ABSTRACT
Background
Functional motor disorders (FMDs) are abnormal movements that are significantly altered by distractive maneuvers and are incongruent with movement disorders seen in typical neurological diseases.
Objective
The objectives of this article are to (1) describe the clinical manifestations of FMDs, including nonmotor symptoms and occurrence of other functional neurological disorders (FND); and (2) to report the frequency of isolated and combined FMDs and their relationship with demographic and clinical variables.
Methods
For this multicenter, observational study, we enrolled consecutive outpatients with a definite diagnosis of FMDs attending 25 tertiary movement disorders centers in Italy. Each patient underwent a detailed clinical evaluation with a definition of the phenotype and number of FMDs (isolated, combined) and an assessment of associated neurological and psychiatric symptoms.
Results
Of 410 FMDs (71% females; mean age, 47 ± 16.1 years) the most common phenotypes were weakness and tremor. People with FMDs had higher educational levels than the general population and frequent nonmotor symptoms, especially anxiety, fatigue, and pain. Almost half of the patients with FMDs had other FNDs, such as sensory symptoms, nonepileptic seizures, and visual symptoms. Patients with combined FMDs showed a higher burden of nonmotor symptoms and more frequent FNDs. Multivariate regression analysis showed that a diagnosis of combined FMDs was more likely to be delivered by a movement disorders neurologist. Also, FMD duration, pain, insomnia, diagnosis of somatoform disease, and treatment with antipsychotics were all significantly associated with combined FMDs.
Conclusions
Our findings highlight the need for multidimensional assessments in patients with FMDs given the high frequency of nonmotor symptoms and other FNDs, especially in patients with combined FMDs.
Keywords: functional neurological disorders, functional dystonia, functional tremor, functional weakness, diagnosis
Functional motor disorders (FMDs) are abnormal movements that are significantly altered by distractive maneuvers and are incongruent with movement disorders seen in typical neurological diseases. 1 FMDs include disorders characterized by either poverty of movement (weakness and slowness) or hyperkinesia (tremor, jerks, and dystonia). 2
Stressful life events 3 , 4 have been associated with FMDs, but a clear psychological causation may be also absent. 5 , 6 Accordingly, in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, 7 psychological stressors preceding the onset of symptoms are not needed for the diagnosis of functional neurological disorders (FNDs), but positive symptoms and signs should be taken into account. 2 Yet, inclusionary approach to FND diagnosis, at least in the movement disorders field, 8 is limited.
Despite being largely misunderstood and underestimated, FMDs are very common 9 , 10 and negatively impact on quality of life and working life. 11 , 12 Knowledge of their clinical features stem from small cohorts of single neurological services. 9 A few studies have provided insights on the frequency of different FMD phenotypes and their association with nonmotor symptoms and other FNDs. 13 , 14 , 15 , 16 However, these reports had a retrospective design 14 or were based on reviews of clinical notes or email contact with the treating neurologists 13 or included cohorts followed up in tertiary referral centers 16 or FND specialist clinics. 14 Finally, it is unclear if people having single (isolated FMD) or multiple (combined FMDs) motor manifestations may differ for associated demographic and clinical variables.
Based on these premises, this cross‐sectional multicenter study in a large Italian cohort of patients with FMDs was designed (1) to describe the clinical manifestations of FMDs, including nonmotor symptoms and the occurrence of other FNDs, and (2) to report the frequency of isolated and combined FMDs and their relationship with demographic and clinical variables.
Methods
For this cross‐sectional study, data were extracted from the Italian Registry of Functional Motor Disorders (IRFMD; investigators listed in Appendix 1) managed by the Department of Neurosciences, Biomedicine and Movement Sciences, University of Verona, and by the Italian Academy for the Study of Parkinson's Disease and other Movement Disorders (Accademia LIMPE‐DISMOV, Italian Academy for the Study of Parkinson's Disease and other Movement Disorders). The IRFMDs prospectively collects data on symptoms, natural history, risk factors, and comorbidity in FMDs.
Participants
Consecutive outpatients with FMDs were recruited from 25 tertiary movement disorders centers (11 in northern, 5 in central, and 6 in southern Italy and 3 in Sardinia/Sicily) between September 1, 2018, and August 31, 2019. Patients identified with 1 (isolated FMD) or multiple FMDs (combined; eg, dystonia + tremor) underwent standardized clinical assessments.
To be recorded in the IRFMD, a patient's medical and medication history had to be documented by medical records or statements from informed relatives. Patient information was recorded using a web‐based, encrypted, and anonymized system in the website of the Italian Academy for the Study of Parkinson's Disease and other Movement Disorders (https://www.accademialimpedismov.it), which complied with General Data Protection Regulation.
Patients were assessed at each center in a single session by a neurologist specialized in movement disorders. Inclusion criteria were age ≥ 10 years; a clinically definite diagnosis of FMDs based on Gupta and Lang diagnostic criteria 17 with the presence of distractibility maneuvers and a demonstration of positive signs 2 ; and the presence of 1 or more clinical symptoms, including tremor, 18 weakness, 19 jerks, 20 dystonia, 21 gait disorders, 22 parkinsonism, 23 and facial motor disorders. 24 Exclusion criteria were the presence of cognitive or physical impairments that precluded signing the informed consent form for participation in the study.
