ABSTRACT
Background
Levodopa‐carbidopa intestinal gel (LCIG) treatment has shown variable effect on dyskinesia in Parkinson's disease (PD).
Objective
To identify PD patients who are likely to have troublesome dyskinesia under LCIG treatment and describe the pharmacokinetic‐dynamic profile and dyskinesia phenomenology of those patients.
Methods
PD patients were assessed for clinical and therapeutic variables, before LCIG treatment (T0) and at last outpatient visit (T1). Sub‐groups of patients with and without “troublesome dyskinesia” (UPDRS IV, item 33 ≥2), matched for disease and LCIG treatment duration, underwent a pharmacokinetic‐dynamic assessment.
Results
We included 53 PD patients. After a mean of 51.7 ± 34.1 months of LCIG treatment, “off‐time” was significantly reduced, whereas, dyskinesia duration/disability did not change. The multivariate regression model, adjusted for LCIG treatment duration, showed that being female increases the risk of presenting troublesome dyskinesia at T1 (odds ratio [OR] = 9.2; 95% confidence interval [CI] = 2.4–37.4) that was also significantly associated to longer off periods at T1 (OR= 4.4; 95% CI = 1.1–14.3). Female patients showed a higher risk for a higher dyskinesia score at T1 (sum of the items 32 and 33: P = 0.001). Patients with troublesome dyskinesia showed a tendency for a lower motor benefit and the appearance of more severe dyskinesia despite similar levodopa plasma concentration.
Conclusion
Dyskinesia should be carefully monitored in patients undergoing LCIG, with particular caution for female patients. Whether combined clinical and pharmacodynamic assessments could be helpful to manage patients with troublesome dyskinesia under LCIG treatment needs further evaluation in a larger group of patients.
Keywords: Parkinson's disease, levodopa/carbidopa intestinal gel, dyskinesia, gender
Long‐term therapy with levodopa (l‐dopa) in Parkinson's disease (PD) has been associated with the development of motor fluctuations and dyskinesia. Indeed, l‐dopa‐induced dyskinesia (LID) occurs in 40% of patients after 4 years of l‐dopa treatment. 1 , 2 At present, 1 treatment option with proven benefit in clinical trials for troublesome motor complications resistant to standard oral treatment is l‐dopa‐carbidopa intestinal gel infusion (LCIG). 3 , 4 , 5
LCIG has shown an optimal efficacy in the reduction of motor fluctuations at both medium and long‐term follow‐up. 3 , 6 , 7 Conversely, effects on dyskinesia can be heterogeneous, with some patients showing a significant increase of dyskinesia within the first years of treatment and other patients developing diphasic dyskinesia, never experienced before, 6 , 8 , 9 , 10 with associated possible treatment discontinuation. 11 So far, no study has specifically investigated the pre‐treatment prognostic factors for a poor outcome in terms of dyskinesia management under chronic LCIG treatment. Equally, the pharmacokinetic‐dynamic properties of this therapy, along with its relationship with dyskinesia occurrence and phenomenology, have been explored in only a few small studies. 12 , 13
Our study aims to investigate the baseline features related to the presence of “troublesome dyskinesia” among PD patients under chronic LCIG treatment and the pharmacokinetic‐dynamic profile of those patients.
Methods
Consecutive patients were recruited from the Movement Disorders outpatient clinic of 2 Italian university hospitals (Department of Biomedical and NeuroMotor Sciences of the University of Bologna and the Department of Neuroscience “Rita Levi Montalcini” of the University of Turin).
Inclusion criteria were idiopathic PD 14 and treatment with LCIG (Duodopa, AbbVie, North Chicago, IL) for at least 6 months. Exclusion criteria were a Mini Mental State examination (MMSE) score ≤24 in combination with the absence of a caregiver participating at the clinical evaluation and UPDRS or MDS‐UPDRS missing data.
Study Design and Outcome Measures
The study was divided into: (1) a cross‐sectional and retrospective assessment of clinical and therapeutic data, including all patients; and (2) a pharmacokinetic‐dynamic monitoring randomly applied to a sub‐group of patients with troublesome dyskinesia and to a group of patients without troublesome dyskinesia, matched for age, disease duration, and LCIG treatment duration.
The Unified PD rating scale (UPDRS) part II‐III or the Movement Disorder Society‐Sponsored revision (MDS) of the UPDRS part II‐III, the UPDRS part IV, Hoehn & Yahr (H&Y) scale, Schwab and England score (SE), 15 MMSE, body weight (BW), body max index (BMI), and l‐dopa equivalent daily dose (LEDD), 16 were assessed at 2 time points, 1 week before starting LCIG treatment (T0) and during the last outpatient visit on LCIG therapy (T1). The treating neurologists (M.F., L.S., and M.Z.) filled out the UPDRS and MDS‐UPDRS scales based on clinical visits for part III and interviewing the patients and caregivers for part II and IV, as historical information based on the previous week. The pharmacokinetic‐dynamic monitoring was performed within the following 2 weeks after the T1 visit with a follow‐up clinical evaluation after 1 month (T2).
Clinical and Laboratory Assessment
At T0, the assessment was carried out in 2 conditions: the practically defined “OFF condition” (at least 12 hours after the last l‐dopa/aromatic amino acid decarboxylase inhibitor [LDDCI] intake, 48 hours after the last intake of dopamine agonists, controlled‐release LDDCI, selegiline or rasagiline, or 12 hours after the last intake of entacapone) and “ON condition” (after the administration of 1.5× the usual l‐dopa morning dose). At T1, the assessment was carried out in daily‐ON condition (during LCIG infusion). Validated conversion tables were applied to convert data from the UPDRS part II‐III of the T0 evaluation to the MDS‐UPDRS part II‐III, for 30 patients. 17 Clinical phenotypes (ie, akinetic‐rigid [AK] and tremor dominant [TD]) were defined in concordance with clinical history.
Patients were considered having troublesome dyskinesia if they scored ≥2 at the UPDRS‐IV item 33.
