ABSTRACT
Objective
To review studies assessing the efficacy of occupational therapy interventions on quality of life in patients with Parkinson's disease.
Method
We followed the international guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist. Databases (PubMed, Physiotherapy Evidence Database, OTsekeer, Scopus, Cinhal, and Web of Science) were searched to identify randomized controlled trials of occupational therapy interventions in patients with Parkinson's disease up to 2019 and with no restriction on language. The primary outcome of the meta‐analysis was the evaluation of quality of life following occupational therapy interventions. Because of the heterogeneity of the studies, we examined the data by using a random effect model.
Results
We identified 15 randomized controlled trials that met the inclusion criteria, and 4 of these were included in the meta‐analysis. Studies with a follow‐up of 2 to 3 months showed that occupational therapy interventions significantly improved the quality of life in patients with Parkinson's disease, with a mean difference of −2.08 (95% confidence interval, −2.52 to −1.64; P < 0.00001). Studies reporting a longer follow‐up (6–12 months) also showed that occupational therapy interventions improved the quality of life, with a mean difference of −2.56 (95% confidence interval, −3.52 to −1.61; P < 0.00001).
Conclusion
Different occupational therapy interventions may be effective in improving the quality of life in patients with Parkinson's disease. However, because of the limited number of studies available, the strength of the evidence should be considered moderate.
Keywords: Parkinson disease, occupational therapy, quality of life, meta‐analysis, systematic review
Parkinson's disease (PD) is a chronic and progressive neurodegenerative disease characterized by the presence of bradykinesia, tremor, and rigidity and several nonmotor symptoms. 1 Although pharmacological dopaminergic therapy improves the symptoms of the disease, patients with PD show limitations in autonomy and when performing daily life activities that cannot be overcome with pharmacological therapy alone. Different rehabilitation approaches such as physical exercise, self‐management strategies, activities of daily living (ADL), training, and cognitive–behavioral interventions can be useful for patients with PD. 2 , 3 , 4 The scientific evidence for the effectiveness of specific rehabilitative treatments is, however, limited. 5 Occupational therapy (OT) is an allied treatment planned to assure the maximum degree of autonomy to the patient. OT may be useful to enable patients to engage in meaningful roles and activities, adapt the living environment with all the necessary devices and precautions to decrease the risk of falls or accidents, and improve domestic life and functional mobility and mantainance of work abilities. OT therefore may give a significant contribute to the overall management of patients with PD and may have a significant impact on the quality of life of patients with PD. 6 , 7 The scientific evidence underlying the efficacy of OT for improving the quality of life in patients with PD is still unclear, and the only meta‐analysis specifically assessing the effect of OT in PD is rather old. 8
The aim of this article was therefore to evaluate the scientific evidence underlying the effect of different OT interventions on quality of life measures in patients with PD. We also aimed to review possible differences in the effect of OT in studies with short‐term and long‐term follow‐up.
Methods
To conduct the systematic review, we followed the international guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses checklist.
Search Strategy
We searched 6 electronic databases—PubMed, Physiotherapy Evidence Database [PEDro], OTsekeer, Scopus, Cinhal, and Web of Science—from inception to March 2019. We used the same search terms (“Occupational therapy,” “Parkinson's disease,” and “Randomized controlled trial”) for each database consulted. The search strategy was adapted for multiple databases. No language restrictions were imposed. Inclusion criteria were randomized controlled trial (RCT) that evaluated different OT interventions in patients with PD. We included studies assessing OT interventions versus interventions that do not include OT, studies comparing different OT techniques, and OT interventions associated with other methods (eg, use of transcranial stimulation).
Study Selection and Quality Assessment
Relevant studies were selected by 2 of the authors (an occupational therapist and a physical therapist) who independently screened the articles, titles, and abstracts according to the eligibility criteria. For the meta‐analysis study, following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement, we excluded the studies that did not report comparable outcomes. The Cochrane Collaboration's tool for assessing risk of bias by Review Manager 10 and PEDro scores from the PEDro website were used to assess the quality of the studies selected. Each score on the PEDro website is generated by 2 accredited raters scoring the trial; any discrepancies in rating are resolved by a third accredited rater (https://www.pedro.org.au/).
Statistical Analysis
We performed a meta‐analysis using Review Manager software developed by Cochrane Collaboration. 9 The mean difference was used as the effect size for continuous outcomes. We used a random effect model as we expected a random effect size from the studies. The overall effect sizes were calculated based on the pooled proportions and 95% confidence intervals (CIs). The differences between the studies were calculated through the overall effect size (z), with a statistical significance threshold of P < 0.05. The presence and level of heterogeneity were assessed using the χ2 test and the I2 index. Data used for statistical analysis were divided according to 2 points in time. First, we considered the results obtained in a follow‐up within 2 to 3 months, and then we considered the results obtained in a follow‐up of 6 to 12 months.
