At the time of this manuscript going to press, Europe remains the epicenter of the COVID-19 pandemic and new cases and deaths in the UK continue to demonstrate an exponential rise.1 London has the highest number of reported UK cases.2 Clinical reports indicate that older adults with comorbidities such as diabetes and hypertension are most at risk of severe COVID-19.3,4 Overwhelming inflammation and cytokine associated lung injury are potential pathological features. Secondary hemophagocytic lymphohistiocytosis-like syndrome with raised pro-inflammatory cytokines has been associated with adverse outcomes.5
King’s College Hospital is a teaching hospital in South London, caring for approximately 500 adults and 500 children with sickle cell disease (SCD). South London currently has some of the highest numbers of confirmed cases of COVID-19 in England2 and a large local SCD cohort. It is thought that patients with SCD might demonstrate a more severe illness if infected with SARSCoV- 2 due to associated functional hyposplenia, high prevalence of concomitant chronic respiratory disease and increased levels of inflammation.6
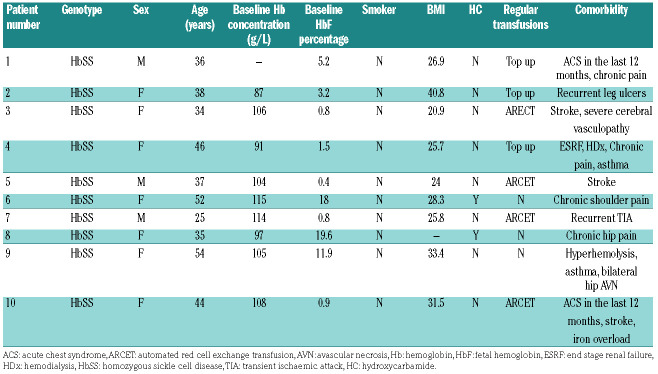
In this report we describe the clinical features of the first 10 confirmed cases of COVID-19 in patients with SCD in the King’s College Hospital. At the time of this report, there were 22,141 confirmed cases of COVID-19 in the UK, of which the majority of cases were from the boroughs of Lambeth and Southward in South London.2 All patients underwent real-time quantitative PCR assay from RNA extracted from nasopharyngeal swabs using a locally validated procedure recommended by Public Health England.7 All patients had homozygous SCD (HbSS) and presented with symptoms such as cough, fever, coryza and associated acute sickle vaso-occlusive pain. None had any recent travel history (Table 1).
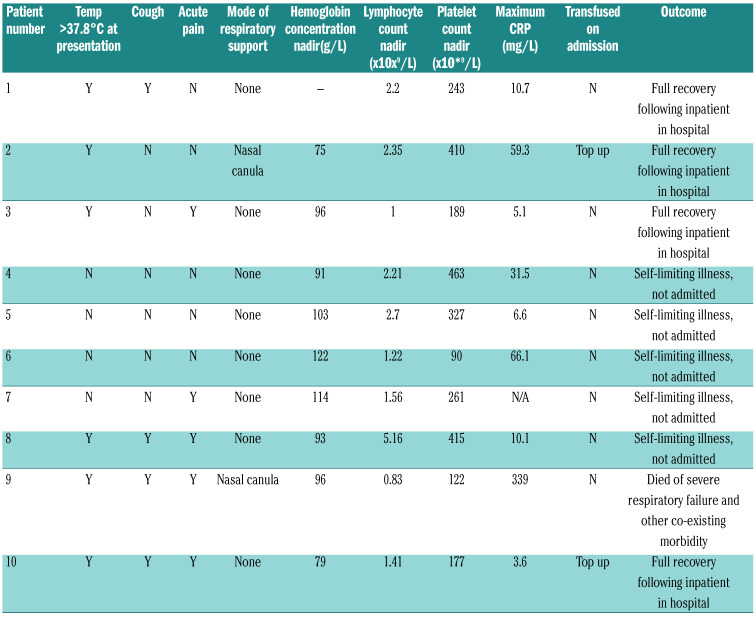
Apart from patient 9 who has severe pre-morbid disease with intensive care admission within the last 12 months due to SCD-related cerebrovascular disease, all patients had relatively mild clinical symptoms related to COVID-19 (Table 2).
In this series, seven patients were female, and the median age was 37 years (range: 25-54 years). No children were seen with SCD and COVID-19. All but two patients were on some disease modification treatment, either hydroxycarbamide (2 of 10) or transfusions (6 of 10). Patients on top-up transfusions were on a 4-weekly program with a post-transfusion hemoglobin target of 120-130 g/L. One patient was on an angiotensin converting enzyme inhibitor (patient 6), and this was not discontinued during the period of illness. Two patients had a history of overt strokes and one patient had a history of recurrent transient ischemic attacks. All patients had ongoing comorbidities, ranging from end stage renal failure to hyperhemolysis. All admitted patients received standard thromboprophylaxis with low molecular weight heparin injection as per hospital venous thromboembolism prevention guidelines.