Two subgroups of patients, 1 with troublesome dyskinesia and 1 with no troublesome dyskinesia, matched for disease duration and LCIG treatment duration, underwent the pharmacokinetic‐dynamic assessment, within the 2 weeks after the T1 visit. On the same day, patients and caregivers were interviewed about dyskinesia timing and phenomenology over the previous week. The pharmacokinetic‐dynamic assessment was performed after a 12‐hour washout of l‐dopa and any concomitant antiparkinsonian drugs. Blood venous samples (2 mL) for measurements of plasma l‐dopa concentrations were drawn by an indwelling catheter immediately before the usual morning dose—C0—and 3 times thereafter, during the on‐going continuous dose, C1, 45 minutes after the end of the morning dose, and C2, C 3, at 45‐minute intervals. Blood specimens were collected and processed for plasma l‐dopa concentration and 3‐O‐methyldopa (3‐OMD) analysis as previously reported. 18 Patients' motor responses were assessed by the MDS‐UPDRS part III (bradykinesia score [Bradik]: sum of the items 3.4–3.8) and the Rush Dyskinesias Rating Scale (RDRS), which were video‐recorded simultaneously with each blood sample collection. Bradykinesia and RDRS scores were rated by an independent clinician (AR), blinded to patients' therapeutic conditions, by video assessment. The magnitude of the effect, elicited by the LCIG infusion, was estimated as the difference (∆) between the first motor performance (Bradik0) and each following motor assessments (Bradik1 , Bradik2, and Bradik 3) expressed as percentage. After 1 month (T2), patients with troublesome dyskinesia were re‐evaluated clinically and completed the Patient's Global Impression‐Improvement (PGI‐I) to rate any changes in terms of troublesome dyskinesia.
Study Endpoints and Statistical Analysis
As primary endpoint, we investigated the T0 prognostic factors for the presence or development of new troublesome dyskinesia at T1. Secondary endpoints included the comparison of the pharmacokinetic‐dynamic profile of patients with and without troublesome dyskinesia and a phenomenological description of dyskinesia.
Continuous variables were described as mean ± standard deviation. Time course comparisons (T0 vs. T1) of paired data sets were performed using Student's t test, considering both all patients and stratifying for gender. Two group comparisons (patients with troublesome dyskinesia vs. “no/mild dyskinesia”) were performed using the t test or Mann–Whitney U test (continuous variables) and chi‐square or exact Fisher (categorical variable), as appropriate. McNemar's test was adopted for paired comparisons.
An univariate analysis including age, age at disease onset, disease duration, gender, LEDD/kg, BMI, ∆LEDD/kg (LEDD/kg at T1‐LEDD/kg at T0), MDS‐UPDRS part III/II, UPDRS IV total score and single items, HY, S&E, and MMSE, was run to evaluate independent factors associated to the presence or the development of new troublesome dyskinesia at T1, not considering the patients who already had troublesome dyskinesia at T0 for new development analysis. Second, all the significant factors were included in a logistic regression analysis, adjusting for LCIG treatment duration, to identify risk factors associated with troublesome dyskinesia at T1. Equally, a multiple linear regression analysis, adjusting for LCIG treatment duration, was performed to identify risk factors contributing to a higher dyskinesia score (sum of the items 32 and 33) at T1, as dependent variable.
A subgroup analysis on patients who underwent the pharmacokinetic‐dynamic assessment was carried out. Differences were tested using Mann–Whitney U‐test and exact Fisher test for continuous and categorical variables, respectively.
All P values reported are 2‐tailed and a P ≤ 0.05 was considered statistically significant. SPSS 24.0 statistical software (SPSS, Chicago, IL) was used.
Results
Prognostic Factors for Troublesome Dyskinesia
We enrolled 53 patients from a global sample of 64 patients (82%). Six were excluded because of a follow‐up <6 months, 3 were excluded because of missing data at T0, and 2 caregivers declined to participate, because patients were HY 5 and homebound. The duration of LCIG therapy was 51.7 ± 34.1 months (range = 10–120 months). Demographic, clinical, and therapeutic data are detailed in Table 1. Women were comparable to men for all clinical and therapeutic T0/T1 variables (statistical data not shown), with the exception of a lower BMI and more severe dyskinesia (UPDRS IV, item 33) at T1. Overall, motor complications (UPDRS‐IV, total score) globally improved, although such an improvement was principally related to motor fluctuations reduction, because dyskinesia scores (items 32 and 33) did not show statistically significant changes (Fig. 1; Table 1).
TABLE 1.
Demographic, clinical, and therapeutic data of PD patients in treatment with LCIG
| Clinical features (n = 53) | |||
| Age (yr) | |||
| at T0 | 67.6 ± 7 | ||
| at T1 | 71.8 ± 7.9 | ||
| Women: n/total (%) | 20 (37) | ||
| Age at disease onset (yr) | 55.2 ± 7.5 | ||
| Man/woman | 52.9 ± 8.1/53.6 ± 6.5 | ||
| Disease duration (yr) | |||
| at T0 | 14.4 ± 6.2 | ||
| at T1 | 18.6 ± 6.8 | ||
| Duodopa therapy duration (mo) | 51.7 ± 34.1 | ||
| Clinical phenotype n (%) a | AK = 29 (55) | ||
| TD = 19 (36) | |||
| Mixed = 5 (9) | |||
| Baseline vs. follow‐up comparisons | T0 (n = 53) | T1 (n = 53) | P value (T0 vs. T1) |
| IMAO‐B, n (%) | 13 (24) | 3 (5) | 0.257 |
| DAA, n (%) | 38 (71) | 17 (32) | 0.001 |
| LEDD DAA, mg | 155 ± 145 | 34.1 ± 64.1 | <0.001 |
| Total LEDD, mg | 1373 ± 496 | 1528 ± 412 | 0.037 |
| LEDD/kg (mg/kg) | 20.2 ± 8.3 | 24.4 ± 7.5 | 0.003 |
| LEDD/kg (man) | 19.6 ± 4.9 | 23.1 ± 6.1 | 0.001 |
| LEDD/kg (woman) | 21.2 ± 12.2 | 26.4 ± 9.3 | 0.067 |
| H&Y | 2.8 ± 0.9 | 3.1 ± 1.2 | <0.001 |
| Number per stage | 2 = 30; 3 = 5; 4 = 18 | 2 = 26; 3 = 2; 4 = 15; 5 = 10; | |
| SE | 62 ± 21 | 53 ± 21 | <0.001 |
| MDS.UPDRS II (Med On) | 17.8 ± .8.2 | 26.4 ± 14.7 | <0.001 |
| MDS.UPDRS III Med Off | 56.1 ± 16.3 | NA | / |
| MDS.UPDRS III Med On | 33.4 ± 16.7 | 45.6 ± 18.1 | <0.001 |
| UPDRS IV, total score (items 32–42) | 9.3 ± 3 | 4.8 ± 3 | <0.001 |
| MMSE | 26 ± 6.9 | 22.9 ± 7.5 | <0.005 |
| Weight (kg) | 69.5 ± 14.3 | 64.3 ± 13.3 | <0.001 |
| % WL (∆WL/weight at T0)*100 | / | 6 ± 12 | |
| BMI (kg/m2) | 25.5 ± 4.7 | 23.5 ± 4.1 | <0.001 |
| BMI (kg/m2): men/women b | 26.5 ± 4.5/23.7 ± 4.6 | 24.4 ± 4/22.1 ± 3.8 | <0.001/0.1 |
Values are presented as mean ± standard deviation (SD) if not otherwise specified.