Outcome Measures
The primary outcome was the quality of life. We collected posttreatment outcomes as reported from follow‐ups ranging from 2 to 3 months and 6 to 12 months after treatment based on the follow‐up times provided by the studies. Some studies reported results for both short‐term and long‐term follow‐up.
Results
Search Results
The study selection process is shown in Figure 1. A total of 143 records were identified and screened through the initial search strategy; 128 records were excluded based on irrelevant titles and abstracts. The remaining 15 RCTs met the eligibility criteria and were included in qualitative synthesis.
FIG 1.
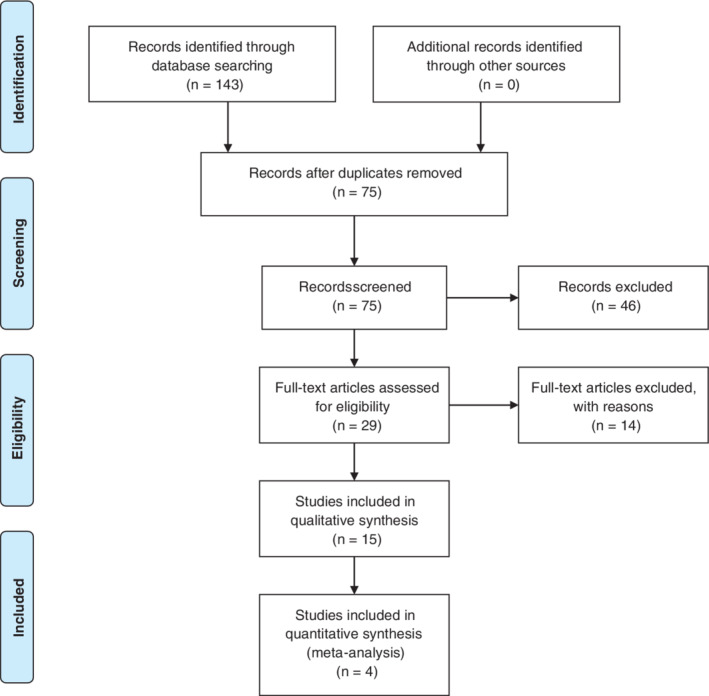
Flowchart of the studies included in the analysis.
RCTs that reported quality of life as an outcome measure were analyzed for quantitative synthesis. The only rating scale that was comparable across the studies reviewed was the Parkinson's Disease Questionnaire (PDQ)–3910 that was used in 5 reports. We included in the meta‐analysis only 4 studies because we excluded the study by Sturkenboom and colleagues 7 that reported only median and interquantile range values.
Characteristics of Studies Included in the Analysis
A summary of study characteristics is shown in Table 1. The Hoehn and Yahr stages of patients included in the studies ranged from I to V (in some studies the Hoehn and Yahr stage was not reported for all the patients assessed by OT interventions). Study samples varied from a minimum of 23 individuals 11 to a maximum of 762, 12 with most studies having a large sample size. In all studies except 1, 13 the men outnumbered the women in the sample groups. The mean and standard deviation age ranged from a minimum of 59.3 (11.3) years to a maximum of 74.1 (6.0) years. The types of OT provided can be categorized as occupational community therapy, 13 , 14 , 15 multidisciplinary approaches (OT, physiotherapy, and others treatments), 12 , 16 , 17 , 18 cognitive enhancement, 19 , 20 , 21 OT with brain stimulation, 11 virtual reality interventions, 22 self‐management interventions (combination of OT, physiotherapy, and speech therapy),23 and OT limited to upper limbs. 24 , 25 The most frequently used treatments were OT interventions used in combination with other rehabilitation strategies. The schedule of treatment ranged from a minimum of a single 15‐minute session to a maximum of 3 sessions, each lasting 1 hour per day, for 12 weeks. Patients in control groups either received no treatment or received standard treatments (conventional OT or simple physical exercises). Study follow‐ups ranged from 1 month to 12 months. No follow‐up was available for 5 of the studies included in the qualitative analysis.
TABLE 1.