The IRFMD was structured in the following 3 main sections: demographic data, clinical history and diagnosis, and clinical manifestations. Demographic data included age, gender (male/female), and education level (years). We further classified education level as primary (including the first 5 grades of school), secondary (from Grade 6 to high school diploma), and tertiary (university). The second section included clinical history and diagnosis. This part documented the number of physicians, investigations, and previous diagnoses (“organic” and “nonorganic”) predating the final diagnosis of FMDs. The third section screened for the following clinical manifestations of FMDs: (1) onset of FMDs (acute, defined as abrupt with deterioration within a few days or weeks; slowly progressing) and disease duration, (2) presence of spontaneous remissions, (3) phenotypes (tremor, weakness, dystonia, jerks, parkinsonism, gait disorders, facial movement disorders), (4) presence of other FNDs (sensory functional symptoms, nonepileptic seizures [PNES], visual and cognitive functional symptoms, fibromyalgia, functional bowel disorders), (5) patients' self‐reported nonmotor symptoms (anxiety, fatigue, pain, headache, insomnia, panic attacks, and depersonalization/derealization), (6) certified neurological comorbidities as per a neurologist's diagnosis (migraine, neuropathy, hyperkinetic motor and seizures, Parkinson's disease and/or parkinsonism, multiple sclerosis, and chronic cerebrovascular diseases), (7) certified psychiatric comorbidities as per a psychiatrist's diagnosis (anxiety, major depression, somatoform disorder, eating disorders, fugue state, personality disorder, posttraumatic stress disorder, bipolar disorder, sexual dysfunction, schizophrenia, impulse‐control disorder/obsessive compulsive disorder, gender dysphoria), (8) childhood predisposing factors (psychological trauma, physical trauma) and precipitating factors (psychological trauma, surgery, physical trauma, general anesthesia, infections, adverse drug reactions) and positive family history for neurological diseases, and (9) investigations and therapies (medication history and previous physiotherapy, cognitive behavioral therapy, transcranial magnetic stimulation, and hypnosis).
Information about education levels of the Italian population was obtained from the website of the Italian National Institute of Statistics (http://www.istat.it); the statistics regard primary, secondary, and tertiary education levels of the population > 6 years old (year of reference, 2011).
Standard Protocol Approval, Registration, and Patient Consents
Approval was obtained by the institutional ethics committee of the coordinator center (University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Project Number. 1757CESC) and confirmed by the committees of each participating center. All patients (or their guardians) were informed about the nature of the study and gave their written consent to participate (consent for research). Participants were free to withdraw from the registry at any time.
Statistical Analysis
Data are expressed as mean ± standard deviation and range for continuous variables and as counts and percentages for categorical variables. The distribution of the patients' education levels was compared with that of the general population using a chi‐square test. For group comparisons, we employed unpaired t tests for continuous variables and chi‐square tests or Fisher tests (in case of expected frequencies <5) for categorical variables. Logistic regression models were created to estimate the unadjusted and adjusted odds ratios (ORs; 95% confidence interval [CI]) of combined FMDs (dependent variable) in relation to sociodemographic and clinical characteristics (independent variables). All tests were significant at P < 0.05. Statistical analyses were performed using SPSS statistical software (version 20; IBM‐SPSS, Armonk, NY).
Results
Demographic Data
We enrolled 410 patients with FMDs, 119 men (29%; mean age, 45.6 ± 15.1 years; range, 10–84) and 291 women (71%; mean age, 47 ± 16.1; range, 10–85). Gender distribution was comparable among northern, central, and southern Italy (P = 0.81; Table S1).
Patients with FMDs reported 11.7 ± 3.8 years of schooling. Compared with the general population, the percentage of patients with FMDs who attained only primary education was lower, whereas the percentage of those with secondary and tertiary education levels was significantly greater (Fig. 1).
FIG. 1.
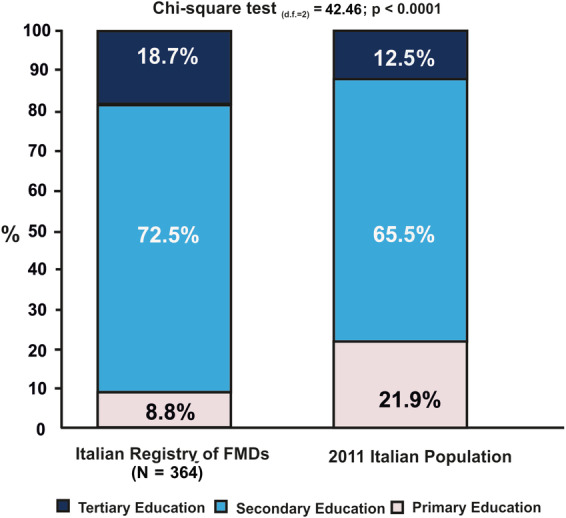
Education levels in the Italian population aged > 6 years (reference year, 2011) and the Italian Registry of FMDs population (age range 10–85) (total sample is 364; 46 missing values). FMDs, functional motor disorders.
Diagnosis of FMDs
The majority (N = 257/410, 62.7%) had the diagnosis of FMDs in a hospital setting, and most of them (N = 322/410, 78.5%) were from a neurologist specialized in movement disorders and far fewer from a general neurologist (N = 71/410, 17.3%), a general physician (N = 2/410, 0.5%), a psychiatrist (N = 14/410, 3.4%), or a physiotherapist (N = 1/410, 0.2%). Of the patients with FMDs, 78% (N = 320/410) had been seen by 1 or more physicians (total sample, mean 2.7 ± 2.5 [range, 1–25]; isolated FMDs, mean 2.2 ± 1.6 [range 1–12]; combined FMDs, mean 3.3 ± 3.1 [range 1–25]) prior to receiving a definite diagnosis. Previously, patients received at least an “organic” (74.4%, N = 238/320) and/or a “nonorganic” diagnosis (24.7%, N = 79/320). “Organic” diagnoses included idiopathic/primary dystonia, Parkinson's disease/parkinsonism, acute/chronic cerebrovascular disease, essential tremor, inflammatory nervous system diseases, disk herniation, epilepsy, ataxia, and migraine. “Nonorganic” diagnoses included nonspecific anxiety syndrome, conversion disorder, somatization, depression, “nonorganic” disease, psychogenic disorder, hysteria, and stress. Of 320 patients, 62 (19.4%) did not receive any diagnosis.
Clinical Manifestations
Acute onset occurred in the majority of cases (N = 290/410, 70.7%). Approximately half experienced spontaneous remissions during the course of the disease (N = 214/410, 52.2%).
Figure 2 reports the overall frequency of different FMD phenotypes, their body distribution, and associated nonmotor symptoms as well as other FNDs. Table 1 reports neurological and psychiatric comorbidities, predisposing and precipitating factors, investigations, and treatments.
FIG. 2.