Clinical phenotypes were the same at baseline and follow‐up.
Statistically significant difference for women versus men, P values: <0.05 (BMI) and <0.001 (UPDRS IV, item 33 at T1). Statistical significant results are in bold.
SE, Schwab and England ADL Scale; H&Y, Hoehn Yahr stage; LEDD, l‐dopa equivalent daily dose; MMSE, Mini Mental State Examination; BMI, body mass index; WL, weight loss; /: not performed.
FIG 1.
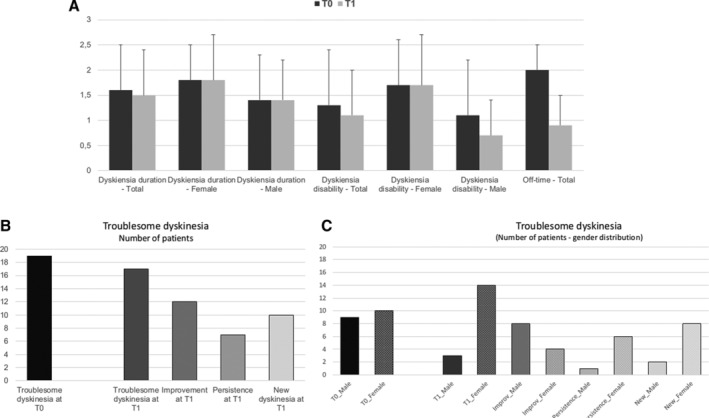
Motor complications comparison at baseline (T0) and follow‐up (T1). (A) Items 32 (dyskinesia duration) and 33 (dyskinesia disability), including male and female values and 39 (OFF‐time) at T0 and T1. (B) Distribution of single scores for item 32 (dyskinesia duration) and item 33 (dyskinesia disability) at T0 and T1. (C) Distribution (number of patients) of troublesome dyskinesia at baseline (T0) and follow‐up (T1), dyskinesia persistence, improvement. and new development for all patients (and stratified per gender). (D) Dyskinesia duration: 0 = none; 1%–25% of day; 2 = 26%–50% of day; 3 = 51%–75% of day; 4 = 76%–100% of day. Dyskinesia disability: 0 = not disabling; 1 = mildly disabling; 2 = moderately disabling; 3 = severely disabling; 4 = completely disabling.
At T0, 19 (35%) patients had troublesome dyskinesia. Between T0 and T1, 12 patients no longer reported troublesome dyskinesia, but 10 subjects reported new troublesome dyskinesia, and 7 continued to have them, which results in 17 (32%) patients with troublesome dyskinesia at T1 (Fig. 1C). LEDD/kg significantly increased at T1 and around half of the patients reported a weight loss of a mean (SD) 6% ± 12% of their baseline BW.
Table 2 and Figure 2 show clinical data comparisons of patients with troublesome dyskinesia at T1 versus patients with no troublesome dyskinesia at T1. The use of amantadine did not confound the findings. Indeed, only 3 patients with troublesome dyskinesia at T1 were taking amantadine, because the rest had previously stopped it because of AEs or inefficacy. Both the presence and the development of new troublesome dyskinesia at T1 were associated to women and longer duration of OFF time at T1 (by UPDRS IV, item 39) (P < 0.01). The presence of troublesome dyskinesia was also correlated with a lower BW at T0 and T1 (P < 0.01), although LEDD/kg was not significantly related to troublesome dyskinesia presence or development at T1.
TABLE 2.
Demographic, clinical and therapeutic data of patients who present “troublesome dyskinesia” at T1 vs. patients who had no “troublesome dyskinesia” at T1
| Troublesome dyskinesia at T1 (n = 17) | No troublesome dyskinesia at T1 (n = 36) | P values patients with “troublesome dyskinesia” vs. patients without “troublesome dyskinesia” | |||
| Clinical features | |||||
| Age (yr) | |||||
| at T0 | 68.5 ± 4.5 | 66.8 ± 7.5 | 0.8 | ||
| at T1 | 72.2 ± 8.1 | 71.2 ± 8.1 | |||
| Women: n (%) | 14 (82%) | 6 (16%) | <0.001 | ||
| Age at disease onset (yr) | 55.2 ± 6.4 | 51.8 ± 8.1 | 0.1 | ||
| Disease duration (yr) | |||||
| at T0 | 13.7 ± 4.3 | 15.2 ± 5.5 | 0.5 | ||
| at T1 | 17.5 ± 6.7 | 19.4 ± 6.8 | |||
| Duodopa therapy duration (mo) | 46.6 ± 27.7 | 53.6 ± 37.7 | 0.1 | ||
| Clinical Phenotype n (%) | AK = 10 (58) | AK = 19 (52) | 0.3 | ||
| TD = 4 (24) | TD = 15 (43) | ||||
| Mixed = 3 (18) | Mixed = 2 (5) | ||||
| Baseline vs. follow‐up comparisons | T0 | T1 | T0 | T1 | |
| LEDD (l‐dopa), mg | 1170 ± 633 | 1373 ± 419 a | 1131 ± 472 | 1538 ± 405 a | 0.8 at T0 and 0.16 at T1 |
| Total LEDD, mg | 1333 ± 633 | 1406 ± 402 | 1408 ± 380 | 1515 ± 415 | 0.6 |
| Total LEDD/kg (mg/kg) | 21.4 ± 11.1 | 26.2 ± 8.7 a | 19.5 ± 5.1 | 23.1 ± 6.4 a | 0.1 |
| Amantadine (a = no; b = yes; c = stopped because of AEs; d = stopped because of inefficacy) | NA | b = 3; c = 9; d = 5 | NA | a = 34; b = 2; | |
| H&Y | 2.9 ± 1.1 | 3.1 ± 1.1 | 2.6 ± 0.9 | 3.1 ± 0.9 a | 0.8 |
| SE | 62.2 ± 19.1 | 53.3 ± 13.2 a | 61.2 ± 23.1 | 53.1 ± 26.5 a | 0.9 |
| MDS.UPDRS II (Med On) | 16.2 ± 4.7 | 24.4 ± 11.3 a | 18.2 ± 9.1 | 28.1 ± 12.6 a | 0.2 |
| MDS.UPDRS III Med Off | 53.3 ± 12.4 | NA | 57.2 ± 13.8 | NA | 0.5 |
| MDS.UPDRS III Med On | 30.8 ± 20.3 | 45.3 ± 13.5 a | 35.1 ± 14.1 | 47.6 ± 19.4 a | b = 0.4 |
| UPDRS IV, Total score | 10.4 ± 2.4 | 7.8 ± 2.2 a | 8.6 ± 3.1 | 3.1 ± 1.9 a | 0.06 (T0) and <0.001 (T1) |
| MMSE | 26.7 ± 7.5 | 25.3 ± 3.3 a | 26.1 ± 6.6 | 21.1 ± 9.2 a | 0.9 (T0) and 0.053 (T1) |
| Weight (kg) | 63.4 ± 13.3 | 56.8 ± 12.2 a | 73.3 ± 13.8 a | 68.7 ± 12.1 | 0.015 (T0) and 0.001 (T1) |
| WL (kg) – n (%) | / | 6.4 ± 9.8–14 (82) | / | 4.3 ± 8.5–17 (47) | 0.3 |
| BMI (kg/m2) | 24.3 ± 4.3 | 22.3 ± 3.2 a | 26.2 ± 4.1 | 24.1 ± 4.3 a | 0.008 (T0) and 0.006 (T1) |
Values are presented as mean ± standard deviation (SD) if not otherwise specified.
