neuropathies and critical care neuropathy were excluded. Symptomatic codes were included but were excluded in a sensitivity analysis. We assessed the prevalence of PN in the full cohort and followed those who did not have PN at inclusion until PN or censoring at death as recorded in the Swedish Cause of Death Registry, lymphoproliferative disease as recorded in the Swedish Cancer Registry, or end of follow-up. We then estimated hazard ratios (HR) using Cox proportional hazard regression adjusting for age, sex, and year of inclusion. PN is common in the general population but is often undetected.6 Individuals with MGUS, who are under medical surveillance, might therefore have more diagnoses of a PN that might otherwise have stayed subclinical in the control population. To mitigate this bias, we also stratified the cohort by diabetes mellitus (DM) and repeated the analysis. DM patients are under regular medical surveillance, similar to that of patients with MGUS. Furthermore, PN is a wellknown feature of DM, and DM patients often undergo PN screening during follow-up, presenting a more appropriate comparison group.
In a secondary analysis, we assessed the association of PN and MGUS progression and death. We included all participants with MGUS and considered PN as the exposure. We then followed them until death or the diagnosis of MM, Waldenström’s macroglobulinemia (WM), and AL in four separate analyses while censoring at the other endpoints or loss to follow-up. In order to prevent immortal time bias in those participants who developed PN after MGUS diagnosis, we included PN as a timedependent covariate in a Cox proportional hazard regression model. The models were adjusted for age, sex, and year of MGUS diagnosis, as well as for DM when assessing risk of death.
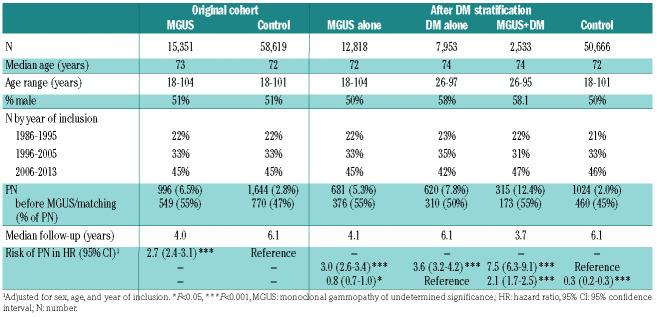
A total of 15,351 participants with MGUS and 58,619 matched controls were included in the study. The prevalence of PN was higher in participants with MGUS than controls (6.5% vs. 2.8%) (Table 1). The reported prevalence of PN varies widely but more recent observational studies estimate the prevalence at 15-20%.7 Therefore, these findings, based on clinical diagnoses of PN, indicate under-recognition of PN during the clinical care of individuals with MGUS.
Individuals with MGUS had 2.7-fold risk of PN compared to matched controls (HR=2.7; 95% confidence interval [95%CI]: 2.4-3.1; P<0.001). After stratification for DM, we found MGUS and DM to be associated with higher risk of PN as compared to controls without MGUS and DM (MGUS alone: HR=3.0; 95%CI: 2.6-3.4; P<0.001 and DM alone: HR=3.6; 95%CI: 3.2-4.2; P<0.001).
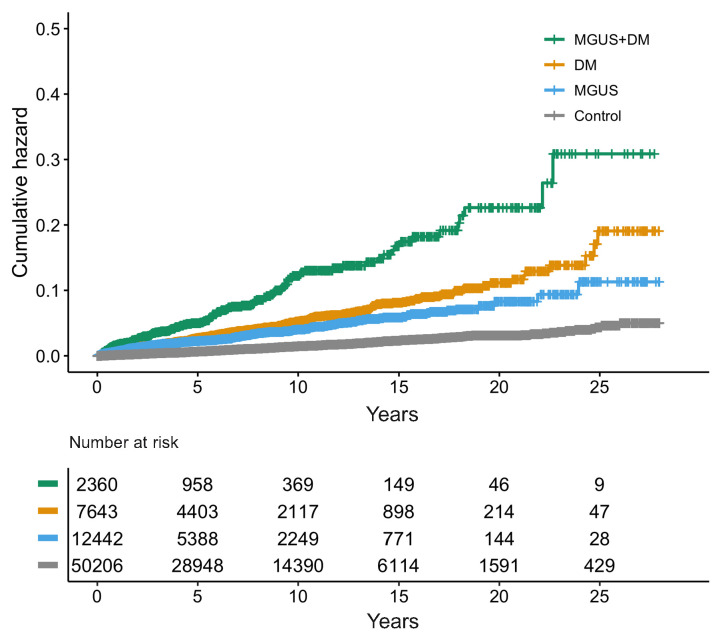
MGUS was associated with a 0.8-fold risk of PN as compared to DM (HR=0.8; 95%CI: 0.7-0.9, P=0.02). Participants with MGUS and DM had a 2.1-fold risk of PN as compared to those with DM alone (MGUS and DM: HR= 2.1; 95%CI: 1.7-2.5; P<0.001) (Table 1 and Figure 1). Although these findings could suggest a synergistic effect of MGUS and DM, it is more likely that excess PN caused by MGUS is being detected during DM or MGUS follow-up in individuals with DM and MGUS as compared to those with DM alone. These findings indicate that PN is truly associated with MGUS, contradicting previous findings that questioned this.4
Table 1.