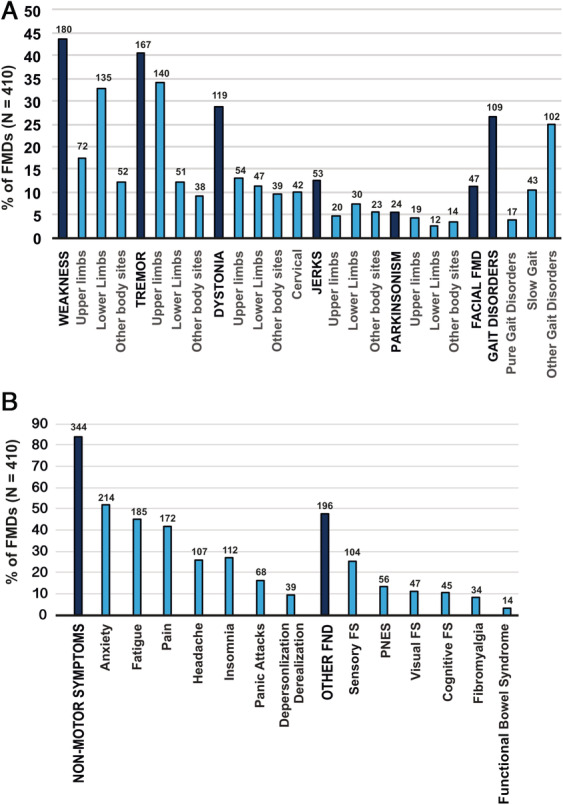
Clinical symptoms reported in patients with FMDs; patients can have 1 FMD (isolated; eg, only tremor or weakness) or more FMDs (eg, weakness + tremor + gait disorders). (A) The different FMD phenomenologies and their body distribution. (B) Patient self‐reported nonmotor symptoms and other FNDs. The bar represents the percentage, whereas the number above shows the absolute value. FMDs, functional motor disorders; FND, functional neurological disorder; FS, functional symptoms; PNES, nonepileptic seizures.
TABLE 1.
Neurological and psychiatric comorbidities, predisposing and precipitating factors, investigations, and treatments in patients with functional motor disorders (N = 410)
| Variables | N | % |
|---|---|---|
| Neurological comorbidities | 70 | 17.1 |
| Migraine | 26 | 6.3 |
| Parkinsonism | 13 | 3.2 |
| Neuropathy | 11 | 2.7 |
| Hyperkinetic motor disorders | 8 | 2.0 |
| Seizures | 8 | 2.0 |
| Multiple sclerosis | 5 | 1.2 |
| Stroke | 5 | 1.2 |
| Psychiatric comorbidities | 165 | 40.2 |
| Anxiety disorders | 110 | 26.8 |
| Major depressive disorders | 55 | 13.4 |
| Somatoform disorder | 19 | 4.6 |
| Eating disorder | 10 | 2.4 |
| Fugue state | 9 | 2.2 |
| Personality disorder | 8 | 2.0 |
| Posttraumatic stress disorder | 6 | 1.5 |
| Bipolar disorder | 5 | 1.2 |
| Impulse control disorder/obsessive compulsive disorder | 5 | 1.2 |
| Sexual dysfunction | 4 | 1.0 |
| Schizophrenia | 3 | 0.7 |
| Gender dysphoria | 1 | 0.2 |
| Familiarity for neurological diseases | 94 | 22.9 |
| Childhood Predisposing factors | 38 | 9.3 |
| Psychological trauma | 25 | 6.1 |
| Physical trauma | 8 | 2.0 |
| Both psychological and physical trauma | 5 | 1.2 |
| Precipitating factors | 206 | 50.2 |
| Psychological trauma | 114 | 27.8 |
| Surgery | 63 | 15.4 |
| Physical trauma | 50 | 12.2 |
| General anesthesia | 33 | 8.0 |
| Infections | 18 | 4.4 |
| Adverse drug reactions | 16 | 3.9 |
| Instrumental investigations | 386 | 94.1 |
| Magnetic resonance imaging | 357 | 87.1 |
| Computerized tomography | 149 | 36.3 |
| Dopamine transporter single‐photon emission computed tomography | 61 | 14.9 |
| Electroencephalography | 48 | 11.7 |
| Neurophysiological tests | 101 | 24.6 |
| Other tests | 51 | 12.4 |
| Oral medications | 209 | 50.9 |
| Antidepressants a | 135 | 32.9 |
| Benzodiazepines b | 111 | 27.1 |
| Antiepileptics c | 73 | 17.8 |
| Antipsychotic drugs d | 35 | 8.5 |
| Other drugs | 77 | 18.8 |
| Other treatments | 180 | 43.9 |
| Physiotherapy | 116 | 28.3 |
| Botulinum toxin injection | 52 | 12.7 |
| Cognitive behavioral therapy | 42 | 10.2 |
| Other therapies | 40 | 9.8 |
Amitriptyline, duloxetine, and paroxetine.
Clonazepam.
Pregabalin, gabapentin, and valproic acid.
Quetiapine and olanzapine.
The majority of patients had functional weakness (43.9%), tremor (40.7%), dystonia (29%), and gait disorders (26.6%), with most of them having 1 or more body districts affected. Lower limbs were more frequently affected in functional weakness (32.9%), whereas the upper limbs were more frequently involved in functional tremor (34.1%). Hemiparesis was found in a smaller proportion of patients (N = 14 on the right side; N = 22 on the left side). Facial motor disorders occurred in 11.4%. For other motor phenotypes, abnormal movements affected with similar frequency all body parts.
Patients with FMDs often reported nonmotor symptoms (83.9%) with anxiety (52.1%), fatigue (45.1%), and pain (41.9%) being the most frequent. Among other FNDs (occurring in 47.8% of patients), sensory symptoms were the most frequent (25.3%). Interestingly, 17.1% of patients with FMDs had other comorbid neurological conditions, such as migraine and parkinsonism. Family history for neurological disorders was positive in 22.9%. A diagnosis of psychiatric disease was reached in 40.2%, and childhood life stressors occurred only in 9.3%. Yet, a psychological trauma during the lifetime occurred in 27.8% of patients, and a physical trauma occurred in 12.2%. Remarkably, 94.1% had undergone instrumental investigations before reaching the final diagnosis.