Characteristics of included studies
| Participants | Hoehn and Yahr Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Experimental | Control | Experimental | Control | Intervention | Control | Outcome | Follow‐Up | Results |
| Diez‐Cirarda et al, 2017 19 |
15 M 8, F 7 Mean age (SD): 66.20 (4.99) |
15 M 10, F 5 Mean age (SD): 67.60 (7.39) |
15% I, 85% II | I 25%, I 66%, I 9% |
REHACOP intervention; 3 times a week and 1 hour a day |
Usual occupational therapy; 3 times a week and 1 hour a day | Brain activation in resting‐state fMRI | None | The experimental group had an increase in brain connectivity between the temporal frontal lobes |
| Taghizadeh et al, 201824 |
20 M NA, F NA Mean age (SD): 61.05 (13.9) |
20 M NA, F NA Mean age (SD): 59.1 (11.2) |
90% <III, 10% III | 85% <III, 15% III | Sensory stimulation; 2 weeks | Usual therapy; 2 weeks | MTPD (2 point), WPST, WEST, HAST, HORT | None | Sensory improvement of tactile performance and proprioception |
| Goedeken et al, 2018 20 |
25 M 12, F 13 Mean age (SD): 63.8 (4.6) |
27 M 13, F 14 Mean age (SD): 62.7 (5.5) |
9% I, 87% II, 4% III | 10% I, 84% II, 6% III | Implementation intentions strategy; 1‐day treatment session | Verbal rehearsal (encoding strategy group); 1‐day treatment session | PRMQ‐Pro | 1 month | VR group decreased from pre‐ to posttraining, whereas the experimental group remained stable (P = 0.03) |
| Forogh et al, 2017 11 |
12 M 5, F 7 Mean age (SD): 61.33 |
11 M 4, F 7 Mean age (SD): 64.81 |
100% II–III | 100% II–III | Transcranial stimulation with occupational therapy; 20 minutes plus 8 sessions conventional occupational therapy | Fake transcranial stimulation with occupational therapy; 20 minutes and 8 sessions | FSI, ESS | 3 months | Fatigue reduction in short term, but not in long term |
| Mateos‐Moset et al, 2016 25 |
30 M 18, F 12 Mean age (SD): 72.60 (8.86) |
30 M 20, F 10 Mean age (SD): 69.97 (9.59) |
100% II–III | 100% II–III | Hand exercise; 15 minutes of activity | Usual therapy for upper limbs; 15 minutes of activity |
Perdue Pegbord Test (Manual Dexterity) COTNAB Dynamometer |
None | Significant improvements in manual dexterity values (P < 0.05); the values of the force (P < 0.05) have also increased |
| Clarke et al, 2016 12 |
381 M 240, F 63 Mean age (SD): 70 (9.1) |
381 M 258, F 68 Mean age (SD): 70 (9.3) |
67% ≤II, 33% >II | 67% ≤II, 33% >II |
Physical therapy and occupational therapy; 8‐week and 263‐minute average treatment |
None | NEADL, PDQ‐39, EUROQoL, SF‐12 caregiver | 3, 9, and 15 months | Minimal improvement in PDQ‐39 and EuroQoL per patient as well as SF‐12 for cargiver |
| Monticone et al, 2015 16 |
35 M 24, F 11 Mean age (SD): 74.1 (6.0) |
35 M 22, F 13 Mean age (SD): 73.4 (7.0) |
23% II, 77% III–IV | 20% II, 80% III–IV |
Physical therapy and occupational therapy; 90 minutes for 8 weeks |
Physical therapy; 90 minutes for 8 weeks | MDS‐UPDRS, BBS, FIM, PDQ‐39 | 2 and 12 months | Significant improvement in mobility, balance, ADL, and QoL |
| Peña et al, 2014 21 |
22 M 13, F 9 Mean age (SD): 67.5 (65.2–69.8) |
22 M 14, F 8 Mean age (SD): 68.1 (64.9−71.3) |
100% I–II | 91% I–II, 9% III | REHACOP; 12 weeks | Usual occupational therapy; 12 weeks |
Processing Speed Verbal Memory Visual Memory, GDS, NPI‐Q, WHO‐DAS‐II |
None | REHACOP shows statistically and clinically significant changes in processing speed, visual memory, mind theory, and functional disability |
| Sturkenboom et al, 201414 |
124 M 119, F 72 Mean age (range): 71.0 (63.3–76.0) 117 caregivers |
67 M 41, F 26 Mean age (range): 70.0 (63.0–75.0) 63 caregivers |
62% I–II, 38% III–V | 70% I–II, 30% III–V | Occupational therapy at home; 10 weeks | Other treatments; 10 weeks | COPM, ACS Utrecht Scale, PDQ‐39, EuroQoL, Fatigue SS, BDI, ZBI | 3 and 6 months | Occupational therapy did not lead to a perceived self‐improvement in the AVQs |
| Sturkenboom 201315 |
27 M 19, F 8 Mean age (SD): 67.7 (11.8) 26 caregivers |
13 M 10, F 3 Mean age (SD): 68.5 (9.6) 13 caregivers |
70% I–II, 30% III–V | 85% I–II, 15% III–V | Occupational therapy at home; 10 weeks | Other treatments; 10 weeks | COPM, ZBI, AMPS | 3 months | The differences were not significant (P > 0.05), and the variability within the group was high |
| Foster et al, 201313 |
26 M 15, F 11 Mean age (SD): 69.0 (7.8) |
