Abstract
Background
To date, 10 patients with GTPase Regulator Associated with Focal Adhesion Kinase 1/Rho GTPase Activating Protein 26‐Immunoglobulin (GRAF1/ARHGAP26‐IgG) associated neurological disorders have been described, most with ataxia.
Objective
To report the clinical, oncological, and radiological associations of GRAF1 autoantibodies.
Methods
We identified 17 patients whose serum and/or cerebrospinal fluid IgG was confirmed to target GRAF1/ARHGAP26‐IgG by both tissue‐based immunofluorescence and transfected cell‐based assay. Clinical information was available on 14 patients.
Results
The median age at neurological symptom onset was 51 years, and 8 (47%) were men. The predominant clinical features were subacute progressive cerebellar ataxia (13) or peripheral neuropathy (2). Magnetic resonance imaging brain (7 available) showed cerebellar atrophy (4, 1 also cerebrum and brainstem atrophy). Of 7 cerebrospinal fluids available for testing, 5 showed pleocytosis with oligoclonal bands in 3. Squamous cell carcinoma was observed in 3 patients (head and neck [2], lung [1]).
Conclusion
GTPase Regulator Associated with Focal Adhesion Kinase 1 autoimmunity manifests commonly with subacute ataxia and cerebellar degeneration with a potential association with squamous cell carcinoma. Peripheral neuropathy may also be encountered. Cases in this series responded poorly to immunotherapy.
Keywords: GTPase regulator, ataxia, neuropathy, tissue‐based immunofluorescence, paraneoplastic, GRAF1
Immunoglobulin‐G (IgG) autoantibodies specific for ρ GTPase activating protein 26 (ARHGAP26, also known as GTPase regulator associated with focal adhesion kinase [GRAF1]) have been reported in 10 patients to date. 1 , 2 , 3 , 4 , 5 , 6 Neurological manifestations in decreasing order of frequency included the following: gait ataxia (gait, 7, limb 4), dysarthria (5), nystagmus (5), dizziness (3), cognitive impairment (3), depression (3), hyperkplexia (2), ocular flutter (1), oscillopsia (1), and recurrent psychoses (1). Half of the patients had a tumor, including ovarian cancer (1), breast cancer (1), melanoma (1), B cell lymphoma (1) prostate adenocarcinoma (1), and gastric adenocarcinoma (1), suggesting that this is a paraneoplastic antibody biomarker. 1 , 2 , 3 , 4 , 5 , 6 Here we describe the clinical and oncological associations of an additional 14 GRAF1–IgG‐seropositive patients.
Methods
The Mayo Clinic institutional review board approved this study (08–06647). This is a retrospective, clinical‐serological cohort study approved by the institutional review board of Mayo Clinic, with a waiver of consent for clinical data obtained as part of serologic test validation (study 08‐00647). All Mayo Clinic patients whose medical records were analyzed provided written consent for medical research.
Patients
Archived specimens from 119 patients referred to the Mayo Clinic Neuroimmunology Laboratory (2011–2019) immunolabeled murine brain synapses in a pattern potentially compatible with GRAF1–IgG (Fig. 1). Of these patients, 17 tested positive for GRAF1–IgG by cell based assay (CBA). Clinical information (limited in some) was available in 14 patients and was obtained by physician telephone interview and case record review.
FIG. 1.