Demographic and Clinical Features in Isolated and Combined FMDs
Isolated (54.1%) FMDs were slightly more frequent than combined (45.8%). Table 2 shows the demographic and clinical features of these 2 groups. Age, gender, and education level were comparable between the 2 groups. Disease duration was longer in combined FMDs. Patients with combined FMDs had higher numbers of consultations prior to the diagnosis. All nonmotor symptoms and other FNDs were more frequent in combined FMDs. Diagnosis of combined FMDs was more frequently done by a neurologist specialized in movement disorders. Precipitating factors (surgery, anesthesia, adverse drug reactions), instrumental investigations, and the use of physiotherapy and medications were more often reported by patients with combined FMDs (Table 2).
TABLE 2.
Demographic and clinical features of patients with combined and isolated FMDs
| Variable | Isolated FMDs, N = 222 | Combined FMDs, N = 188 | P Value, Combined vs. Isolated |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 158 (71.2) | 133 (70.7) | 0.924 |
| Age, y, mean (SD) | 45.5 (16.8) | 47.9 (14.4) | 0.115 |
| Education, y, mean (SD) | 11.7 (3.7) | 11.7 (3.9) | 0.902 |
| Previous consultations, n (%) | 163 (73.4) | 157 (83.5) | 0.014 |
| FMD duration, y, mean (SD) | 4.8 (5.8) | 6.4 (7.7) | 0.020 |
| FMD phenotype, n (%) | |||
| Weakness | 74 (33.3) | 106 (56.4) | <0.001 |
| Tremor | 58 (26.1) | 109 (58) | <0.001 |
| Dystonia | 41 (18.5) | 78 (41.5) | <0.001 |
| Gait disorders | 17 (7.7) | 92 (48.9) | <0.001 |
| Jerks | 18 (8.1) | 35 (18.6) | 0.002 |
| Facial Motor Disorders | 12 (5.4) | 35 (18.6) | <0.001 |
| Parkinsonism | 2 (0.9) | 22 (11.7) | <0.001 |
| Diagnosis of FMDs, n (%) a | |||
| General neurologist | 47(21.2) | 24 (12.8) | 0.025 |
| Movement disorders neurologist | 162 (73) | 160 (85.1) | 0.003 |
| Nonmotor symptoms, n (%) | |||
| Anxiety | 104 (46.8) | 110 (58.5) | 0.018 |
| Fatigue | 77(34.7) | 108 (57.4) | <0.001 |
| Pain | 68 (30.6) | 104 (55.3) | <0.001 |
| Headache | 47(21.2) | 60 (31.9) | 0.014 |
| Insomnia | 44 (19.8) | 68 (36.2) | <0.001 |
| Panic attacks | 27 (12.2) | 41 (21.8) | 0.009 |
| Other FND, n (%) | |||
| Visual functional symptoms | 16 (7.2) | 31 (16.5) | 0.003 |
| Cognitive functional symptoms | 17 (7.7) | 28 (14.9) | 0.020 |
| Fibromyalgia | 12 (5.4) | 22 (11.7) | 0.021 |
| Psychiatric comorbidities, n (%) | |||
| Major depressive disorder | 21 (9.5) | 34 (18.1) | 0.011 |
| Somatoform disorder | 5 (2.3) | 14 (7.4) | 0.013 |
| Precipitating factors, n (%) | |||
| Surgery | 26 (11.7) | 37 (19.7) | 0.026 |
| General anesthesia | 11 (5) | 22 (11.7) | 0.012 |
| Adverse drug reactions | 4 (1.8) | 12 (6.4) | 0.017 |
| DaT‐SPECT | 23 (10.4) | 38 (20.2) | 0.005 |
| Physiotherapy | 51 (23) | 65 (34.6) | 0.009 |
| Oral medications, n (%) | |||
| Antidepressants | 60 (27) | 75 (39.9) | 0.006 |
| Benzodiazepine | 51 (23) | 60 (31.9) | 0.042 |
| Antipsychotic drug | 10 (4.5) | 25 (13.3) | 0.001 |
Before enrollment.
Bold indicates significant values.
Abbreviations: DaT‐SPECT, dopamine transporte imaging with single photon emission computed tomography; FMDs, functional motor disorders; SD, standard deviation; FNDs, functional neurological disorders.
The univariate logistic regression model yielded a significant association between combined FMDs and many clinical and demographic variables (Table 2). After mutually adjusting for the variables reported in Table 3, the multivariate logistic regression model confirmed the association with the following variables: FMD duration (adjusted OR, 1.04; 95% CI, 1–1.08), diagnosis made by a neurologist specialized in movement disorders (adjusted OR, 7.09; 95% CI, 1.57–31.9), pain (adjusted OR, 2.05; 95% CI, 1.21–3.48), insomnia (adjusted OR, 2.09; 95% CI, 1.19–3.68), somatoform disorder (adjusted OR, 3.58; 95% CI, 1.02–12.64), and use of antipsychotics (adjusted OR, 2.85; 95% CI, 1.09–7.38).
TABLE 3.