P value <0.05 comparing T0 versus T1 within each group of patients. Statistical significant results are in bold.
SE, Schwab and England ADL scale; H&Y, Hoehn Yahr stage; LEDD, l‐dopa equivalent daily dose; MMSE, mini mental state examination; BMI, body mass index; WL, weight loss; /: not performed.
FIG 2.
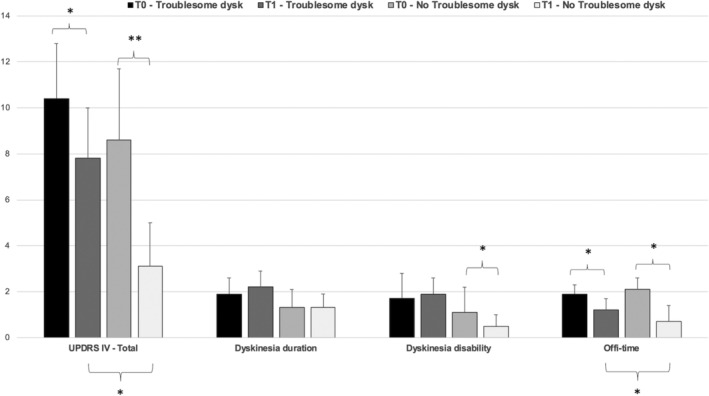
UPDRS‐IV total score and sub‐items (item 32, dyskinesia duration, item 33 dyskinesia disability and item 39, off‐time) at baseline (T0) and follow‐up (T1), stratified for patients with troublesome dyskinesia at T1 (n = 36) and without troublesome dyskinesia at T1 (n = 17). T0 versus T1 statistical significant differences (*P < 0.05 and **P < 0.01) in the upper part of the figure and for groups comparisons (patients with troublesome vs. non troublesome dyskinesia at T1) at the bottom.
The multivariate logistic regression model, including gender, item 39 and BMI at T0, adjusted for LCIG treatment duration, showed that being a woman increases the risk of presenting troublesome dyskinesia at T1 (OR = 9.2, 95% CI = 2.4–37.4). The presence of troublesome dyskinesia at T1 was significantly associated to longer off periods at T1 (OR = 4.4, 95% CI = 1.1–14.3).
Female gender was also significantly correlated to a higher dyskinesia score at T1 (sum of the items 32 and 33: P = 0.001; β = 1.68; 95% CI = 0.3–2.8).
Pharmacokinetic‐Dynamic Assessment
Ten patients (6 with troublesome dyskinesia) underwent the pharmacokinetic‐dynamic assessment. Clinical, therapeutic, and pharmacokinetic‐dynamic data are detailed in Table S1.
l‐dopa plasma kinetics did not differ among groups (Table S1). No statistically significant difference was found in the magnitude of LCIG effect over repeated measurements within each group, though a lower motor benefit was found in the group with troublesome dyskinesia for the ∆Bradik0 ‐ 2 (%) (P = 0.04). As expected, patients with troublesome dyskinesia had higher RDRS scores, although only at the fourth assessment. This is the case despite having similar l‐dopa doses. Even if no statistical correlation were performed because of the small group of patients, graphically, among patients with troublesome dyskinesia, we do not find a linear correlation between the bradykinesia score or dyskinesia severity and the l‐dopa concentration (Fig. 3).
FIG 3.