26 M 15, F 11 Mean age (SD): 69.3 (9.4) |
77% <III, 23% III ≤ X ≤ IV | 81% <III, 19% III ≤ X ≤ IV | Community based tango. 1 hour group dance 2 times a week for 12 months | Usual therapy |
BDI UPDRS ACS |
3, 6 and 12 months | Tango in community is associated with increased participation in activities between individuals with PD |
| Ma et al, 201122 |
17 M 8, F 9 Mean age (SD): 64.7 (8.4) |
16 M 10, F 6 Mean age (SD): 68.1 (7.3) |
16 stage II, 1 stage III |
13 stage II 3 stage III |
Virtual reality; 60 exercises of 1 hour | Usual therapy; 60 exercises of 1 hour | Movements time (minutes) | None | In the short term, improvement of the speed of movement in discrete‐aiming tasks |
| Tickle‐Degnen et al, 201023 |
37 M 26, F 11 Mean age (SD): 65.8 (8.3) 39 M 28, F 11 Mean age (SD): 67.6 (10.3) |
41 M 27, F 14 Mean age (SD): 65.6 (8.3) |
86% <III, 14% ≥III (27 hours) 87% <III, 13% ≥III |
77% <III, 23% ≥III | Self‐management interventions (occupational therapy, speech therapy, and physical therapy); 6 weeks; 2 experimental groups of 18 hours and 27 hours | None | PDQ‐39 | 2 and 6 months | People with PD had a statistically significant improvement in quality of life |
| Wade et al, 200317 |
53 M 30, F 23 Mean age (SD): 71.3 (8.6) |
41 M 26, F 15 Mean age (SD): 70.4 (7.6) |
None | None | Multidisciplinary approach (occupational therapy and physical therapy); a full day of treatment once a week for 6 weeks | None; they received intervention 6 months later | PDQ‐39; SF‐36; stand, walk, sit time; 9‐hole peg test; UPDRS | 6 months | Significant differences were recorded for the synthesis scores of the SF‐36 mental components; a significant deterioration was recorded in the PDQ‐39 |
| Gauthier et al, 198718 |
30 M NA, F NA Mean age (SD): 60.9 (6.9) |
29 M NA, F NA Mean age (SD): 65.3 (7.5) |
37% II, 63% III–V | 41% II, 59% III–V | Specific occupational therapy for Parkinson's problems; 5 weeks of 20 total hours | Usual occupational therapy. 5 weeks; 20 total hours of treatment | Barthel Extrapiramidal Symptom Rating Scale, Purdue Pegboard Test, Brudburn PsychologicWellbeing | 6 and 12 months | No significant differences |
Abbreviations: M, male; F, female; SD, standard deviation; REHACOP, Cognitivity Rehabilitation Program in Psychosis; fMRI, functional magnetic resonance imaging; NA, not available; MTPD, moving 2‐point discrimination; WPST, wrist proprioception sensation test; WEST, Weinstein enhanced sensory test; HAST, hand active sensation test; HORT, haptic object recognition test; PRMQ‐Pro, Prospective and Retrospective Memory Questionnaire Prospective Scale; FSI, Fatigue Severity Index; ESS, Epworth Sleeping Scale; COTNAB, Chessington Occupational Therapy Neurological Assessment Battery; NEADL, Nottingham Extended Activities of Daily Living; PDQ‐39, Parkinson Disease Questionnaire 39; EuroQoL, EuroQuality of Life; SF‐12, Short‐form Health Survey 12; MDS‐UPRDS, Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale; BBS, Berg Balance Scale; FIM, Functional Independence Measure; ADL, activities of daily leaving; QoL, quality of life; GDS, Geriatric Depression Scale; NPI‐Q, Neuropsychiatric Inventory–Questionnaire; WHO‐DAS‐II, World Health Organization Disability Assessment Schedule II; COPM, Canadian Occupational Performance Measure; ACS, Activity Card Short; Fatigue SS, severity scale; BDI, Beck Depression Inventory; ZBI, Zarit Burden Interview; AMPS, Assessment of Motor and Process Skills; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale.
Trial Quality
The risk of bias for each study was assessed using the Cochrane Collaboration risk‐of‐bias tool. All studies scored well in terms of methodological quality, with an average score of 4. A score of 3, denoting low quality, was given only to the study of Ma and colleagues. 22 It was not necessary to stratify the statistical analysis for the quality of the studies. We also assessed the quality of each study based on its PEDro score (Table 2). According to the PEDro criteria, study quality can be classified as low quality (scores 0–3), medium quality (scores 4–7), and high quality (scores 8–10, with a score of 10 reflecting the highest quality). Of the studies included in the systematic review, 4 obtained a score of 8, 5 obtained a score of 7, and 6 obtained a score of 6.