Clinical and demographic variables associated with combined FMDs
| Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Independent Variable | Total Sample | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Patients, n | 410 | ||||||
| Gender, males vs. females a | 1.02 | 0.66–1.56 | 0.92 | 1.33 | 0.78–2.26 | 0.29 | |
| Age, y | 1.01 | 0.99–1.02 | 0.12 | 1.01 | 0.99–1.03 | 0.29 | |
| Education, y c | 1 | 0.95–1.06 | 0.90 | 1.04 | 0.97–1.12 | 0.21 | |
| Previous consultations, yes vs. no a | 1.83 | 1.13–2.98 | 0.01 | 1.20 | 0.64–2.26 | 0.56 | |
| FMD duration, y | 1.04 | 1.01–1.07 | 0.021 | 1.04 | 1.01–1.08 | 0.028 | |
| Diagnosis of FMDs b | |||||||
| General neurologist, yes vs. no a | 0.54 | 0.32–0.93 | 0.03 | 4.25 | 0.85–21.08 | 0.07 | |
| Neurologist specialized in movement disorders, yes vs. no a | 2.12 | 1.28–3.48 | 0.003 | 7.09 | 1.57–31.9 | 0.011 | |
| Nonmotor symptoms | |||||||
| Anxiety, yes vs. no a | 1.60 | 1.08–2.37 | 0.019 | 0.71 | 0.41–1.22 | 0.21 | |
| Fatigue, yes vs. no a | 2.54 | 1.70–3.79 | <0.001 | 1.58 | 0.94–2.65 | 0.08 | |
| Pain, yes vs. no a | 2.80 | 1.87–4.20 | <0.001 | 2.05 | 1.21–3.48 | 0.008 | |
| Headache, yes vs. no a | 1.74 | 1.12–2.72 | 0.01 | 1.05 | 0.59–1.84 | 0.87 | |
| Insomnia, yes vs. no a | 2.29 | 1.47–3.57 | <0.001 | 2.09 | 1.19–3.68 | 0.010 | |
| Panic attacks, yes vs. no a | 2.01 | 1.18–3.42 | 0.010 | 1.81 | 0.89–3.66 | 0.09 | |
| Other FNDs | |||||||
| Visual functional symptoms, yes vs. no a | 2.54 | 1.34–4.81 | 0.004 | 2 | 0.88–4.54 | 0.09 | |
| Cognitive functional symptoms, yes vs. no a | 2.11 | 1.12–3.99 | 0.022 | 1.43 | 0.64–3.19 | 0.38 | |
| Fibromyalgia, yes vs. no a | 2.32 | 1.11–4.82 | 0.024 | 0.71 | 0.27–1.88 | 0.49 | |
| Psychiatric comorbidities | |||||||
| Major depressive disorder, yes vs. no a | 2.11 | 1.18–3.78 | 0.012 | 1.39 | 0.64–3.04 | 0.40 | |
| Somatoform disorder, yes vs. no a | 3.49 | 1.23–9.88 | 0.018 | 3.58 | 1.02–12.64 | 0.047 | |
| Precipitating factors | |||||||
| Surgery, yes vs. no a | 1.85 | 1.07–3.18 | 0.027 | 1.23 | 0.49–3.09 | 0.66 | |
| General anesthesia, yes vs. no a | 2.54 | 1.19–5.39 | 0.015 | 1.59 | 0.47–5.36 | 0.45 | |
| Adverse drug reactions, yes vs. no a | 3.72 | 1.18–11.72 | 0.025 | 4.15 | 0.93–18.59 | 0.06 | |
| Oral medications | |||||||
| Antidepressants, yes vs. no a | 1.79 | 1.18–2.72 | 0.006 | 1.43 | 0.82–2.50 | 0.20 | |
| Benzodiazepine, yes vs. no a | 1.57 | 1.01–2.43 | 0.043 | 1.03 | 0.57–1.85 | 0.91 | |
| Antipsychotics drugs, yes vs. no a | 3.25 | 1.52–6.96 | 0.002 | 2.85 | 1.09–7.38 | 0.031 | |
Reference category.
The diagnosis of FMDs before enrollment in this study.
A total of 46 missing values for the education variable.
Bold indicates significant values; significant associations at P < 0.05.
Abbreviations: FMDs, functional motor disorders; OR, odds ratio; CI, confidence interval; FNDs, functional neurological disorders.
Discussion
The results of this large, multicenter, cross‐sectional study provide novel insights on patients with FMDs. Weakness and tremor were the most frequent FMD phenotypes, more often affecting, respectively, the lower and upper limbs. People with FMDs had higher educational levels and frequent nonmotor symptoms, especially anxiety, fatigue, and pain. Almost half of the patients with FMDs had other FNDs, such as sensory symptoms, PNES, and visual symptoms. When stratifying based on the presence of 1 or more FMDs, patients with combined FMDs showed a higher burden of nonmotor symptoms and more frequent occurrences of other FNDs. Multivariate regression analysis showed that FMD duration, pain, insomnia, and diagnosis of somatoform disease were significantly associated with combined FMDs. Moreover, the diagnosis of combined FMDs was more likely to be delivered by a movement disorders neurologist. Finally, treatment with antipsychotics was significantly associated with having combined FMDs.
Demographic and Clinical features of FMDs
Although confirming the higher prevalence of female sex 25 in the whole sample, the proportion of patients with FMDs with a higher education level was greater than in the general population. There are no previously published data on educational level in FMDs except 2 studies performed in small cohorts (N = 42 26 and N = 30 27 ), which reported primary education levels in most of the subjects. However, in a recent study of 132 subjects, 57% of patients with FMDs had a college or higher degree. 28 This is an opposite trend compared with neurodegenerative and cardiovascular diseases, which are associated with poor education. 29 Interestingly, it was demonstrated that higher educational level is associated with lower severity of motor impairment in Parkinson's disease. 30 Higher education level might be correlated with higher socioeconomic status, but it is uncertain if these could be considered risk factors for FMDs and how it could modify their phenotype. Moreover, our data should be carefully interpreted as representative of the Italian population, as Italy has the lowest percentage of subjects aged 15 to 64 years who attained a higher educational level (2018 data, https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=edat_lfs_9903&lang=en). Cross‐cultural comparisons of educational level are needed.
Phenomenological descriptions of FMDs have been extensively reported in the literature, 2 , 13 , 14 , 16 , 24 , 31 , 32 , 33 , 34 , 35 but most of these studies have a retrospective design and/or were based on tertiary movement disorders referral centers or a specialistic FND clinic. Indeed, data on the prevalence of different phenotypes from large cohorts are missing. A small, single‐center, retrospective cases series (N = 28) from a movement disorders clinic identified tremor and dystonia as the most frequent FMD manifestations. 36 A large, multicenter Scottish study reporting 209 patients diagnosed with conversion disorder showed that among 56 patients with FMDs, functional weakness was the most frequent clinical manifestation (62.5%) followed by movement disorders (16.1%). 37 However, in the same study there was no mention about the frequency of each FMD phenotype. Among 410 patients, we showed that weakness, tremor, and dystonia were the most represented phenotypes (43.9, 40.7, and 29%, respectively), with weakness affecting mainly the leg. 19 The predilection of different phenotypes for specific body parts has been never investigated, but it might be related to the nature of trigger or risk factors associated with them.
