Abstract
Accurate pain assessment methods are necessary to ensure animal welfare and reliable data collection in animal research. The Rat Grimace Scale (RGS), a facial expression pain scale, allows effective identification of pain. However, the potential confounds of this method remain mostly unexplored. General anesthesia, which is used in many laboratory procedures, suppresses thermoregulation and results in hypothermia. We investigated the effects of isoflurane-induced hypothermia on RGS scores. Twenty (10 male and 10 female) Sprague–Dawley rats each received 30 min of anesthesia, followed by 30 min of observation after the return of sternal recumbency. Rats were randomized to receive warming with an electric heating pad or no warming during both periods. Unwarmed rats became hypothermic within 15 min after isoflurane exposure began and returned to normothermia within 15 min after returning to sternal recumbency. Warmed rats did not deviate from the normothermic range. The RGS scores of unwarmed rats were significantly higher than baseline levels for 3 h after anesthesia and were higher than those of warmed rats at 5 and 180 min after anesthesia. Hypothermia resulted in a larger proportion of rats crossing a predetermined analgesic intervention threshold. Our findings show that hypothermia induced by isoflurane anesthesia presents a confound to accurate RGS scoring. These results emphasize the importance of maintaining normothermia to avoid inflated pain scores and to obtain accurate pain assessment.
Abbreviations: RGS, Rat Grimace Scale; PA, post anesthesia
The use of laboratory rodents in research is widespread and plays an important role in the advancement of science and medicine. To ensure humane use of animals, accurate pain assessment methods are necessary to allow appropriate pain management. Traditional methods of pain assessment focus on nociceptive tests, which are limited to assessing evoked responses. Recent developments have focused on analyzing behaviors to assess ongoing pain, which is more clinically relevant than evoked pain.13,20 One such approach is the Rat Grimace Scale (RGS).19 The RGS assesses changes in 4 action units (orbital tightening, nose/cheek appearance, ear and whisker positions) to identify pain and is sensitive to analgesic administration.19 Ongoing RGS development has extended to a wide range of experimental conditions,3,7 including models of chronic pain,1,6 reassessment of analgesic efficacies,22 identification of an analgesic intervention threshold9 and successful application for real-time pain assessments.5 However, the limitations of this method have not been fully explored.
General anesthesia is often used in animal research to facilitate animal manipulation and is mandatory for invasive surgical procedures. Recently, a short period (12 min) of general anesthesia with isoflurane was associated with an increase in pain scores, as assessed using the RGS.8 While these findings describe an important confound to the use of the RGS, the mechanism(s) underlying this observation are unknown.
The induction of general anesthesia disrupts thermoregulation, leading to hypothermia.11,12,14,17,23 In humans, hypothermia results in a host of adverse effects, including prolonged recovery and discomfort.18 The incidence of similar adverse effects in animals has received minimal investigation, although hypothermia has been shown to delay recovery from anesthesia.4,10,16 The objective of this study was to determine if hypothermia induced by general anesthesia confounds postanesthetic pain assessment of rats with the RGS. We hypothesized that rats allowed to become hypothermic under isoflurane anesthesia would display higher RGS scores after anesthesia than would normothermic animals.
Materials and Methods
All experiments were approved by the University of Calgary Health Sciences Animal Care Committee (protocol ID: AC13-0124), in accordance with the Canadian Council on Animal Care guidelines.2
Animals.
Twenty 10-13-wk-old Sprague–Dawley rats (Rattus norvegicus, 178 to 355g, male [n = 10], female [n = 10]) were purchased from Charles River Laboratories (Senneville, Quebec, Canada) or were obtained from surplus stock (original source, Charles River Laboratories, as above) at the University of Calgary Animal Resource Center. Sentinel rats were negative for rat parvoviruses Toolan H1 virus, Kilham rat virus, rat minute virus and protoparvovirus NS-1, rat sialodacryoadenitis virus, rat theilovirus, Pneumocystis carnii, Sendai virus, pneumonia virus of mice, reovirus, Mycoplasma pulmonis, Lymphocytic choriomeningitis virus, adenovirus, hantavirus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, rat rotavirus, Bordetella bronchiseptica, Corynebacterium kutscheri, Klebsiella oxytoca, Klebsiella pneumoniae, Rodentibacter pneumotropicus, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus β hemolytic and Streptococcus pneumoniae, Proteus mirabilis, Salmonella and other bacteria, and endo- and ectoparasites. Rats were allowed to acclimatize for a minimum of 7 d before the experiment began. Animals were housed in pairs in standard polycarbonate cages (47 × 25 × 21 cm, RC8D-UD, Alternate Design Manufacturing and Supply, Siloam Springs, AZ) with wood chips and shredded paper as bedding, and a plastic tube for enrichment. The housing environment was climate controlled: temperature (23 °C), humidity (22%) and light cycle (12h light on/12h light off with lights on at 0730. Food (Prolab 2500 Rodent 5p14, LabDiet, PMI Nutrition International, St Louis, MO) and tap water were provided ad libitum.