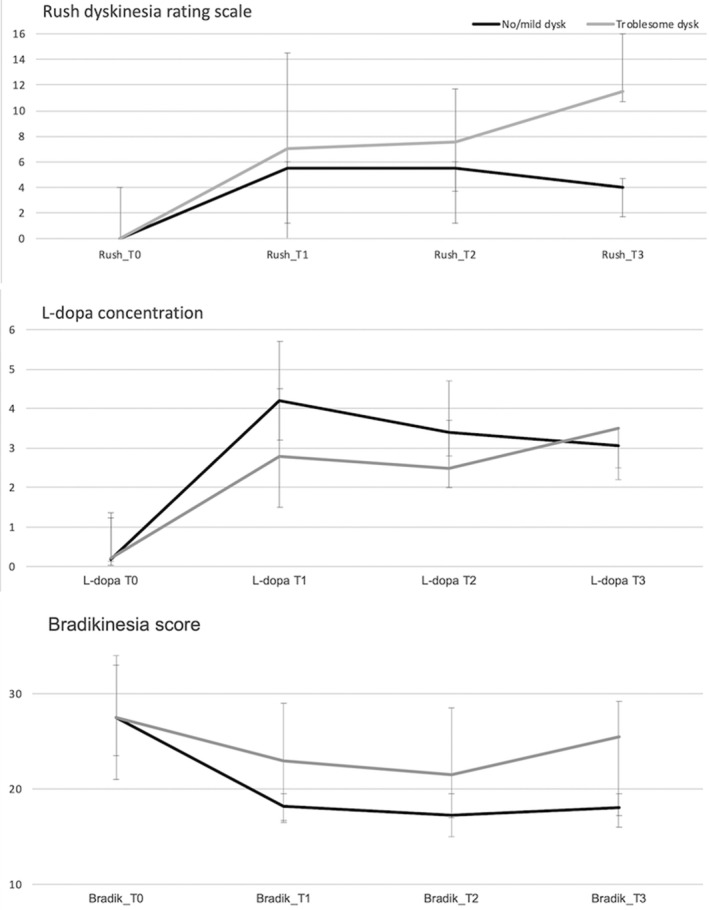
Median l‐dopa concentration (μg/mL) and related Rush Dyskinesias Rating Scale (RDRS) and Bradykinesia scores among patients with troublesome dyskinesia (gray line) and patients with no/mild dyskinesia (black line), before the usual morning dose (T0) and 3 times after the morning dose, during the on‐going continuous dose, at 45‐minute intervals (T1, T2, T3). Bars represent the IQR, 25th to 75th percentile.
Phenomenological description of dyskinesia, timing, therapeutic strategy, and changes after 1 month, are detailed in Table 3. Dyskinesia phenomenology was heterogeneous varying from peak‐dose‐like generalized choreic dyskinesia (Video S1A, Pt 5), to slow choreic movement of lower limbs during ON‐time (Video S1B, Pt 4) and to superimposition of peak‐dose‐like choreic dyskinesia of the truck and upper limbs on rapid at time ballistic movement of lower limbs (Video S1C, Pt 3, ON‐time). Overall, a mild improvement in term of dyskinesia (PGI‐I = 3) was obtained in 4 of 6 patients, based both on dyskinesia's timing and correlation with morning dose/extra bolus and dyskinesia phenomenology, as reported in patient's clinical history.
TABLE 3.
Dyskinesia phenomenological/timing description and therapeutic strategy among a subgroup of patients with “troublesome dyskinesia”
| Patients | Troublesome dyskinesia phenomenology (T1) | Dyskinesia/off‐periods timing, during the 24 hr a | Dyskinesia severity and l‐dopa concentration (μg/mL) | Therapeutic strategy | After 1 mo (T2) |
| Pt‐1 | Lower limbs>>upper limbs, choreic and rarely ballistic movements | Sub‐continuous, with bouts of aggravation, alternated with unpredictable severe long‐lasting off‐time |
RDRS0 = 0; C0 = 0.21 RDRS1 = 0; C1 = 1.09 RDRS2 = 5; C2 = 2.63 RDRS3 = 15; C 3 = 2.72 |
Start 24 hr infusion, lowering the day time continuous dose | Improvement of day time dyskinesia, worsening off‐time, including morning akinesia (PGI‐I = 3) |
| Pt‐2 b | Lower limbs, choreic | Sub‐continuous, no clear modification before and after the extra‐dose; off‐time early in the morning and after lunch |
RDRS0 = 0; C0 = 1.26 RDRS1 = 8; C1 = 4.9 RDRS2 = 7; C2 = 5.7 RDRS3 = 9; C 3 = 5.4 |
Start 24 hr infusion, lowering the day time continuous dose | 24‐hr infusion not tolerated for mechanical reason with negative impact on sleep efficiency (PGI‐I = 5) |
| Pt‐3 | Severe choreic movements, generalized, rarely predominantly in the trunk and lower limbs | Sub‐continuous with aggravation after the morning dose, during meals and in the late afternoon/night; severe off in the afternoon |
RDRS0 = 4; C0 = 0.13 RDRS1 = 14; C1 = 2.95 RDRS2 = 16; C2 = 2.32 RDRS3 = 17; C 3 = 2.41 |
Lowering the morning bolus dose (∆1 cc), increase afternoon continuous dose (0,2 cc/hr) | Improvement (PGI‐I = 3) |
| Pt‐4 | Lower limbs and truck, mainly slow and choreic, more rapid in the afternoon | Before/after LCIG starting in the morning and during the whole afternoon |
RDRS0 = 0; C0 = 0.2 RDRS1 = 4; C1 = 4.42 RDRS2 = 8; C2 = 3.74 RDRS3 = 9; C 3 = 4.39 |
Increase continuous dose (0.2 cc/hr), lowering (0.5 cc) and anticipate the morning dose | Improvement (PGI‐I = 3) |
| Pt‐5 c | Choreic lower limbs >>upper limber; severe and painful off‐dystonia of the lower limb | The whole afternoon; Off‐dystonia in the morning/night, rarely in the afternoon |
RDRS0 = 0; C0 = 0.12 RDRS1 = 0; C1 = 1.74 RDRS2 = 0; C2 = 1.83 RDRS3 = 11; C 3 = 4.64 |
Increase continuous dose (0.2 cc/h) and morning bolus dose (0.5 cc/hr) | Improvement (both dyskinesia and off‐dystonia) (PGI‐I = 3) |
| Pt‐6 b | Severe choreic movements, generalized, >>upper limbs and trunk | Sub‐continuous with bouts of aggravation during meals and late‐afternoon, alternated with unpredictable off‐time |
RDRS0 = 4; C0 = 1.67 RDRS1 = 16; C1 = 2.79 RDRS2 = 10; C2 = 2.16 RDRS3 = 10; C 3 = 1.85 |
24 hr infusion, lowering the day time continuous dose | Worsening of dyskinesia in the afternoon/night (PGI‐I = 5) |
Dyskinesia timing during the last week before the assessment, as referred by the patients and caregivers.
Pt‐2 and Pt‐6 stopped the infusion only 8 hours before the assessment because of severe “off.”
Pt‐5 after study ending an abdominal X‐ray found that the tube was erroneously placed at a gastric level PGI‐I: Patient's Global impression‐Improvement (with PGI‐I 3: minimally improved; PGI‐I 4: no change; PGI‐I 5: minimally worse).
At the same time, we used the pharmacokinetic‐dynamic assessment to perform a combined patient–physician observation of dyskinesia phenomenology to understand which kind of movement were perceived as “troublesome” by the patients (Table 3).