In our sample, the clinical spectrum of FMDs was often enriched by a constellation of physical and psychiatric symptoms, such as anxiety, pain, and fatigue. Pain and fatigue are common symptoms in patients with FMDs and should be recognized when planning treatment strategies. 13 , 14 , 15 In addition, patients often had functional sensory symptoms (25.3%) and PNES (13.6%), which represent the most frequent FND manifestations in outpatient clinics. 37
A formal psychiatric diagnosis, more frequently anxiety and depression, was delivered in 40.2% of subjects, and only a minority of them reported childhood predisposing trauma. Frequently, FMDs had precipitating factors, including psychological trauma or surgery and physical trauma. These findings reflect the complex interplay between life events, physical triggers, and biological features that lead to the development of FMDs. In addition, they further support to discard the dichotomy between mental and brain disorders which has been swept away by several evidences for a biological model of FMDs generation. 38
Diagnostic Challenges in Patients with FMDs
Most of the patients (78%) reported multiple consultations and numerous tests before receiving a diagnosis of FMDs. These data might be explained by so‐called “doctor shopping,” a patient practice that we believe might reflect several issues associated with the FMD diagnosis, including miscommunication between physicians and patients, a reluctance/failure to accept the diagnosis, and the absence of a clear therapeutic plan and treatment goals. 39 Likewise, misdiagnosis might be a significant determinant for multiple consultations and investigations. In our sample, this hypothesis is supported by the evidence that two thirds of patients had a previous diagnosis of an “organic” disease, and people with combined FMDs received more frequently the diagnosis from a movement disorders neurologist. Indeed, misdiagnosis may arise from lack of expertise for these disorders or poor diagnostic agreement especially when dealing with jerks and functional gait disorders. 40
On a different perspective, our data demonstrate that FMDs may also occur during the course of other neurological diseases as has already been reported with Parkinson's disease 41 and epilepsy. 42
High Burden of Associated Symptoms in Combined FMDs
This is the first study to estimate the overall frequency of isolated and combined FMDs and to analyze the association with clinical variables. Some symptoms (weakness, tremor, dystonia, and gait disorders) occurred more frequently in combination than in isolation, a pattern known so far only for functional gait disorders. 22 Patients with facial motor disorders are also known to develop often dystonia in the upper limbs, albeit most of the time they have a facial onset. 24
Many factors were associated with combined FMDs, but only a few survived multivariate logistic regression analysis. All of these factors may reflect the challenges in diagnosis and the treatment of patients with FMDs. Specifically, the fact that combined FMDs had a long duration of symptoms and needed more frequently a movement disorders neurologist to reach a diagnosis is in keeping with the tortuous diagnostic pathway and the difficulty in evaluating complex phenotypes (eg, dystonia combined with tremor and/or gait disorders). The high frequency of pain and insomnia in this group of patients might be related to each other as chronic pain and sleep disorders are often comorbid. 43 Many factors may contribute to pain generation in combined FMDs, such as a long duration of symptoms; yet pain in combined FMDs seems to be an independent variable from the co‐occurrence of headache and fibromyalgia. Still, our data do not clarify how pain and insomnia impact the level of disability and quality of life in patients with FMDs and which strategies might be successful in their treatment.
Novel and interesting associations with combined FMDs are a more frequent diagnosis of somatoform disorder and the use of antipsychotics. Clinical trials on antipsychotics in FMDs are lacking, although there are anecdotical reports of antipsychotic use in a few patients with PNES. 44 These data might be also explained by the results of a recent Cochrane review, which disclosed low‐quality evidence in favor of combination treatment for selective serotonin reuptake inhibitors and antipsychotics in patients with somatoform disorders. 45 These are data to verify in prospective cohorts assessed with detailed psychiatric assessment given the potential for antipsychotic medications to cause drug‐induced movement disorders. 46
Limitations, Strengths, and Final Remarks
The main limitation of our study is the lack of a control group as well the use of many variables based on clinical records or patient interviews. Yet the cross‐sectional design allowed us to have a standardized collection of clinical data in all centers. Moreover, we could not determine the severity of recorded symptoms as we did not employ any rating instrument for them. Finally, the frequency of psychological stressor might be underestimated as we did not include a formal psychiatric interview.
The main strength of our work is represented by the large multicenter sample of patients with FMDs that is representative of the whole Italian national territory. This allowed us to provide novel knowledge on a wide range of motor disturbances and their associated symptoms. Moreover, the diagnosis of definite FMDs 17 was confirmed by a movement disorder neurologist. That is, our findings highlight the need for multidimensional assessment in patients with FMDs given the high frequency of nonmotor symptoms and other FNDs, especially in patients with combined FMDs. Future prospective studies are needed to clarify how these factors affect quality of life and prognosis in different FMD phenotypes to develop specific management strategies.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.T.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
F.M.: 1A, 1C, 2A, 2C, 3B
E.M.: 1C, 2B, 2C, 3B
R.E.: 1C, 2C, 3B
P.B.: 1C, 2C, 3B
R.C.: 1C, 2C, 3B
S.M.: 1C, 2C, 3B
A. Pilotto: 1C, 2C, 3B
A. Padovani: 1C, 2C, 3B
L.M.R.: 1C, 2C, 3B
R. Eleopra: 1C, 2C, 3B
M.Z.: 1C, 2C, 3B
A.N.: 1C, 2C, 3B
C.D.: 1C, 2C, 3B
C.A.: 1C, 2C, 3B
F.B.: 1C, 2C, 3B
A.P.: 1C, 2C, 3B
B.D.: 1C, 2C, 3B
O.G.: 1C, 2C, 3B
N.M.: 1C, 2C, 3B
E.O.: 1C, 2C, 3B
V.D.S.: 1C, 2C, 3B
A.A.: 1C, 2C, 3B
G.F.: 1C, 2C, 3B
A.T.: 1C, 2C, 3B
M.Z.: 1C, 2C, 3B
G.C.‐B.: 1C, 2C, 3B
M.P.: 1C, 2C, 3B
M.E.: 1C, 2C, 3B
A.P.: 1C, 2C, 3B
P.M.: 1C. 2C. 3B
F.S.: 1C, 2C, 3B
M.C.M.: 1C, 2C, 3B
A.A.: 1C, 2C, 3B
G.D.: 1A, 1C, 2A, 3B
C.G.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
Disclosures
Ethical Compliance Statement
Approval was obtained by the Institutional Ethics Committee of the Coordinator Centre (University of Verona, Azienda Ospedaliera Universitaria Integrata Verona, Project Number. 1757CESC) and confirmed by the committees of each participating centers. All patients (or their guardians) were informed about the nature of the study and gave their written consent to participate (consent for research). Participants were free to withdraw from the registry at any time. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
The authors declare that there are no conflicts of interest to report.