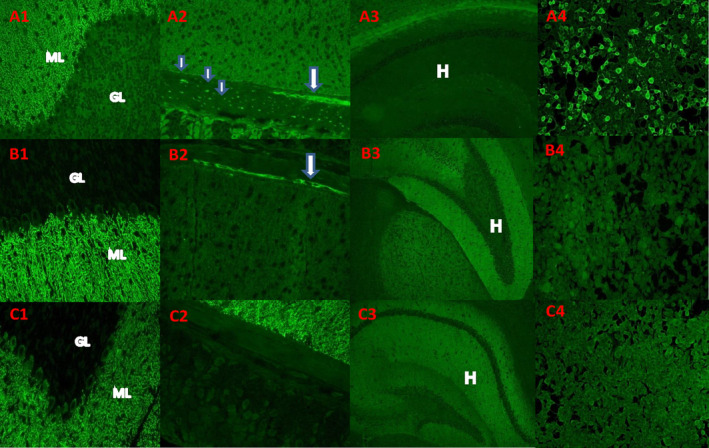
Examples of “medusa‐head” immunostaining pattern and IgG reactivity with ARHGAP26 (GRAF)‐transfected cells. (A) IgG in serum of patient 5 bound to neural elements in fixed cryosectioned mouse tissues. (A1) Staining is prominent in the molecular layer (ML) of the cerebellar cortex in purkinje neuronal dendritic arbors and (A2) in the gastric smooth muscle myenteric ganglia (arrow) and nerve fibers (small arrow). (A3) Hippocampus was not immunoreactive. (A4) human embryonic kidney 293 (HEK293) cells transfected with ARHGAP26/GRAF were highly reactive with patient's serum IgG; interspersed nontransfected cells were not stained. (B, C) The medusa head–like pattern of staining observed in 104 cases lacking ARHGAP26‐IgG specificity and excluded from this report: IgG in 2 such patients' sera yielded a medusa‐head pattern of staining that shares some similarities with ARHGAP26‐IgG, but does not bind to HEK293 cells transfected with ARHGAP26/GRAF (B4, C4). Unlike ARHGAP26‐IgG, these patients' serum IgG stains hippocampus brightly (B3, C3). Patient C serum does not stain myenteric ganglia or nerve fibers in the stomach smooth muscle (C2).
Serological Testing
Specimens were evaluated by indirect immunofluorescence assay (IFA) on a composite substrate of mouse hippocampus, cerebral cortex, cerebellum, basal ganglia, thalamus, kidney, and stomach. GRAF1‐specific IgG was identified by the characteristic indirect IFA cytoplasmic staining pattern (Fig. 1).
GRAF1–IgG specificity was confirmed (in all patients) molecularly by cell‐based assay (CBA) on human embryonic kidney 293 cells that were transfected with GRAF1 complementary DNA fixed with 1% formalin and stored at 4°C (Euroimmun AG, Lubeck, Germany). Controls were tested on GRAF1‐transfected CBA and nontransfected cells using sera from healthy adults (100), healthy children (50), patients with other antineuronal antibodies (Purkinje cell antibody type 1; 5), patients with Purkinje cell antibody type 2 (10), hypergammaglobulinemia (25), patients with multiple sclerosis (9), and patients with Sjogrens/lupus (30). Controls were also tested using cerebrospinal fluid (CSF) from healthy adults (25) and healthy children (25). Interrater agreement was 100%. Positive serum samples were titrated in doubling dilutions to ascertain the end dilution that remained positive by IFA.
All specimens underwent comprehensive paraneoplastic neural autoantibody evaluation that includes testing for cation channel antibodies (voltage‐gated calcium channels [P/Q‐type and N‐type], voltage‐gated potassium channels, and nicotinic acetylcholine receptors [muscle type and ganglionic type]); skeletal muscle striational antibodies; antineuronal nuclear autoantibodies types 1, 2 (anti‐Ri), and 3; Purkinje‐cell cytoplasmic autoantibodies types 1 (anti‐Yo), 2, and Tr (DNER, delta/notch‐like epidermal growth factor–related receptor); antiglial/neuronal nuclear antibody type 1; collapsin response‐mediator protein‐5 IgG; amphiphysin‐IgG; and glutamic acid decarboxylase, NMDA‐R (N‐methyl‐D‐aspartate receptor), GABAB‐R (Gamma‐aminobutyric acid B receptor), and AMPA‐R (α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor) antibodies.
Results
A total of 17 specimens (11 sera and 6 CSF) from 17 patients were confirmed by CBA to be GRAF1–IgG positive. All 17 specimens had an identical characteristic indirect IFA cytoplasmic staining (Fig. 1). Only 1 of the control specimens tested positive for GFAP1–IgG, but by CBA only (seropositivity requires antibody detection by both IFA and CBA). GRAF1–IgG end point titers by IFA in serum ranged from 1:7680–1:61440 and CSF from 1:64–1:1024.
Of the patients, 8 (53%) were men, median onset age was 51 years (range 14–76), and median follow‐up was 23 months (range 0–63). A total of 4 patients had evidence of cancer (33%), with squamous cell carcinoma being confirmed in 3.
Among the 17 patients, clinical histories were available in 14 (Table 1). Of the patients, 12 had a subacute/progressive pancerebellar syndrome (isolated in 8; patient 12) with associated large fiber neuropathy (2), autonomic neuropathy with severe gastroparesis (1), pseudobulbar affect (2), cognitive dysfunction (1), long tract signs (2), and parkinsonism (static in nature and without dysautonomia on autonomic reflex testing [making multisystem atrophy–C unlikely]) (Table 1). The 2 patients without cerebellar signs/symptoms had a small fiber neuropathy and large fiber neuropathy, respectively (patients 3 and 4). All the patients with cerebellar ataxia had a subacute onset and were progressive despite immunotherapy, except patient 8 who stabilized to premorbid function.