Discussion
The management of troublesome motor complications in advanced PD is still an unmet clinical need for which LCIG is one of the currently effective therapeutic options. 3 In our cohort, after ~4 years of LCIG treatment, we still find a substantial benefit in terms of motor fluctuations if compared to baseline, although with a concomitant deterioration of motor performances, activities of daily living (ADLs), and cognitive scores, despite a significant higher dose of antiparkinsonian medication. Indeed, the only score that still detects an improvement is the UPDRS‐IV, mainly related to the reduction of OFF‐time. Ten patients evolved to HY 5, which additionally highlights the severity of the disease progression. Indeed, worsening of motor performances, as per the MDS‐UPDRS part III even in the Med On condition and ADLs scores (MDS‐UPDRS part II and SE), was significant and equally present among patients with troublesome and non‐troublesome dyskinesia (Table 2). Such an aggravation of motor symptoms and ADLs, while On‐medication, is also reported among PD patients submitted to subthalamic‐deep brain stimulation (STN‐DBS) at 5 years follow‐up, showing the fact that device‐aided therapies are symptomatic treatments mainly of the off periods and do not improve symptoms during the on period, which may get worse because of disease progression. 19 Additionally, patients selected for LCIG may present axial signs in the Med On condition, at baseline, which is not the case for STN‐DBS‐treated patients, which tends to aggravate faster with disease progression. Nevertheless, our finding seems to suggest only a slightly superior deterioration of motor symptoms and patients' independency in ADLs, if compared to few previously published studies with long‐term follow‐up of 3 to 4 years of LCIG treatment. 3 , 20
Overall, score for dyskinesia duration (item 32) and disability (item 33) do not change significantly, despite a tendency for lower frequency of patients with severely disabling dyskinesia (item 33 ≥3). Nevertheless, troublesome dyskinesia (item 33 ≥2) endured in 13% of patients and 18% still developed new troublesome dyskinesia. Lower efficacy on dyskinesia management has been previously suggested among patients with mild baseline dyskinesia at around 24 months follow‐up when compared to patients with more severe dyskinesia at baseline. 6 Additionally, a retrospective study on 208 LCIG‐treated PD patients found that patients with baseline mixed dyskinesia, which is both peak‐dose and continuous atypical biphasic‐like, present the worst outcome. 10 A few case series have also reported the possible development of new troublesome dyskinesia after starting LCIG treatment. 8 , 9 In this context, the identification of any risk factors for troublesome dyskinesia could be relevant, because it has seldom 10 been specifically investigated for LCIG‐treated PD patients.
In our cohort, female gender was the only significant baseline feature related to the persistence and development of new troublesome dyskinesia that were also associated with longer off‐time at T1. Patients with lower BW may also present troublesome dyskinesia, and we found a statistically significant decrease of BW and BMI at follow‐up if compared to baseline. Among PD patients, weight loss is usually related to multiple factors, such as dysphagia, difficulties in self‐feeding, intestinal hypomotility, depression, and cognitive impairment. However, in LCIG‐treated patients, it can be strictly related to dyskinesia, in particular with the percentage of the waking day spent with dyskinesia. 21 Even though a lower BW does not keep significance at the logistic regression analysis for troublesome dyskinesia. At the same time, we know that young age at disease onset, female gender, low BW, AK phenotype, greater motor disability, and higher LEDD are recognized risk factors for LID among PD patients on oral treatment. 1 , 22 , 23 , 24 , 25 However, we found no difference at T0 between men and women for all clinically relevant variables for LID, including LEDD/kg, therefore indicating an apparent pure gender‐related relationship in our cohort. At the same time, it has never been investigated if the higher prevalence of dyskinesia among women could be partly influence by a gender‐related difference in dyskinesia awareness.
Patients who still report troublesome dyskinesia seem to represent a subgroup of patients with a more severe disease and motor fluctuations, because they also display longer off‐periods during LCIG treatment, despite similar LEDD/kg that cannot be increased because of the presence of dyskinesia. Equally, other therapeutic strategies (eg, the use of amantadine) have been tried with poor success because of inefficacy or AEs.
The pharmacokinetic‐dynamic assessment revealed similar kinetic profiles comparing patients with and without troublesome LID. This could be in line with the finding of a previous study that found similar plasma kinetics among PD patients with different motor response throughout different disease stages. 26 Moreover, a tendency for a lower motor benefit and the appearance of more severe dyskinesia was observed among patients with troublesome dyskinesia, despite similar l‐dopa plasma concentration, suggesting an erratic dose–effect relationship and more severe dopaminergic denervation with consequent altered striatal output among those patients. Therapeutic strategies for these involuntary movements can be particularly challenging due to the complexity of concomitant motor fluctuations and the difficulties in interpreting the timing of dyskinesia and motor function during a continuous treatment delivering. Indeed, in patients treated with LCIG, the association between l‐dopa plasma concentration and biphasic‐type dyskinesia is not well investigated. A case report of 2 patients has described biphasic‐like dyskinesia, with rapid repetitive movement of the lower limbs, associated with a high l‐dopa concentration, 9 suggesting that a dichotomic separation in peak‐dose and diphasic dyskinesia can be challenging in LCIG‐treated patients. This was the case for our patients, who also presented mixed and continuous dyskinesia types that were difficult to classify and treat. Concomitantly, a 24‐hour infusion has shown an efficacy on motor fluctuations/nocturnal akinesia reduction, although data on troublesome dyskinesia are heterogeneous, 27 as shown in our patients. We performed a pharmacokinetic‐dynamic assessment to verify if it could be helpful in the correct management of LCIG‐treated patients. Indeed, previous studies have shown a predictable motor response even among difficult‐to‐treat patients. 28 However, interindividual variability may occur, 28 and no generalizable recommendations are yet possible. We believe that a combined clinical and pharmacodynamic approach, including 1‐day long observation of patients' clinical status and dyskinesia phenomenology, could be helpful in any case of troublesome and unresponsive dyskinesia under LCIG treatment.