Financial Disclosures for the Previous 12 Months
Francesca Morgante reports receiving speaking fees from Abbvie, Medtronic, Zambon, Bial, Merz; travel grants from the International Parkinson's Disease and Movement Disorder Society; advisory board fees from Merz; consultancy fees from Merz and Bial; research support from Boston Scientific, Merz, and Global Kynetic; royalties for the book entitled Disorders of Movement, Springer Verlag; is a member of the editorial board of Movement Disorders, Movement Disorders Clinical Practice, and European Journal of Neurology. Roberto Erro reports receiving honoraria from UCB, Bial, the International Society for Parkinson's Disease and Movement Disorders and the American Academy of Neurology. Paolo Barone reports receiving consultancy fees as a member of the advisory board for Zambon, Lundbeck, UCB, Chiesi, AbbVie, and Acorda. Roberto Ceravolo reports receiving speaking fees from Abbvie, Zambon, Lusofarmaco, UCB, and General Electric. Andrea Pilotto has served on the advisory board of Z‐cube (technology division of Zambon Pharmaceuticals); he received honoraria from Z‐cube s.r.l., Biomarin, Zambon, Nutricia, and Chiesi Pharmaceuticals. He received grant funding from the Ministry of Health and H2020 calls and independent research support from Vitaflo Germany and Zambon Italy. Alessandro Padovani is a consultant and has served on the scientific advisory board of GE Healthcare, Eli Lilly, and Actelion Ltd Pharmaceuticals; he has received speaker fees from Nutricia, PIAM (PIAM Pharma & Integrative Care), Lansgstone Technology, GE Healthcare, Eli Lilly, UCB Pharma, and Chiesi Pharmaceuticals; and grants from the Ministry of the University, H2020, JPND (EU Joint Programme ‐ Neurodegenerative Disease Research), CARIPLO restricted grants; and independent research support from Zambon, Italy. Maurizio Zibetti reports receiving speaker fees and grants from Medtronic, Zambon Pharma, UCB Pharma, and AbbVie. Fabrizio Stocchi report receiving research/grant support from Zambon; he has received honoraria/consulting fees/compensation for advisory boards from Bial, Chiesi, Neuroderm, Britannia, Sunovion Pharmaceuticals Inc., Lundbeck, Zambon, Cynapsus, Biogen, and Kyowa. All other authors have no disclosures to report.
Supporting information
Table S1. Gender distribution of FMDs among Italian regions.
Appendix S1. Coinvestigators. Italian Registry of Functional Motor Disorders Study Group.
Acknowledgments
We thank Laura Vacca, MD, PhD, and Francesco Paolo Bonifacio, MD, for their assistance in data collection.
Relevant disclosures and conflicts of interest are listed at the end of this article.
Contributor Information
Michele Tinazzi, Email: michele.tinazzi@univr.it.
Christian Geroin, Email: christian.geroin@univr.it.
References
- 1. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol 2012;11:250–260. [DOI] [PubMed] [Google Scholar]
- 2. Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kletenik I, Sillau SH, Isfahani SA, LaFaver K, Hallett M, Berman BD. Gender as a risk factor for functional movement disorders: the role of sexual abuse. Mov Disord Clin Pract 2020;7:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholson TR, Aybek S, Craig T, et al. Life events and escape in conversion disorder. Psychol Med 2016;46:2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kranick S, Ekanayake V, Martinez V, Ameli R, Hallett M, Voon V. Psychopathology and psychogenic movement disorders. Mov Disord 2011;26:1844–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta‐analysis of case‐control studies. Lancet Psychiatry 2018;5:307–320. [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Publishing . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 8. LaFaver K, Lang AE, Stone J, et al. Opinions and clinical practices related to diagnosing and managing functional (psychogenic) movement disorders: changes in the last decade. Eur J Neurol 2020;27(6):975–984. [DOI] [PubMed] [Google Scholar]
- 9. Carson A, Lehn A. Epidemiology. Handb Clin Neurol 2016;139:47–60. [DOI] [PubMed] [Google Scholar]
- 10. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?‐the diagnoses made in 3781 new patients. Clin Neurol Neurosurg 2010;112:747–751. [DOI] [PubMed] [Google Scholar]
- 11. Rask MT, Rosendal M, Fenger‐Gron M, Bro F, Ornbol E, Fink P. Sick leave and work disability in primary care patients with recent‐onset multiple medically unexplained symptoms and persistent somatoform disorders: a 10‐year follow‐up of the FIP study. Gen Hosp Psychiatry 2015;37:53–59. [DOI] [PubMed] [Google Scholar]
- 12. Anderson KE, Gruber‐Baldini AL, Vaughan CG, et al. Impact of psychogenic movement disorders versus Parkinson's on disability, quality of life, and psychopathology. Mov Disord 2007;22:2204–2209. [DOI] [PubMed] [Google Scholar]
- 13. Gelauff JM, Rosmalen JGM, Gardien J, Stone J, Tijssen MAJ. Shared demographics and comorbidities in different functional motor disorders. Parkinsonism Relat D 2020;70:1–6. [DOI] [PubMed] [Google Scholar]
- 14. Aybek S, Lidstone SC, Nielsen G, et al. What is the role of a specialist assessment clinic for FND? Lessons from three national referral centers. J Neuropsychiatry Clin Neurosci 2020;32:79–84. [DOI] [PubMed] [Google Scholar]
- 15. Gelauff JM, Kingma EM, Kalkman JS, et al. Fatigue, not self‐rated motor symptom severity, affects quality of life in functional motor disorders. J Neurol 2018;265:1803–1809. [DOI] [PubMed] [Google Scholar]