Baseline characteristics of study participants in the original cohort and after additional stratification for diabetes mellitus (DM) as well as results of a Cox proportional hazard regression model of risk of peripheral neuropathy (PN) for each group.
Figure 1.
Kaplan-Meier graph illustrating the cumulative hazard of peripheral neuropathy (PN) throughout the study period by assigned study group. MGUS: monocloncal gammopathy of undetermined significance; DM: diabetes mellitus.
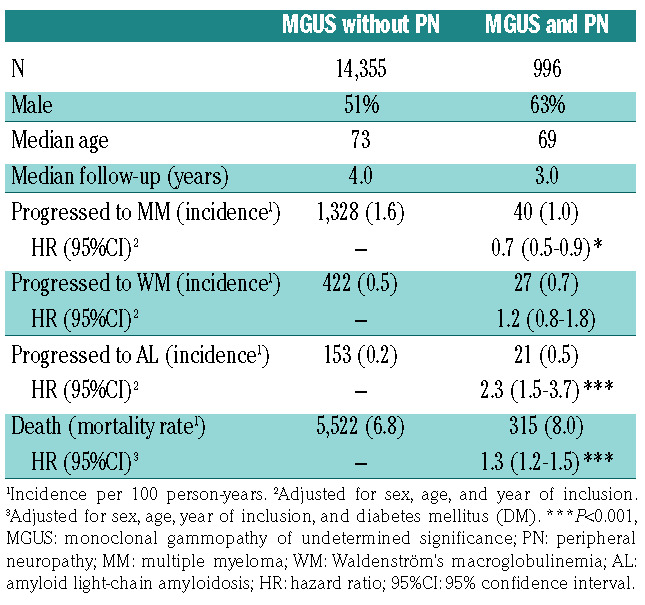
In the secondary analysis, 1,368 participants progressed to MM, 449 progressed to WM, and 173 progressed to AL (Table 2). PN was associated with lower risk of MM (HR=0.7; 95%CI: 0.5-0.9; P=0.02) but was not associated with WM progression (HR=1.3; 95%CI: 0.9-1.9; P=0.2). PN has been shown to be more common in IgM MGUS3 that rarely progresses to MM, but rather to WM,8 potentially leading to selection bias. Unfortunately, isotype data are not available for this cohort making it difficult to interpret these results. However, these findings could indicate that PN is unlikely to be associated with increased risk of MM or WM, suggesting that the development of PN in MGUS might be unrelated to progression of the underlying plasma cell disorder.
Interestingly, we found PN to be associated with a 2.9- fold risk of MGUS progression to AL (HR=2.9; 95%CI: 1.8-4.6; P<0.001). Furthermore, we found that nine out of the 11 individuals (82%) with PN at diagnosis who later progressed to AL did so within a year of MGUS diagnosis. Diagnosis of AL can be difficult, leading to under-recognition and a delay in diagnosis of AL.9 Furthermore, virtually all cases of AL are preceded by MGUS,10 so it is likely that these participants had AL, not MGUS, at inclusion. These findings stress the importance of a thorough evaluation for AL in individuals with MGUS and PN, especially at MGUS diagnosis.
We found PN to be associated with a 1.3-fold risk of death in MGUS (HR=1.3; 95%CI: 1.2-1.5; P<0.001). When associated with other disorders, PN can lead to falls and fractures11-13 which might contribute to this increased risk of death. However, PN is also associated with various other diseases that might lead to increased risk of death, such as other cancers and alcohol misuse.6 Therefore, it is unclear whether this represents a causal relationship. Further studies are needed to validate these findings.
Our study has several strengths. We included a nationwide population of 15,351 MGUS cases and 58,619 matched controls diagnosed over a 28-year period. Data were acquired with high accuracy and completeness from well-established registries. As far as we know, this is the largest study of MGUS-associated PN so far. Secondly, by including clinical data from routine care, the study provides an insight into the real-world care of individuals with MGUS. Finally, by also stratifying participants for DM, we mitigated detection bias that would otherwise have affected the results of this type of study.