TABLE 2.
Physiotherapy Evidence Database scores of the study included in the systematic review
| Studies | Eligibility Criterita | Random Allocation | Concealed Allocation | Group Similar at Baseline | Participant Blinding | Therapist Blinding | Assessor Blinding | <15% Dropouts | Intention to Treat Analysis | Between‐Group Difference Reported | Point Estimate and Variability Reported | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Díez‐Cirarda et al, 2017 19 | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| Taghizadeh et al, 2018 24 | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Goedeken et al, 2017 20 | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Forogh et al, 2017 11 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Mateos‐Moset et al, 2016 25 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Clarke et al, 2016 12 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Monticone et al, 2015 16 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Peña et al, 2014 21 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
| Sturkenboom et al, 2014 14 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 8 |
| Sturkenboom et al, 2013 15 | Yes | Yes | Yes | Yes | No | No | No | Yes | No | Yes | Yes | 6 |
| Foster et al, 2013 13 | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
| Ma et al, 2011 22 | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Tickle‐Degnen et al, 2010 23 | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | 7 |
| Wade et al, 2003 17 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | 7 |
| Gauthier et al, 1987 18 | Yes | Yes | No | Yes | No | No | Yes | Yes | No | Yes | Yes | 6 |
Meta‐Analysis of Primary Outcomes
Quality of life was investigated as primary outcome. PDQ‐39 was the only comparable outcome that could be included in the meta‐analysis.
Efficacy of OT on Quality of Life Assessed by PDQ‐39
Of the 15 studies, 4 were used for the meta‐analysis. 11 , 15 , 18 , 22
PDQ‐39 Total Score
At 2 to 3 months of follow‐up (Fig. 2), the 456 patients in the experimental sample group had a clinically and statistically significant improvement in quality of life when compared with the 457 patients in the control group (−2.08; 95% CI, −2.52 to −1.64; P < 0.00001), with no evidence of heterogeneity (I2, 39%; P = 0.19). For studies with 6 to 9 months of follow‐up (Fig. 3), the 509 patients in the experimental sample group also showed a clinically and statistically significant improvement when compared with the 498 members of the control group (−2.56; 95% CI, −3.52 to −1.61; P < 0.00001), with a statistically significant heterogeneity (I2, 63%; P = 0.04).
FIG 2.
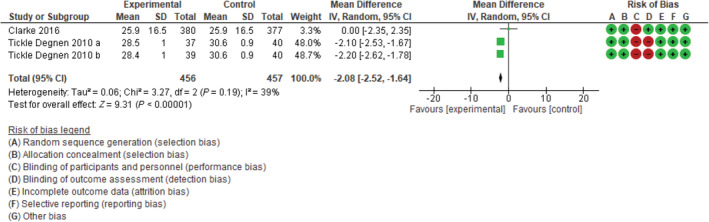
Forest plot: total index of Parkinson's Disease Questionnaire 39 at 2 to 3 months of follow‐up. CI, confidence interval; SD, standard deviation.
FIG 3.
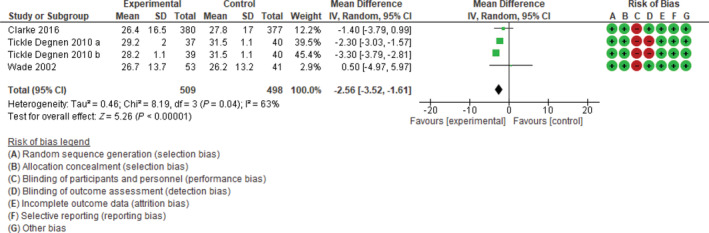
Forest plot: total index Parkinson's Disease Questionnaire 39 at 6 to 9 months of follow‐up. CI, confidence interval; SD, standard deviation.
PDQ‐39 Mobility
In studies with short‐term follow‐up (Supporting Information Fig. S1), the 491 patients in the experimental sample had a clinically and statistically significant improvement when compared with the 492 patients in the control group (−3.47; 95% CI, −5.78 to −1.16; P = 0.003), with very high and significant heterogeneity (I2, 88%; P < 0.00001). For studies with 6 to 12 months of follow‐up, the forest plot (Supporting Information Fig. S2) shows that the 491 patients in the experimental sample had a clinically and statistically significant improvement when compared with the 492 patients in the control group (−4.95; 95% CI, −8.90 to −1.01; P = 0.01), with statistically significant heterogeneity (I2, 96%; P < 0.00001).