- 16. Cubo E, Hinson VK, Goetz CG, et al. Transcultural comparison of psychogenic movement disorders. Mov Disord 2005;20:1343–1345. [DOI] [PubMed] [Google Scholar]
- 17. Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol 2009;22:430–436. [DOI] [PubMed] [Google Scholar]
- 18. Schwingenschuh P, Katschnig P, Seiler S, et al. Moving toward "laboratory‐supported" criteria for psychogenic tremor. Mov Disord 2011;26:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain 2010;133:1537–1551. [DOI] [PubMed] [Google Scholar]
- 20. Dreissen YEM, Cath DC, Tijssen MAJ. Functional jerkstics and paroxysmal movement disorders. Handb Clin Neurol 2016;139:247–258. [DOI] [PubMed] [Google Scholar]
- 21. Ganos C, Edwards MJ, Bhatia KP. The phenomenology of functional (psychogenic) dystonia. Mov Disord Clin Pract 2014;1:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baik JS, Lang AE. Gait abnormalities in psychogenic movement disorders. Mov Disord 2007;22:395–399. [DOI] [PubMed] [Google Scholar]
- 23. LaFaver K, Espay AJ. Diagnosis and treatment of functional (psychogenic) parkinsonism. Semin Neurol 2017;37:228–232. [DOI] [PubMed] [Google Scholar]
- 24. Fasano A, Valadas A, Bhatia KP, et al. Psychogenic facial movement disorders: clinical features and associated conditions. Mov Disord 2012;27:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baizabal‐Carvallo JF, Jankovic J. Gender differences in functional movement disorders. Mov Disord Clin Pract 2020;7:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feinstein A, Stergiopoulos V, Fine J, Lang AE. Psychiatric outcome in patients with a psychogenic movement disorder: a prospective study. Neuropsychiatry Neuropsychol Behav Neurol 2001;14:169–176. [PubMed] [Google Scholar]
- 27. Binzer M, Kullgren G. Motor conversion disorder. A prospective 2‐ to 5‐year follow‐up study. Psychosomatics 1998;39:519–527. [DOI] [PubMed] [Google Scholar]
- 28. Perry CG, Holmes KG, Gruber‐Baldini AL, et al. Are patients with psychogenic movement disorders more likely to be healthcare workers? Mov Disord Clin Pract 2017;4:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect 2005;113:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kotagal V, Bohnen NI, Muller ML, et al. Educational attainment and motor burden in Parkinson's disease. Mov Disord 2015;30:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stone J, Vermeulen M. Functional sensory symptoms. Handb Clin Neurol 2016;139:271–281. [DOI] [PubMed] [Google Scholar]
- 32. Stone J, Warlow C, Sharpe M. Functional weakness: clues to mechanism from the nature of onset. J Neurol Neurosurg Psychiatry 2012;83:67–69. [DOI] [PubMed] [Google Scholar]
- 33. Schrag A, Trimble M, Quinn N, Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain 2004;127:2360–2372. [DOI] [PubMed] [Google Scholar]
- 34. Baizabal‐Carvallo JF, Alonso‐Juarez M, Jankovic J. Functional gait disorders, clinical phenomenologyand classification. Neurol Sci 2020;41:911–915. [DOI] [PubMed] [Google Scholar]
- 35. Gelauff EM, Carson A, Ludwig L, Tijssen MAJ, Stone J. The prognosis of functional limb weakness: a 14‐year case‐control study. Brain 2019;142:2137–2148. [DOI] [PubMed] [Google Scholar]
- 36. Factor SA, Podskalny GD, Molho ES. Psychogenic movement disorders: frequency, clinical profile and characteristics. J Neurol Neurosurg Psychiatry 1995;59:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stone J, Carson A, Duncan R, et al. Symptoms “unexplained by organic disease” in 1144 new neurology out‐patients: how often does the diagnosis change at follow‐up? Brain 2009;132:2878–2888. [DOI] [PubMed] [Google Scholar]
- 38. Edwards MJ, Adams RA, Brown H, Parees I, Friston KJA. Bayesian account of 'hysteria. Brain 2012;135:3495–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dallocchio C, Marangi A, Tinazzi M. Functional or psychogenic movement disorders: an endless enigmatic tale. Front Neurol 2015;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morgante F, Edwards MJ, Espay AJ, Fasano A, Mir P, Martino D. Diagnostic agreement in patients with psychogenic movement disorders. Mov Disord 2012;27:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wissel BD, Dwivedi AK, Merola A, et al. Functional neurological disorders in Parkinson disease. J Neurol Neurosurg Psychiatry 2018;89:566–571. [DOI] [PubMed] [Google Scholar]
- 42. Wissel BD, Dwivedi AK, Gaston TE, et al. Which patients with epilepsy are at risk for psychogenic nonepileptic seizures (PNES)? A multicenter case‐control study. Epilepsy Behav 2016;61:180–184. [DOI] [PubMed] [Google Scholar]
- 43. Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter‐relate? Insights from the longitudinal and cognitive‐behavioral clinical trials literature. Sleep Med Rev 2004;8:119–132. [DOI] [PubMed] [Google Scholar]
- 44. Alessi R, Valente KD. Psychogenic nonepileptic seizures: should we use response to AEDS as a red flag for the diagnosis? Seizure 2014;23:906–908. [DOI] [PubMed] [Google Scholar]
- 45. Kleinstauber M, Witthoft M, Steffanowski A, van Marwijk H, Hiller W, Lambert MJ. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst Rev 2014;CD010628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Factor SA, Burkhard PR, Caroff S, et al. Recent developments in drug‐induced movement disorders: a mixed picture. Lancet Neurol 2019;18:880–890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Gender distribution of FMDs among Italian regions.
Appendix S1. Coinvestigators. Italian Registry of Functional Motor Disorders Study Group.