TABLE 1.
Mayo Clinic GRAF1–IgG positive cases
| Peripheral Neuropathy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No./Sex/Age | Cerebellar (Cbl) Ataxia | Small Fiber | Large Fiber | Other Symptoms/Signs | Evidence of Cancer | MRI Brain | CSF | GRAF1–IgG Serum(s) or CSF IFA Titer | Immunotherapy | Follow‐Up (months) | Outcome |
| 1/M/76 | + | − | − | Peritracheal & hilar nodes FDG‐avid by PET‐CT | NA | Pleocytosis OCB positive | S:30720 | Pulse steroid, IVIG, cyclophosphamide | 5 | Wheelchair bound | |
| 2/M/55 | + | + | + | Severe gastroparesis | Squamous cell carcinoma in solitary node (histopathology) | Cerebral and cerebellar atrophy | Pleocytosis | S:7680 | Oral steroid IVIG, PLEX | 7 | Wheelchair bound, GJ tube feeding |
| 3/F/50 | − | + | − | Burning feet, orthostatic intolerance and episodic diaphoresis | NA | Pituitary microadenoma | ND | S:15360 | None | 60 | Ambulatory |
| 4/M/47 | − | − | + | − | Negative (PET‐CT) | ND | ND | S:61440 | PLEX | 7 | Ambulatory |
| 5/F/46 | + | − | − | Pseudobulbar affect cognitive dysfunction | Negative (PET‐CT) | Cerebellar and brainstem atrophy | ND | S:15360 | PLEX | 26 | Wheelchair bound |
| 6/M/62 | + | − | − | Pseudobulbar affect | Lingual tonsil and inguinal nodes FDG‐avid by PET‐CT | Cerebellar and cerebrum atrophy | NA | S:15360 | Pulse steroid, IVIG | 36 | Wheelchair bound |
| 7/F/50 | + | − | − | Mild parkinsonism longtract signs LEs | None | Cerebellar atrophy | None | S:30720 | None | 20 | Walking frame |
| 8/F/14 | + | − | − | − | Negative (PET‐CT) | Normal | Pleocytic lymphocytosis, elevated protein, OCB negative | S:30720 CSF:64 | Pulse steroid, IVIG, PLEX, cyclophosphamide | 63 | Wheelchair bound |
| 9/F/51 | + | − | − | − | Negative (PET‐CT) | Unavailable | Unavailable | S:1920 CSF:128 | Pulse Steroid, PLEX | 0 | Unavailable |
| 10/M/63 | + | − | − | − | Nasopharyngeal squamous cell carcinoma(histopathology) | Cerebellar and brainstem atrophy | Pleocytic lymphocytosis | CSF:256 | Pulse steroid, IVIG, PLEX | 24 | Wheelchair bound |
| 11/F/35 | + | − | + | − | Unavailable | Normal | Normal | CSF:1024 | Unavailable | Unavailable | Unavailable |
| 12/M/50 | + | − | − | Longtract signs LEs | Negative (PET‐CT) | Normal | No pleocytosis, OCB positive, Increased protein and IgG index | S:7680 CSF: 256 | Pulse steroid, oral steroid, IVIG | 3 | Wheelchair bound |
| 13/F/67 | + | − | − | − | Squamous cell lung cancer with retroperitoneal metastases. Remote history of mullerian fallopian tube cancer 10 years prior | Normal | Pleocytotic, elevated protein, OCB positive | CSF: 512 | PLEX, IVIG | NA | NA |
| 14/F/64 | + | − | − | − | CT no solid organ malignancy. Blastic bony lesions in spine. Past history of breast cancer. PET‐CT not completed | T2 hyperintensity and DWI restriction of bilateral colliculi, basal ganglia, putamen, thalamus | NA | Serum IFA positive, not titer | None | 1 | Rapid deterioration with coma and death within 1 mo |
GRAF1, GTPase Regulator Associated with Focal Adhesion Kinase 1; IgG, immunoglobulin‐G; Cbl, Cerebellar; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; IFA, immunofluorescence assay; M, male; F, female; FDG, fluorodeoxyglucose; PET‐CT, positron emission tomography–computed tomography; S, serum; NA, not available; OCB, oligoclonal band; IVIG, intravenous immunoglobulin; PLEX, plasma exchange; GJ, gastro‐jejunal; ND, not done; LEs, lower extremities; CT, computed tomography; DWI, diffusion weighted image.