PDQ‐39 ADL
In studies with 2 to 3 months of follow‐up (Supporting Information Fig. S3), there was no statistically significant differences between the experimental sample and the control groups (−5.40; 95% CI, −10.61 to 0.19; P = 0.04), with a high heterogeneity (I2, 98%; P < 0.00001). In studies with longer follow‐up, the forest plot (Supporting Information Fig. S4) shows a clinically and statistically significant improvement in the experimental sample compared with the control group (−6.48; 95% CI, −10.53 to −2.44; P = 0.002), with a statistically significant heterogeneity (I2, 95%; P < 0.00001).
PDQ‐39 Emotional Well‐Being
The forest plot for the 2 to 3 months fo follow‐up (Supporting Information Fig. S5) shows a clinically and statistically significant improvement in the experimental sample (−3.01; 95% CI, −5.16 to −0.86; P = 0.006), with very high and significant heterogeneity (I2, 87%; P < 0.00001). For long‐term follow‐up (6–12 months), the forest plot (Supporting Information Fig. S6) shows a clinically and statistically significant improvement in the experimental sample compared with the control group (−6.39; 95% CI, −9.28 to −3.49; P < 0.0001), with statistically significant heterogeneity (I2, 93%; P < 0.00001).
PDQ‐39 Stigma
The forest plot for the 2 to 3 months of follow‐up (Supporting Information Fig. S7) shows no improvement in the experimental sample (−0.93; 95% CI, −3.73 to 1.88; P = 0.52), with very high and significant heterogeneity (I2, 92%; P < 0.00001). For the long‐term follow‐up (6–12 months), the forest plot (Supporting Information Fig. S8) shows a clinically and statistically significant improvement of the experimental sample when compared with the control group (−4.37; 95% CI, −6.85 to −1.89; P = 0.0006), with a statistically significant heterogeneity (I2, 92%; P < 0.00001).
PDQ‐39 Social Support
In studies with 2 to 3 months of follow‐up (Supporting Information Fig. S9), no statistically significant improvement was observed in the experimental sample (−0.80; 95% CI, −2.79 to 1.18; P = 0.43), with very high and significant heterogeneity (I2, 87%; P < 0.00001). Similar results were observed at 6 to 12 months of follow‐up, showing no improvement in the experimental sample (−1.44; 95% CI, −4.62 to 1.73; P = 0.37; Supporting Information Fig. S10), with a statistically significant heterogeneity (I2, 96%; P < 0.00001).
PDQ‐39 Cognition
In the studies with 2 to 3 months of follow‐up (Supporting Information Fig. S11), a clinically and statistically significant improvement was shown in the experimental sample (−1.90; 95% CI, −3.46 to −0.35; P = 0.02), with very high and significant heterogeneity (I2, 78%; P = 0.003). For the long‐term follow‐up (6–12 months), the forest plot (Supporting Information Fig. S12) shows a clinically and statistically significant improvement of the experimental sample compared with the control group (−3.14; 95% CI, −5.69 to −0.60; P = 0.02), with a statistically significant heterogeneity (I2, 91%; P < 0.00001).
PDQ‐39 Communication
In studies with short‐term follow‐up, a clinically and statistically significant improvement was observed in the experimental sample (−4.50; 95% CI, −6.22 to −2.79; P < 0.00001), with a very high and significant heterogeneity (I2, 77%; P = 0.005; Supporting Information Fig. S13). This result was confirmed in studies with 6 to 12 months of follow‐up (−2.14; 95% CI, −5.15 to 0.87; P = 0.16; Supporting Information Fig. S14), with a statistically significant heterogeneity (I2, 91%; P < 0.00001).
PDQ‐39 Bodily Discomfort
The forest plot for the short‐term follow‐up (Supporting Information Fig. S15) shows a nonstatistically significant improvement of the experimental sample (−3.70; 95% CI, −8.22 to 0.82; P = 0.11), with very high and significant heterogeneity (I2, 95%; P < 0.00001). In the studies with 6 to 12 months of follow‐up, there was a clinically and statistically significant improvement of the experimental sample when compared with the control group (−5.37; 95% CI, −8.45 to −2.30; P = 0.0006; Supporting Information Fig. S16), with a statistically significant heterogeneity (I2, 89%; P < 0.00001).
Discussion
Qualitative synthesis of the studies reviewed demonstrate that OT, combined with other treatments (physiotherapy and speech therapy), led to statistically significant improvements, in the short‐term and long‐term follow‐ups, in patient's mobility, balance, and independence in everyday life activities. Furthermore, specific interventions, such as cognitive enhancement and interventions for upper limbs, resulted in clinically and statistically significant improvements in attention and memory skills and in mobility. Further studies are needed to see whether OT interventions combined with virtual reality and with brain stimulation are also effective.