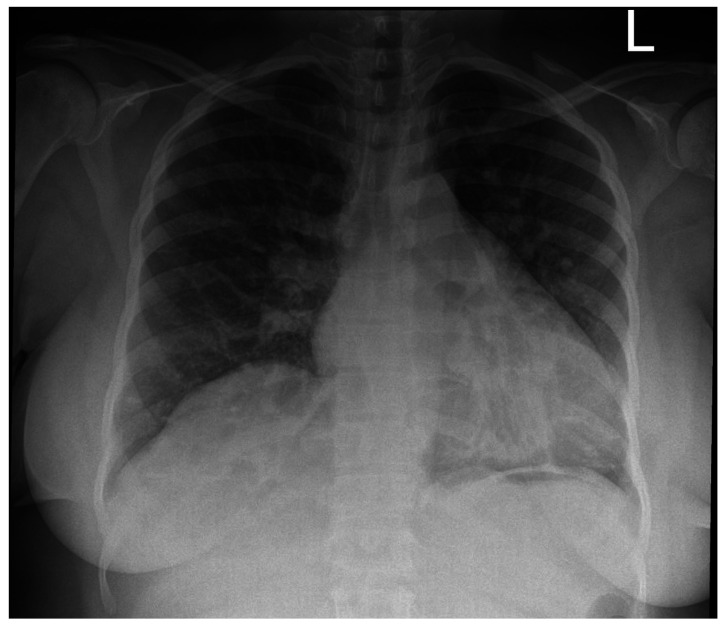
The mean number of days from onset of symptoms to PCR testing was 2.5 days. Of the seven patients in this cohort needing hospital admission, the mean number of days from the onset of symptoms to hospital admission was two days. Nine of 10 patients made a full recovery. Two patients presenting with cough and hypoxia received early top up transfusions. See Figure 1 for the chest radiograph of patient 2 who was hypoxic on admission and received an additive transfusion. All in-patients received broad spectrum antibiotics to cover community acquired pneumonia. No COVID-19 specific treatment was given. The lymphocyte count fell significantly during infection compared to the baseline, from a median of 3.7 to 1.9 x109/L (P=0.037, Wilcoxon signed-rank test).
One patient died of respiratory complications following COVID-19. She had multiple comorbidities, including a history of brittle asthma and hyperhemolysis with multiple red cell alloantibodies, making it difficult to transfuse her. Escalation to ventilation was deemed unsuitable due to existing comorbidities.
Table 1.
Characteristics of COVID-19 positive patients with sickle cell disease.
Table 2.
COVID-19 clinical features in SCD patients.
Based on our small early cohort of 10 individuals with HbSS who have tested positive for COVID-19, patients seem to be experiencing a relatively mild course despite having significant associated comorbidities such as end stage renal failure, severe cerebral vasculopathy and recurrent painful episodes. Half were managed at home with regular telephone contact by the clinical team.
Our first (and so far) only fatality was in an individual of >50 years with poor pre-infection performance status and severe pre-existing lung disease, who had had admissions to intensive therapy unit (ITU) within the last 12 months, as well as multiple red cell alloantibodies and a previous history of severe delayed hemolytic transfusion reactions, which precluded transfusion. This patient had lymphopenia, thrombocytopenia and a high C-reactive protein (CRP), which have been identified as poor prognostic markers in patients without SCD.
Seven of 10 patients in this series were female, all patients were non-smokers and all but two were on a disease modifying treatments, such as regular a blood transfusion programme or hydroxycarbamide. These demographic features may have contributed to the mild clinical course in all but one patient.
Figure 1.
Chest radiograph of patient 2 who was hypoxic on admission and received an early additive transfusion. This showed bilateral congestive changes with no additional pulmonary parenchymal pathology and was obtained on day 2 of admission.
SCD is mentioned in the Public Health England list of conditions which should prompt individuals to be shielded from infection, by rigorous self-isolation. Our series shows that patients with SCD can have a relatively mild course with COVID-19. It is difficult to speculate why this might be the case, and it may be postulated that most of our patients were already on some form of disease modification, which may have helped with the host response. It is unclear whether hyposplenism has played a role in the apparent lack of a hyperimmune syndrome and this is likely to be an area of research in the future. The one patient who died was in a poor prognostic group, based on risk factors identified in the general population. So far, we have seen no children with COVID-19 and SCD, suggesting that this may be a mild condition in children with SCD, as has been found in the general population. It is not entirely clear why more women are represented in this group. It is possible that with time, this ratio may become more skewed to the male sex. The relatively low case fatality is evidence that affected individuals should not be excluded from potentially lifesaving measures including respiratory support and artificial ventilation, particularly as our well-studied cohort have a median survival of 67 years.8
Supplementary Material
References
- 1.World Health Organisation. Coronavirus disease (COVID-19) situation summary. 2020 27/3/2020. Available from: https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd. [Google Scholar]
- 2.Public Health England. Total UK COVID-19 cases update 2020 27/3/20. Available from: https://www.arcgis.com/apps/opsdashboard/index.html#/f94c3c90da5b4e9f9a0b19484dd4bb14. [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. [DOI] [PubMed] [Google Scholar]
- 7.Public Health England. Guidance and standard operating procedure COVID-19 virus testing in NHS laboratories. 2020. https://www.england.nhs.uk/coronavirus/publication/guidanceand-standard-operating-procedure-covid-19-virus-testing-in-nhslaboratories/ [Google Scholar]
- 8.Gardner K, Douiri A, Drasar E, et al. Survival in adults with sickle cell disease in a high-income setting. Blood. 2016;128(10):1436-1438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.