The retrospective analysis of some data and the small cohort of included patients are the main limitation of our findings. The last could have influenced the logistic regression results giving high OR values, as well as the groups' comparisons of our pharmacological assessment. A deeper pharmacokinetic‐dynamic assessment, in a larger sample, should be performed, including all‐day long l‐dopa concentration and individualized assessment of clinical/pharmacological response to extra bolus. Additionally, based on our data availability, we have adopted the UPDRS item 33 to define troublesome dyskinesia, and we acknowledge this is an arbitrary criterion, although the scale is universally adopted in dyskinesia‐specific trials. 29 , 30 However, there is no consensus on how to define the outcome in terms of troublesome dyskinesia. Indeed, trials variably adopt the threshold of ≥1 or ≥4 hour/day 31 of troublesome dyskinesia, as well as the total hour/day with troublesome dyskinesia, as based on the patient's diary or changes in Unified Dyskinesia Rating Scale and the Abnormal Involuntary Movement Scale total score, depending on study design. 32 Finally, LCIG treatment duration was quite heterogeneous in this cohort, including patients with a follow‐up duration ranging from 1.5 to 6 years, even though this was taken into account by adjusting the logistic regression model for LCIG treatment duration.
Conclusions
Being a woman, and, to a lesser extent, having a lower BW may be associated with a less favorable outcome in terms of LID management under chronic LCIG treatment. This sub‐group of PD patients should be carefully monitored once started on this device‐aided therapy. Individualized adjustment of dose parameters, based on dyskinesia response to morning dose/extra bolus, may be particularly helpful. A specific trial that combines a pharmacokinetic‐dynamic assessment, all‐day long clinical observation, and patient's diaries with different treatment strategies should be performed to elaborate a therapeutic algorithm for LCIG patients with troublesome dyskinesia.
Authors' Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.F.: 1A, 1C, 2A, 3A
M.Z.: 1B, 2A, 3B
G.C.‐B.: 1B, 2A, 3B
M.C.: 1A, 2A, 3B
L.S.: 1B, 1C, 3B
S.M.: 1B, 1C, 3B
A.R.: 1B, 1C, 3B
P.B.: 2A, 2 B, 2C
G.I.: 1C, 3B
G.G.: 1C, 3B
M.G.R.: 1C, 3B
C.A.A.: 1C, 3B
P.C.: 1B, 2A, 3B
L.L.: 1B, 2A, 3B
Disclosures
Ethical Compliance Statement Section
The Ethical Committee of the “A.O.U. Città della Salute e della Scienza” of Turin, approved the study (CS2/1243; protocol number 0043547). Patients provided written informed consent to participate in the study and publication of related videos. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
The study had no specific funding. The authors report no conflict of interest.
Financial Disclosures for the Previous 12 Months
Dr. Margherita Fabbri: Honoraria to speak: AbbVie; Grants: AbbVie. Maurizio Zibetti: Honoraria to speak and grants: Medtronic, Lundbeck, UCB Pharma and AbbVie. Giovanna Calandra‐Buonaura: Honoraria to speak: Zambon Italia, Abbvie SRL and UCB Pharma S.P.A. Manuela Contin, Luisa Sambati, Susan Mohamed, Paola Berchialla, Gabriele Imbalzano, Professor Pietro Cortelli, and Giulia Giannini have no disclosures. Alberto Romagnolo: Grant support and speaker honoraria from AbbVie, speaker honoraria from Chiesi Farmaceutici and travel grants from Lusofarmaco and UCB Pharma. Mario Giorgio Rizzone: Honoraria to speak and grants: Zanbom. Carlo Alberto Artusi: Honoraria to speak and grants: Zanbom and AbbVie. Prof. Leonardo Lopiano: Honoraria to speak and grants: Medtronic, UCB Pharma, AbbVie and Doc Generici.
Supporting information
Table S1. Clinical, therapeutic, and pharmacokinetic‐dynamic data of the patients. Values are presented as median (IQR, 25th–75th percentile) if no otherwise specified. H&Y, Hoehn & Yahr; LEDD, l‐dopa equivalent daily dose; C0, l‐dopa concentration before the morning bolus dose; C1, C2, C3, l‐dopa concentration during the on‐going continuous dose at 45‐minute intervals each; 3‐OMD, 3‐O‐methyldopa; Bradik, bradykinesia score (sum of the items 3.4–3.8 of the MDS‐UPDRS part III); RDRS, Rush Dyskinesias Rating Scale.
Video S1. (A) (Med Off) Pt‐5, before the morning bolus; (Med On) during the fourth assessment, with generalized choreic dyskinesia. (B) (Med Off) Pt‐4, before the morning bolus; (Med On) during the fourth assessment, with slow choreic dyskinesia, predominantly in the lower limbs and with lesser extend in the trunk. (C) (Med Off) Pt‐3, before the morning bolus, presenting mild dyskinesia of the neck/head and right lower limb; (Med On) during the fourth assessment, with superimposition of choreic peak‐dose on biphasic‐like dyskinesia with rapid lower limbs movements.
Acknowledgments
The authors thank all the patients and their families for participating in this study.