Habituation.
Animals were habituated for at least 3 d before experimentation. Habituation consisted of a minimum 10 min of handling by the experimenters (CK, HR) each day, during which rats received a food reward (Honey Nut Cheerios, General Mills, Golden Valley, MN) and had their rectal temperatures taken 5 times. The rectal thermometer was inserted approximately 2 cm into the rectum. Next, rats were placed in the plexiglass observation box (28 × 15 × 21 cm) used for RGS scoring for a 10-min period. Rats were considered to be habituated when they met all of the following criteria: 1) ate the food reward from the experimenter's gloved hand and in the observation box, 2) the first 2 rectal temperature measurements were within 2 standard deviations of mean baseline temperature observed in a previous study (37.5 ± 0.5 °C)16 and 3) the first 2 rectal temperature measurements were within 0.3 °C of each other. If these criteria were not met within 3 d, additional days for habituation were added until the criteria were met.
Experiments.
A sample size of 10 animals per treatment group was estimated using an α value of 0.05 and a power of 0.8 to detect a mean difference of 0.35 (SD = 0.25) in the RGS (G*Power 3.1.9.2, Germany).5 Twenty rats were block randomized to 2 groups: warmed (n = 10) and unwarmed (n = 10) with 5 males and 5 females in each group. Approximately 20 min before induction of general anesthesia, rats had rectal temperatures taken and were placed in the observation box for 10 min for RGS scoring using a real-time method.5 After baseline measurements were complete, general anesthesia was induced with 5% isoflurane in oxygen (1 L/min) in an induction chamber (30 × 18 × 17 cm). Rats were removed from the induction chamber once the righting reflex was lost. Next, the rats were placed in dorsal recumbency, and anesthesia was maintained for 30 min with 2% isoflurane in oxygen (1 L/min) via a nose cone. Rats in the warmed group were placed on top of an electric heating pad (Hot Dog Patient Warming System, Augustine Temperature Management, MN) set to 40 °C. Unwarmed rats were placed on a thin synthetic sheet (MedPro Disposable Underpad, AMG Medical, Montreal, QC, Canada) without warming. Rectal temperatures were taken every 5 min from the time of nose cone placement to the termination of isoflurane. Rats were then allowed to recover with oxygen. After the spontaneous return of sternal recumbency, warmed rats were transferred to a plastic container (59.7 cm × 42.9 cm × 31.1 cm) containing an identical electric heating pad (set at 37 °C) and remained there for 30 min, followed by transfer to a clean, unheated cage (47 × 25 × 21 cm) with wood chip bedding. Unwarmed rats were placed in a clean, unheated cage after attaining sternal recumbency. Both groups remained in the clean cage for the remainder of the experiment (180 min), with access to food and water.
During the 180 min after return of sternal recumbency (postanesthetic (PA) period), rectal temperatures and RGS data were collected at baseline and 5, 60, 120 and 180 min. During this time, rats were removed from their recovery cages and placed in a plexiglass observation box (28 × 15 × 21 cm). At the 5 min time point, rectal temperatures were taken before and after the 10 min RGS scoring period (5 min PA and 15 min PA respectively). For the other RGS scoring timepoints (60, 120, and 180 mins PA), rectal temperatures were only taken after RGS scoring. All experiments were completed between 0830 to 1730, during the light period.
RGS Scoring.
The real-time RGS scoring method was performed as previously described.5 Each observation period lasted 10 min, with no scoring performed during the first minute. An RGS score was recorded every 30 s, based on a continuous observation period of 15 s, followed by 15 s of no observation. This generated a total of 18 RGS observations for each time period. Scores of the 4 action units (orbital tightening, nose/cheek appearance, ear and whisker positions) were averaged for each observation and all scores over the 10 min observation period averaged to generate a single mean score per time point per rat. Two observers (HR and CK) were present in the room and performed RGS scoring. One observer was blind to treatment (CK) while the other (HR) administered the anesthetic and performed temperature measurements. Scoring reliability between observers was assessed with an intraclass correlation coefficient to see if awareness of the treatment altered assigned scores.