OT in PD had never been assessed in a meta‐analysis that followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses criteria. We found that OT interventions were heterogeneous and were often assessed within a multidisciplinary rehabilitation program. We therefore investigated whether multiple OT interventions could affect positively quality of life, and consequently a random effect model was used to run the meta‐analysis. In the quantitative analysis of the 4 studies included in the meta‐analysis, we confirm that different OT interventions (intended as heterogeneous OT approaches) improve the quality of life in patients with PD assessed through the PDQ‐39. The results of the forest plot demonstrate clinically and statistically significant improvements for the total indexes (P < 0.01) in both short‐term and long‐term follow‐ups. In considering PDQ‐39 subscales, the improvement was significant for mobility (P < 0.01), ADL (P < 0.01), emotional well‐being (P < 0.01), cognition (P < 0.05), and communication (P < 0.01) in short‐term follow‐ups and for mobility (P < 0.05), ADL (P < 0.01), emotional well‐being (P < 0.01), stigma (P < 0.01), cognition (P < 0.05), and bodily discomfort (P < 0.05) in long‐term follow‐ups. Stigma and bodily discomfort subscale scores did not improve in the short‐term studies, but they did improve in the long‐term observations. No significant improvement was reported for social support in both short‐term and long‐term follow‐up studies. It may be speculated that the lack of improvement in social support may be attributed to the fact that the OT interventions assessed in the studies analyzed in the meta‐analysis did not consider social support as a specific therapeutic end point.
The results reported in our article are consistent with systematic reviews 3 , 8 that performed only qualitative analyses and that showed improvements in quality of life, independence in ADL, and mobility with OT in patients with PD. Our meta‐analysis adds quantitative evidence for different OT interventions in quality of life.
Several limitations should be considered in this review. First, we could not include in the statistical analysis several clinical variables such as severity of disease (Hoehn and Yahr stages) and changes in medications because not all the studies examined reported these data. Therefore, we were not able to identify a more specific target population for which any specific intervention could have been effective. A second limitation is that the number of studies included in the systematic review and then in the meta‐analysis is rather limited. Third, most studies did not provide a blinding procedure for both participants and researchers. This is, however, a common limitation for RCTs in rehabilitation because blinding is difficult to obtain and because the control group usually receives a sham intervention. A significant limitation concerns the study design. In fact, although the studies examined are focused on OT as the main intervention in the patients studied, OT was usually used in combination with physiotherapy or other interventions. Consequently, the strength and validity of the evidence that emerged from the statistical analysis, also considering the high heterogeneity between studies, must be considered as limited. Finally, a possible publication bias should be considered as we found that most of the studies reviewed reported positive results.
Conclusions
OT interventions (intended as a miscellanea of different approaches) led to an improvement in the quality of life for patients with PD both in the short and medium‐long follow‐ups. Further RCTs with better descriptions of the clinical characteristics of the patients with PD included and treated only with OT appear necessary. We recommend a comprehensive effort to investigate the effects of specific OT interventions in patients with PD, possibly in multicenter studies. Further research should also investigate the minimum detectable change of the PDQ‐39 to confirm that changes in the scores are significant in clinical practice.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.T.: 1A, 2A, 3B
A.R.: 1C, 2C, 3B
G.F.: 1A, 1B, 2C, 3B
A.B.: 1C, 2C, 3B
E.P.: 1C, 2C. 3B
D.V.: 1C, 2C. 3B
A.F.: 1C, 2B, 3A
M.C.: 1C, 2B, 3A
G.G.: 1A, 2A, 3B
Disclosures
Ethical Compliance Statement
Neither patient consent nor approval from an institutional review board was required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
No specific funding was received for this work, and the authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Supporting information
Figures S1–S16 Forest plots of Parkinson's Disease Questionnaire‐39 subscales for short‐term and long‐term follow‐up.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Hughes AJ, Daniel SE, Ben‐Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–870. [DOI] [PubMed] [Google Scholar]
- 2. Patti F, Reggio A, Nicoletti F, Sellaroli T, Deinite G, Nicoletti F. Effects of rehabilitation therapy on Parkinsonians' disability and functional independence. J Neurol Rehabil 1996;10(4):223–231. [Google Scholar]