Cerebral magnetic resonance imaging (available in 11) showed cerebellar atrophy in 5 (patients 2, 5, 6, and 10), brainstem atrophy in 2 (patients 5 and 10), and cerebrum atrophy 2 (patients 2 and 6). Brain magnetic resonance imaging in 5 patients were normal (with a coincidental pituitary microadenoma in patient 3). CSF analysis (7 available) was inflammatory in 6: pleocytosis (5), supernumerary oligoclonal bands in 3, and a raised IgG index in 1 (Table 1).
Frequency of GRAF1–IgG Autoantibody Detection
During a 12‐month period, the Mayo Clinic Neuroimmunology laboratory detected GRAF1–IgG (IFA and confirmed by CBA) in 0.004% of neurological sera submitted for paraneoplastic autoantibody evaluation (2/52,000). In comparison, approximate detection frequencies in the same period for other neuronal autoantibodies were antineuronal nuclear antibody type 1, 0.2%; Purkinje cell cytoplasmic antibody type 1 (anti‐Yo), 0.08%; antineuronal nuclear autoantibodies 2/anti‐Ri, 0.03%; and Purkinje cell antibody–Tr/DNER, 0.001%.
Comparison with Previously Described GRAF1–IgG Cases
There are now 24 described cases of GRAF1–IgG autoimmunity, including the 14 cases from this study. Of these, 20 (83%) had cerebellar ataxia and 8 of 18 (44%) with MRI data available had cerebellar atrophy (Table 2). Neurocognitive symptoms in 9 patients included depression, flat affect, pseudobular affect, psychosis, reduce verbal fluency, and cognitive impairment (Tables 1 and 2). Of the 24 cases, 10 had a history of malignancy (various types).
TABLE 2.
Previously published GRAF1–IgG positive cases
| Peripheral Neuropathy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient No/Sex/Age/Reference | Cbl Ataxia | Small Fiber | Large Fiber | Other Symptoms/Signs | Evidence of Cancer | MRI Brain | CSF | GRAF1–IgG Serum (s) or CSF IFA Titer | Immunotherapy | Follow‐Up (months) | Outcome |
| 1/F/33/ 1 | + | NA | NA | Diplopia, depression, hyperekplexia | NA | Cerebellar atrophy | Pleocytosis, OCBs positive | S: 1:6000 CSF: 1:200 | IVMP, IVIG, RTX | 16 months | Cerebellar signs and hyperekplexia, stable |
| 2/F/68/ 2 | + | NA | NA | Dizziness, nausea and vomiting | Ovarian cancer | Empty sella, cerebellar atrophy | NA | S:1:32000 | RTX, IVIG, cyclophosphamide | 24 months | Progressive decline |
| 3/M/38/ 2 | + | NA | NA | Weight loss, nausea and vomiting | NA | Cerebellar atrophy | 5 cells, OCBs positive | S:1:3200 | NA | NA | NA |
| 4/M/24/ 3 | + | NA | NA | Oscillopsia, flattened affect, cognitive impairments, weight loss, headache, memory disturbances | NA | Cerebellar atrophy | OCBs positive | S:1:20000 CSF: 1:240 | IVIG, PLEX | 4 years | Severe gait ataxia and cognitive symptoms. Using a walking stroller at 3 years |
| 5/F/34/ 4 | − | NA | NA | Recurrent psychotic symptoms, suicidal thoughts, headache | NA | Normal | Normal | S: 1:1000 CSF: positive | IVIG | NA | NA |
| 6/F/57/ 6 | + | NA | NA | Urticaria, dizziness, abnormal eye movements | History of breast cancer, melanoma | Normal | Normal | S: 1:32 | IVMP | 12 months | Mild ataxia and dizziness, stable |
| 7/F/37/ 6 | + | NA | NA | Hyperekplexia, myoclonic jerks, depression, falls, dysphagia, deficits in verbal fluency | NA | Normal | OCBs positive | S: 1:100 | IVMP | 19 years | Wheelchair bound at 19 years |
| 8/M/84/ 5 | + | NA | NA | Emotional instability, cognitive impairment, hyperekplexia, myoclonic jerks, loss of appetite, weightloss, hyponatraemia | B cell lymphoma | Generalized atrophy | OCBs positive | S:1:1000 | IVMP, PLEX | 3 months | Initial mild improvement in short term memory, agitation, and depression at last follow‐up |
| 9/M/73/ 5 | + | NA | NA | Poor memory | Prostate cancer | NA | NA | S:1:10000 | No | NA | Death from prostate cancer |
| 10/M/77/ 5 | NA | NA | NA | Cognitive impairment | Gastric adenocarcinoma | NA | NA | S: 1:100 | No | NA | NA |
GRA, GTPase Regulator Associated with Focal Adhesion Kinase 1; IgG, immunoglobulin‐G; Cbl, Cerebellar; MRI, magnetic resonance imaging; CSF, cerebrospinal fluid; IFA, immunofluorescence assay; M, male; F, female; NA, not available; OCB, oligoclonal bands; S, serum; IVMP, intravenous methylprednisolone; IVIG, intravenous immunoglobulin; RTX, rituximab; PLEX, plasma exchange.