Statistical analysis.
Data were analyzed with commercial statistical software (Prism 8.3.1, GraphPad Software, La Jolla, CA and MedCalc Software 18.5, Ostend, Belgium). The normothermic range was considered ± 2 SD from the mean baseline temperature of both the warmed and unwarmed groups. This resulted in a range of 36.66 to 38.91 °C. Intraclass correlation coefficients were calculated with an absolute model, and average-measure ICC reported. All data approximated a normal distribution as assessed with a D'Agostino-Pearson omnibus normality test. Two-way ANOVAs and a multiple comparison test with a Bonferroni correction were used to compare between sexes (RGS scores), within treatment (RGS and individual action unit scores), for each timepoint. As the sample size estimate did not account for sex differences, data from both sexes were pooled for analysis (Table 1). Between (RGS scores, individual RGS action unit scores, temperature and food consumption) and within (baseline compared with all other timepoints for RGS scores and temperature), treatment comparisons were made using 2-way ANOVA with a Bonferroni correction for multiple comparisons. Time to sternal recumbency was compared between treatment groups using an unpaired t test. The proportion of rats with an RGS score greater or lower than 0.67 in each treatment group at all timepoints was compared with a Fisher exact test. Data supporting the study results are available in an electronic repository: Pang, Daniel, 2020, “hypothermia, general anesthesia and RGS”, https://doi.org/10.7910/DVN/5DG8XT, Harvard Dataverse, V1, UNF:6:iwsQa+MpXkBAvjwYdMOO6A== [fileUNF].
Table 1.
Comparisons between sex of RGS scores in the warmed (female: n = 5, male: n = 5) and unwarmed (female: n = 5, male: n = 5) groups during all experimental timepoints. 95% confidence intervals (95%CI) are for mean difference.
|
Warmed female compared with male |
Unwarmed female compared with male |
|||
| Timepoints (min) | P value | 95% CI | P value | 95% CI |
| Baseline | >0.9999 | −0.231 to 0.337 | >0.9999 | −0.397 to 0.359 |
| 5 PA | 0.3587 | −0.478 to 0.090 | >0.9999 | −0.455 to 0.301 |
| 60 PA | >0.9999 | −0.311 to 0.257 | >0.9999 | −0.363 to 0.393 |
| 120 PA | >0.9999 | −0.362 to 0.206 | >0.9999 | −0.502 to 0.254 |
| 180 PA | >0.9999 | −0.232 to 0.336 | >0.9999 | −0.294 to 0.462 |
Results
No animals were excluded from the experiment, and all data are included in the analysis. The intraclass correlation coefficient for the 2 observers was excellent (ICC = 0.98) so the average scores from both observers were used for analysis.
Temperature.
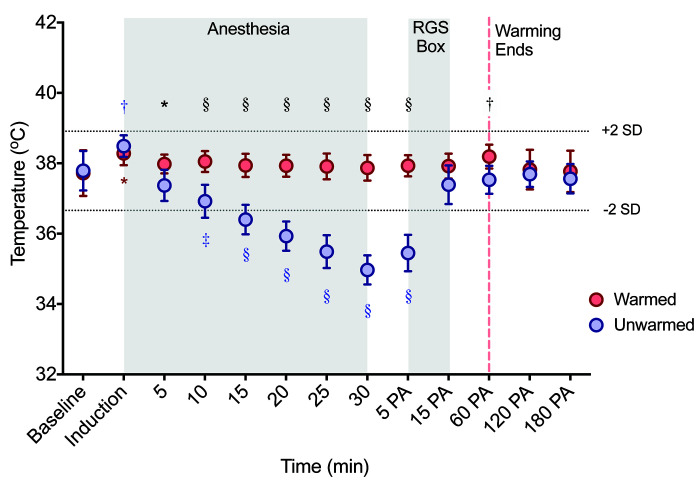
Normothermia was successfully maintained in warmed rats with no significant change over time, except for a slight increase at induction (Figure 1, Table 2). During anesthesia, unwarmed rats became hypothermic beginning at 15 min after induction of general anesthesia (Figure 1, Tables 2 and 3). A significant interaction was detected between time and treatment during and after anesthesia (F (12, 216) = 45.2, P < 0.0001). Rectal temperatures of the unwarmed cohort were lower than warmed rats at all timepoints during anesthesia. After anesthesia, rectal temperatures of unwarmed rats returned to normothermia within 15 min of regaining sternal recumbency (Figure 1, Table 2).