- 3. Foster ER, Bedekar M, Tickle‐Degnen L. Systematic review of the effectiveness of occupational therapy related interventions for people with Parkinson's disease. Am J Occup Ther 2014;68(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cusso ME, Donald KJ, Khoo TK. The impact of physical activity on non‐motor symptoms in Parkinson's disease: a systematic review. Front Med 2016;3:35 10.3389/fmed.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Radder DLM, Sturkenboom IH, van Nimwegen M, Keus SH, Bloem BR, de Vries NM. Physical therapy and occupational therapy in Parkinson's disease. Int J Neurosci 2017;127(10):930–943. [DOI] [PubMed] [Google Scholar]
- 6. Meek C, Morgan E, Walker M, et al. Occupational therapy to optimize independence in Parkinson's disease: the designing and recording of a randomized controlled trial intervention. Br J Occup Ther 2010;73:178–185. [Google Scholar]
- 7. Sturkenboom IH, Graff MJ, Borm GF, et al. Effectiveness of occupational therapy in Parkinson's disease: study protocol for a randomized controlled trial. Trial 2013;14:34 10.1186/1745-6215-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy S, Tickle‐Degnen L. The effectiveness of occupational therapy‐related treatments for persons with Parkinson's disease: a meta‐analytic review. Am J Occup Ther 2001;55(4):385–392. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galeoto G, Colalelli F, Massai P, et al. Quality of life in Parkinson's disease: Italian validation of the Parkinson's Disease Questionnaire (PDQ‐39‐IT). Neurol Sci 2018;39(11):1903–1909. [DOI] [PubMed] [Google Scholar]
- 11. Forogh B, Rafiei M, Arbabi A, Motamed MR, Madani SP, Sajadi S. Repeated sessions of transcranial direct current stimulation evaluation on fatigue and daytime sleepiness in Parkinson's disease. Neurol Sci 2017;38(2):249–254. [DOI] [PubMed] [Google Scholar]
- 12. Clarke CE, Patel S, Ives N, et al. Physiotherapy and occupational therapy vs no therapy in mild to moderate Parkinson's disease: a randomized controlled trial. JAMA Neurol 2016;73(3):291–299. [DOI] [PubMed] [Google Scholar]
- 13. Foster ER, Golden L, Duncan RP, Earhart GM. Community‐based argentine tango dance program is associated with increased activity participation among individuals with Parkinson's disease. Arch Phys Med Rehabil 2013;94(2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sturkenboom IH, Hendriks JC, Graff MJ, et al. Efficacy of occupational therapy for patients with Parkinson's disease: a randomized controlled trial. Lancet Neurol 2014;13(6):557–566. [DOI] [PubMed] [Google Scholar]
- 15. Sturkenboom IH, Graff MJ, Borm GF, et al. The impact of occupational therapy in Parkinson's disease: a randomized controlled feasibility study. Clin Rehab 2013;27(2):99–112. [DOI] [PubMed] [Google Scholar]
- 16. Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In‐patient multidisciplinary rehabilitation for Parkinson's disease: a randomized controlled trial. Mov Disord 2015;30(8):1050–1058. [DOI] [PubMed] [Google Scholar]
- 17. Wade DT, Gage H, Owen C, Trend P, Grossmith C, Kaye J. Multidisciplinary rehabilitation for people with Parkinson's disease: a randomised controlled study. J Neurol Neurosurg Psychiatry 2003;74(2):158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gauthier L, Dalziel S, Gauthier S. The benefits of group occupational therapy for patients with Parkinson's disease. Am J Occup Ther 1987;41(6):360–365. [DOI] [PubMed] [Google Scholar]
- 19. Díez‐Cirarda M, Ojeda N, Peña J, et al. Increased brain connectivity and activation after cognitive rehabilitation in Parkinson's disease: a randomized controlled trial. Brain Imaging Behav 2017;11(6):1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goedeken S, Potempa C, Prager EM, Foster ER. Encoding strategy training and self‐reported everyday prospective memory in people with Parkinson disease: a randomized‐controlled trial. Clin Neuropsychol 2018;32(7):1282–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peña J, Ibarretxe‐Bilbao N, García‐Gorostiaga I, Gomez‐Beldarrain MA, Díez‐Cirarda M, Ojeda N. Improving functional disability and cognition in Parkinson disease: Randomized controlled trial. Neurology 2014;83(23):2167–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma HI, Hwang WJ, Fang JJ, et al. Effects of virtual reality training on functional reaching movements in people with Parkinson's disease: a randomized controlled pilot trial. Clin Rehabil 2011;25(10):892–902. [DOI] [PubMed] [Google Scholar]
- 23. Tickle‐Degnen L, Ellis T, Saint‐Hilaire MH, Thomas CA, Wagenaar RC. Self‐management rehabilitation and health related quality of life in Parkinson's disease: a randomized controlled trial. Mov Disord 2010;25(2):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taghizadeh G, Azad A, Kashefi S, Fallah S, Daneshjoo F. The effect of sensory‐motor training on hand and upper extremity sensory and motor function in patients with idiopathic Parkinson disease. J Hand Ther 2018;31(4):486–493. [DOI] [PubMed] [Google Scholar]
- 25. Mateos‐Moset S, Cabrera‐Martos I, Torres‐Sanchez I, et al. Effect of a single hand exercise session on manual dexterity and strength in persons with Parkinson's disease: a randomized controlled trial. Phys Med Rehabil 2016;8(2):115–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S16 Forest plots of Parkinson's Disease Questionnaire‐39 subscales for short‐term and long‐term follow‐up.