The study also has important limitations. Firstly, PN diagnoses were acquired from diagnostic coding without data on the underlying symptoms or diagnostic tests, relying on detection, and accurate diagnosis of PN by physicians. By stratifying for DM, we mitigated some of the effects of any unequal detection and reporting of PN in the cohort. Secondly, study participants were not screened for MGUS, but were diagnosed during the work-up of other medical problems, leading to biased selection of participants with other medical problems into the MGUS group. Furthermore, MGUS might have been diagnosed as a result of PN. However, this applies to all real-world MGUS populations, and individuals with PN before MGUS diagnosis were excluded from analyses assessing risk of PN. Thirdly, and unfortunately, immunoglobulin isotype data are not available for this cohort, and some isotypes, especially IgM, might be associated with higher risk of PN and skew the average risk for the whole cohort so that it might not be representative for each isotype. Furthermore, this limits the interpretation of analyses of progression to MM and WM. Prospective studies including screening for MGUS and PN are needed to validate these findings. We are currently conducting a population-based screening study for MGUS (clinincaltrials.gov identifier: NCT03327597). A substudy is ongoing assessing the prevalence, symptoms, clinical impact, and associated disease factors of MGUSassociated PN.
Table 2.
Baseline characteristics, rates and incidence of progression, and risk of progression to MM, WM, and AL as well as death for study participants with MGUS with and without PN as assessed by Cox proportional hazard regression with PN as a time dependent covariate.
In conclusion, in this large population-based study, including 15,351 MGUS individuals and 58,619 matched controls, we found that a significant proportion of individuals with MGUS have clinically evident PN (6.5%) and that PN is truly associated with MGUS. In addition, our findings suggest under-recognition of PN in the realworld care of individuals with MGUS. Interestingly, we found PN to be associated with a 2.9-fold risk of AL and that PN is not associated with increased risk of MM or WM. PN was associated with increased risk of death, but multiple confounders make it impossible to establish a causal relationship. When associated with other disorders, PN leads to falls, fractures,11-13 and lower quality of life.14 It is, therefore, reasonable to assume that PN causes considerable morbidity in MGUS that may go unrecognized. Our findings should help increase awareness of MGUS as a cause of PN among all clinicians and promote closer monitoring of individuals with MGUS for symptoms of PN.
Supplementary Material
Funding Statement
Funding: this research was supported by grants from the University of Iceland Research Fund, Icelandic Centre for Research (RANNIS), Landspitali University Hospital Research Fund, and Karolinska Institutet Foundations. Funding support for this publication was provided by the Memorial Sloan Kettering Core Grant (P30 CA008748) and the Perelman Family Foundation in collaboration with the Multiple Myeloma Research Foundation (MMRF) for OL. SR is a PhD candidate at the University of Iceland and this work is submitted in partial fulfilment of the requirement for a PhD.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362-1369. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375(9727):1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosselin S, Kyle RA, Dyck PJ. Neuropathy associated with monoclonal gammopathies of undetermined significance. Ann Neurol. 1991;30(1):54-61. [DOI] [PubMed] [Google Scholar]
- 4.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population- based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristinsson SY, Bjo M, Goldin LR, McMaster ML, Turesson I, Landgren O. Risk of lymphoproliferative disorders among firstdegree relatives of lymphoplasmacytic lymphoma / Waldenström macroglobulinemia patients: a population-based study in Sweden. Blood. 2008;112(8):3052-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31(1):5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner N, Schwärzler A, Göbel G, Löscher W, Gunsilius E. Are neurological complications of monoclonal gammopathy of undetermined significance underestimated? Oncotarget. 2017;8(3):5081-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102(10):3759-3764. [DOI] [PubMed] [Google Scholar]
- 9.Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light chain amyloidosis: patient experience survey from the Amyloidosis Research Consortium. Adv Ther. 2015;32(10):920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss BM, Hebreo J, Cordaro D, Roschewski MJ, Abbott KC, Olson SW. Monoclonal gammopathy of undetermined significance (MGUS) precedes the diagnosis of AL amyloidosis by up to 14 years. Blood. 2011;118(21):1827. [Google Scholar]
- 11.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults. Arch Intern Med. 2005;165(14):1612. [DOI] [PubMed] [Google Scholar]
- 12.Timar B, Timar R, Gaita L, Oancea C, Levai C, Lungeanu D. The impact of diabetic neuropathy on balance and on the risk of falls in patients with type 2 diabetes mellitus: a cross-sectional study. PLoS One. 2016;11(4):e0154654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall TF, Zipp GP, Battaglia F, Moss R, Bryan S. Chemotherapyinduced- peripheral neuropathy, gait and fall risk in older adults following cancer treatment. J Cancer Res Pract. 2017;4(4):134-138. [Google Scholar]
- 14.Benbow SJ, Wallymahmed ME, MacFarlane IA. Diabetic peripheral neuropathy and quality of life. QJM. 1998;91(11):733-737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.