Discussion
Our observations extend the neurological spectrum of GRAF autoimmunity beyond cerebellar ataxia and provide insights into potential cancer associations. Close to one‐third of patients had peripheral somatic or autonomic neuropathy (isolated or with cerebellar ataxia). The course of cerebellar ataxia was progressive and led to wheelchair dependence in all but 1 patient. Immunotherapy did not reverse the neurological disability, but stabilization was observed; however, detailed information about the timing of immunotherapy with respect to symptom onset was lacking for many patients, making it unclear whether early initiation of treatment might have reduced neurological disability.
GRAF1 binds to focal adhesion kinase, a component of the integrin signaling pathway. GRAF1 is an intracellular cytosolic protein that is expressed in a wide variety of normal tissues, including the brain, lung, gastrointestinal tract, nasopharynx, and breast. GRAF1 attenuates GTPase‐mediated cellular response to integrin–extracellular matrix interaction, which include cytoskeleton organization 7 , 8 The finding of squamous cell carcinoma of the head and neck in 2 patients and the lung in 1 accords with the known expression of GRAF1 in epithelial tissues. 9 Furthermore, GRAF1 protein expression has been reported in head and neck cancers and ovarian cancers.
Because GRAF1–IgG is specific for an intracellular cytosolic antigen, it does not exert cell‐specific cytotoxicity, but serves as a surrogate biomarker for CD8+ cytotoxic T cells targeting neural cells displaying Major Histocompatibility Complex (MHC) 1‐complexed GRAF1 peptides on their surface membranes. 10
As a result of this clinical association, we recommend investigation for head and neck tumors (with attention to the nasopharynx) and lung cancer in patients who are found to be GRAF–IgG positive, ideally with a whole‐body positron emission tomography–computed tomography, which identified malignancy in 4 of our cases that had negative conventional computed tomography. 11 Testing for this antibody should be considered in patients presenting with a subacute onset ataxia, especially if a paraneoplastic etiology is on the differential diagnosis.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
S.P.: 1A, 1B, 1C, 3A, 3B
N.A.: 1B, 1C, 3A
K.O.: 1B, 1C, 3A
S.H.: 1C, 3B
A.K.: 1B, 1C, 3B
V.L.: 1A, 1B, 3B
L.K.: 1B, 3B
C.P.: 1B, 3B
A.M.: 1B, 3B
Disclosures
Ethical Compliance Statement
This is a retrospective clinical‐serological cohort study approved by the Institutional Review Board of Mayo Clinic with a waiver of consent for clinical data obtained as part of serological test validation (Study 08–00647). All Mayo Clinic patients whose medical charts were analyzed provided written consent for medical research. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
Mayo Clinic Foundation and Dr. O′Connor's fellowship funded by Euroimmun, AG.