References
- 1. Espay AJ, Morgante F, Merola A, et al. Levodopa‐induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol 2018;84:797–811. [DOI] [PubMed] [Google Scholar]
- 2. Turcano P, Mielke MM, Bower JH, Parisi JE, Cutsforth‐Gregory JK, Ahlskog JE, Savica R. Levodopa‐induced dyskinesia in Parkinson disease: a population‐based cohort study. Neurology 2018;91(24):e2238–e2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernandez HH, Boyd JT, Fung VSC, et al. Long‐term safety and efficacy of levodopa‐carbidopa intestinal gel in advanced Parkinson's disease. Mov Disord 2018;33(6):928–936. [DOI] [PubMed] [Google Scholar]
- 4. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol 2014;13(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antonini A, Fung VS, Boyd JT, et al. Effect of levodopa‐carbidopa intestinal gel on dyskinesia in advanced Parkinson's disease patients. Mov Disord 2016;31(4):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buongiorno M, Antonelli F, Camara A, et al. Long‐term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the Barcelona registry. Parkinsonism Relat Disord 2015;21(8):871–876. [DOI] [PubMed] [Google Scholar]
- 7. Zibetti M, Merola A, Artusi CA, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson's disease: a 7‐year experience. Eur J Neurol 2014;21(2):312–318. [DOI] [PubMed] [Google Scholar]
- 8. Meloni M, Solla P, Mascia MM, Marrosu F, Cannas A. Diphasic dyskinesias during levodopa‐carbidopa intestinal gel (LCIG) infusion in Parkinson's disease. Parkinsonism Relat Disord 2017;37:92–96. [DOI] [PubMed] [Google Scholar]
- 9. Catalan MJ, Escribano PM, Alonso‐Frech F. Dyskinesias in levodopa‐carbidopa intestinal gel infusion era: new challenges, new features. Mov Disord 2017;32(4):624–625. [DOI] [PubMed] [Google Scholar]
- 10. Marano M, Naranian T, di Biase L, et al. Complex dyskinesias in Parkinson patients on levodopa/carbidopa intestinal gel. Parkinsonism Relat Disord 2019;69:140–146. [DOI] [PubMed] [Google Scholar]
- 11. Regidor I, Santos‐Garcia D, Catalan MIJ, et al. Impact of disease duration in effectiveness of treatment with levodopa‐carbidopa intestinal gel and factors leading to discontinuation. J Parkinsons Dis 2019;9(1):173–182. [DOI] [PubMed] [Google Scholar]
- 12. Nyholm D, Johansson A, Aquilonius SM, Hellquist E, Lennernas H, Askmark H. Complexity of motor response to different doses of duodenal levodopa infusion in Parkinson disease. Clin Neuropharmacol 2012;35(1):6–14. [DOI] [PubMed] [Google Scholar]
- 13. Nyholm D, Askmark H, Gomes‐Trolin C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained‐release tablets. Clin Neuropharmacol 2003;26(3):156–163. [DOI] [PubMed] [Google Scholar]
- 14. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 15. Schwab RS, England A. Projection technique for evaluating surgery in Parkinsonʼs disease In: Billingham FH, Donaldson MC, eds. Third Symposium on Parkinsonʼs Disease. Edinburgh, UK: Churchill Livingstone; 1969:152–157. [Google Scholar]
- 16. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 17. Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson's disease rating scale scores to Movement Disorder Society‐unified Parkinson's disease rating scale scores. Mov Disord 2012;27(10):1239–1242. [DOI] [PubMed] [Google Scholar]
- 18. Contin M, Riva R, Martinelli P, Cortelli P, Albani F, Baruzzi A. A levodopa kinetic‐dynamic study of the rate of progression in Parkinson's disease. Neurology 1998;51(4):1075–1080. [DOI] [PubMed] [Google Scholar]
- 19. Limousin P, Foltynie T. Long‐term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol 2019;15(4):234–242. [DOI] [PubMed] [Google Scholar]
- 20. Zibetti M, Merola A, Ricchi V, et al. Long‐term duodenal levodopa infusion in Parkinson's disease: a 3‐year motor and cognitive follow‐up study. J Neurol 2013;260(1):105–114. [DOI] [PubMed] [Google Scholar]
- 21. Fabbri M, Zibetti M, Beccaria L, et al. Levodopa/carbidopa intestinal gel infusion and weight loss in Parkinson's disease. Eur J Neurol 2019;26(3):490–496. [DOI] [PubMed] [Google Scholar]
- 22. Bjornestad A, Forsaa EB, Pedersen KF, Tysnes OB, Larsen JP, Alves G. Risk and course of motor complications in a population‐based incident Parkinson's disease cohort. Parkinsonism Relat Disord 2016;22:48–53. [DOI] [PubMed] [Google Scholar]
- 23. Cilia R, Akpalu A, Sarfo FS, et al. The modern pre‐levodopa era of Parkinson's disease: insights into motor complications from sub‐Saharan Africa. Brain 2014;137(Pt. 10):2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manson A, Stirpe P, Schrag A. Levodopa‐induced‐dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2012;2(3):189–198. [DOI] [PubMed] [Google Scholar]
- 25. Warren Olanow C, Kieburtz K, Rascol O, et al. Factors predictive of the development of levodopa‐induced dyskinesia and wearing‐off in Parkinson's disease. Mov Disord 2013;28(8):1064–1071. [DOI] [PubMed] [Google Scholar]
- 26. Contin M, Riva R, Martinelli P, Albani F, Avoni P, Baruzzi A. Levodopa therapy monitoring in patients with Parkinson disease: a kinetic‐dynamic approach. Ther Drug Monit 2001;23(6):621–629. [DOI] [PubMed] [Google Scholar]
- 27. Morales‐Briceno H, Mahant N, Ha AD, et al. Long‐term safety and efficacy of 24‐hour levodopa‐carbidopa intestinal gel in Parkinson's disease. Mov Disord 2019;34:1747–1748. [DOI] [PubMed] [Google Scholar]
- 28. Westin J, Nyholm D, Palhagen S, et al. A pharmacokinetic‐pharmacodynamic model for duodenal levodopa infusion. Clin Neuropharmacol 2011;34(2):61–65. [DOI] [PubMed] [Google Scholar]
- 29. Ory‐Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 2014;82(4):300–307. [DOI] [PubMed] [Google Scholar]
- 30. Colosimo C, Martinez‐Martin P, Fabbrini G, et al. Task force report on scales to assess dyskinesia in Parkinson's disease: critique and recommendations. Mov Disord 2010;25(9):1131–1142. [DOI] [PubMed] [Google Scholar]
- 31. Poewe W, Chaudhuri KR, Bergmann L, Antonini A. Levodopa‐carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry. Neurodegener Dis Manag 2019;9(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oertel W, Eggert K, Pahwa R, et al. Randomized, placebo‐controlled trial of ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson's disease (EASE LID 3). Mov Disord 2017;32(12):1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical, therapeutic, and pharmacokinetic‐dynamic data of the patients. Values are presented as median (IQR, 25th–75th percentile) if no otherwise specified. H&Y, Hoehn & Yahr; LEDD, l‐dopa equivalent daily dose; C0, l‐dopa concentration before the morning bolus dose; C1, C2, C3, l‐dopa concentration during the on‐going continuous dose at 45‐minute intervals each; 3‐OMD, 3‐O‐methyldopa; Bradik, bradykinesia score (sum of the items 3.4–3.8 of the MDS‐UPDRS part III); RDRS, Rush Dyskinesias Rating Scale.
Video S1. (A) (Med Off) Pt‐5, before the morning bolus; (Med On) during the fourth assessment, with generalized choreic dyskinesia. (B) (Med Off) Pt‐4, before the morning bolus; (Med On) during the fourth assessment, with slow choreic dyskinesia, predominantly in the lower limbs and with lesser extend in the trunk. (C) (Med Off) Pt‐3, before the morning bolus, presenting mild dyskinesia of the neck/head and right lower limb; (Med On) during the fourth assessment, with superimposition of choreic peak‐dose on biphasic‐like dyskinesia with rapid lower limbs movements.