Financial Disclosures for the Previous 12 Months
Sean Pittock reports grants, personal fees, and nonfinancial support from Alexion Pharmaceuticals, Inc.; grants from Grifols, Autoimmune Encephalitis Alliance; grants, personal fees, nonfinancial support, and other from MedImmune, Inc.; consulting support from Astellas; and personal fees for consulting services from UCB Biopharma SRL. Dr. Pittock has patent 9,891,219 (Application 12–573,942) “Methods for Treating Neuromyelitis Optica (NMO) by Administration of Eculizumab to an Individual That Is Aquaporin‐4 (AQP4)‐IgG Autoantibody Positive.” Dr. Pittock has patents pending for Kelch‐like Protein 11 (KLHL11), Septin 5, and Microtubule‐associated protein 1B (MAP1B) as markers of neurological autoimmunity and paraneoplastic disorders. Nora Alfugham, Kevin O'Connor, Amy Kunchok, and Shannon Hinson have nothing to disclose. Vanda A. Lennon receives royalties from Mayo Clinic licensing of diagnostic tests for Aquaporin‐4 (AQP4)‐IgG and is a named inventor on filed patents that relate to functional AQP4/Neuromyelitis opticaImmunoglobulin (NMO) ‐IgG assays and NMO‐IgG as a cancer marker. She has a patent pending for KLHL11, Septin 5, and MAP1B IgGs as markers of neurological autoimmunity and paraneoplastic disorders. Dr. Lennon receives royalties from RSR/Kronus for the sale of aquaporin‐4 antibody testing kits and for commercial aquaporin‐4 autoantibody testing performed outside Mayo Clinic and received research support from MN Partnership for Biotechnology and Medical Genomics. Lars Komorowski is an employee of the Euroimmun AG, a company that develops, produces, and manufactures immunoassays for the detection of disease‐associated antibodies. Christian Probst is an employee of the Euroimmun AG, a company that develops, produces, and manufactures immunoassays for the detection of disease‐associated antibodies. Andrew McKeon has patents pending for KLHL11, Septin 5, and MAP1B as markers of neurological autoimmunity and paraneoplastic disorders. He has consulted for Grifols, Medimmune, Euroimmun, Alexon (no personal compensation), and received research support from Medimmune, Euroimmun, Grifols, and Alexion.
Acknowledgments
We thank and acknowledge our research and administrative staff, Mary Curtis, and Jessica Sagen.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Jarius S, Wandinger KP, Horn S, Heuer H, Wildemann B. A new Purkinje cell antibody (anti‐ca) associated with subacute cerebellar ataxia: immunological characterization. J Neuroinflammation 2010;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jarius S, Martinez‐Garcia P, Hernandez AL, et al. Two new cases of anti‐ca (anti‐ARHGAP26/GRAF) autoantibody‐associated cerebellar ataxia. J Neuroinflammation 2013;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doss S, Numann A, Ziegler A, et al. Anti‐ca/anti‐ARHGAP26 antibodies associated with cerebellar atrophy and cognitive decline. J Neuroimmunol 2014;267(1–2):102–104. [DOI] [PubMed] [Google Scholar]
- 4. Jarius S, Wildemann B, Stocker W, Moser A, Wandinger KP. Psychotic syndrome associated with anti‐ca/ARHGAP26 and voltage‐gated potassium channel antibodies. J Neuroimmunol 2015;286:79–82. [DOI] [PubMed] [Google Scholar]
- 5. Bartels F, Pruss H, Finke C. Anti‐ARHGAP26 autoantibodies are associated with isolated cognitive impairment. Front Neurol 2018;9:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallwitz U, Brock S, Schunck A, Wildemann B, Jarius S, Hoffmann F. From dizziness to severe ataxia and dysarthria: new cases of anti‐ca/ARHGAP26 autoantibody‐associated cerebellar ataxia suggest a broad clinical spectrum. J Neuroimmunol 2017;309:77–81. [DOI] [PubMed] [Google Scholar]
- 7. Doherty GJ, Ahlund MK, Howes MT, et al. The endocytic protein GRAF1 is directed to cell‐matrix adhesion sites and regulates cell spreading. Mol Biol Cell 2011;22(22):4380–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai B, Xie S, Caplan S, Naslavsky N. GRAF1 forms a complex with MICAL‐L1 and EHD1 to cooperate in tubular recycling endosome vesiculation. Front Cell Dev Biol 2014;2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regev M, Sabanay H, Kartvelishvily E, Kam Z, Bershadsky AD. Involvement of rho GAP GRAF1 in maintenance of epithelial phenotype. Cell Adh Migr 2017;11(4):367–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zekeridou A, Lennon VA. Neurologic autoimmunity in the era of checkpoint inhibitor cancer immunotherapy. Mayo Clin Proc 2019;94(9):1865–1878. [DOI] [PubMed] [Google Scholar]
- 11. McKeon A, Apiwattanakul M, Lachance DH, et al. Positron emission tomography‐computed tomography in paraneoplastic neurologic disorders systematic analysis and review. Arch Neurol 2010;67(3):322–329. [DOI] [PubMed] [Google Scholar]