Figure 1.
Rectal temperatures of warmed (n = 10) and unwarmed (n = 10) male and female rats. Temperatures from unwarmed rats were significantly lower than warmed rats at most time points. Broken horizonal lines indicate the normothermic range (between 36.66-38.91 °C). PA = postanesthesia timepoints. Induction is induction of general anesthesia with isoflurane. Data are mean ± SEM (see Tables 1 and 2 for detailed results). Blue and red asterisks near data points indicate within group differences (comparison to baseline) in unwarmed and warmed rats, respectively. Black asterisks above data points indicate between group differences. *P < 0.05 †P < 0.01 ‡P < 0.001 §P < 0.0001
Table 2.
Within group comparisons of rectal temperatures between warmed (n = 10) and unwarmed (n = 10) rats during the peri-anesthetic period.
|
Warmed |
Unwarmed |
|||
| P value | 95% CI | P value | 95% CI | |
| Baseline compared with Induction | 0.0447 | −1.11 to -0.00695 | 0.0037 | −1.25 to -0.147 |
| Baseline compared with 5 min | >0.9999 | −0.813 to 0.293 | 0.3475 | −0.133 to 0.973 |
| Baseline compared with 10 min | >0.9999 | −0.883 to 0.223 | 0.0001 | 0.317 to 1.42 |
| Baseline compared with 15 min | >0.9999 | −0.773 to 0.333 | <0.0001 | 0.837 to 1.94 |
| Baseline compared with 20 min | >0.9999 | −0.763 to 0.343 | <0.0001 | 1.31 to 2.41 |
| Baseline compared with 25 min | >0.9999 | −0.743 to 0.363 | <0.0001 | 1.75 to 2.85 |
| Baseline compared with 35 min | >0.9999 | −0.703 to 0.403 | <0.0001 | 2.27 to 3.37 |
| Baseline compared with 5 min PA | >0.9999 | −0.763 to 0.343 | <0.0001 | 1.79 to 2.89 |
| Baseline compared with 15 min PA | >0.9999 | −0.753 to 0.353 | 0.4492 | −0.153 to 0.953 |
| Baseline compared with 60 min PA | 0.1758 | −1.02 to 0.0830 | >0.9999 | −0.293 to 0.813 |
| Baseline compared with 120 min PA | >0.9999 | −0.653 to 0.453 | >0.9999 | −0.453 to 0.653 |
| Baseline compared with 180 min PA | >0.9999 | −0.603 to 0.503 | >0.9999 | −0.323 to 0.783 |
P values and 95% confidence intervals (95%CI) of mean differences for within group comparisons of rectal temperatures at all experimental time points. PA = postanesthesia. Induction = induction of general anesthesia with isoflurane. Numbers in bold denote significant differences.
Table 3.
Between group comparisons of rectal temperatures for warmed (n = 10) and unwarmed (n = 10) rats during the peri-anesthetic period.
| P value | 95% CI | |
| Baseline | >0.9999 | −0.628 to 0.488 |
| Induction | >0.9999 | −0.768 to 0.348 |
| 5 min | 0.0209 | 0.0520 to 1.17 |
| 10 min | <0.0001 | 0.572 to 1.69 |
| 15 min | <0.0001 | 0.982 to 2.10 |
| 20 min | <0.0001 | 1.44 to 2.56 |
| 25 min | <0.0001 | 1.86 to 2.98 |
| 30 min | <0.0001 | 2.34 to 3.46 |
| 5 min PA | <0.0001 | 1.92 to 3.04 |
| 15 min PA | 0.0780 | −0.0280 to 1.09 |
| 60 min PA | 0.0085 | 0.102 to 1.22 |
| 120 min PA | >0.9999 | −0.428 to 0.688 |
| 180 min PA | >0.9999 | −0.348 to 0.768 |
P values and 95% confidence intervals (95%CI) of mean differences for between group comparisons of rectal temperatures at all experimental timepoints. PA = postanesthesia. Induction = induction of general anesthesia with isoflurane. Numbers in bold denote significant differences.
Rat Grimace Scale.
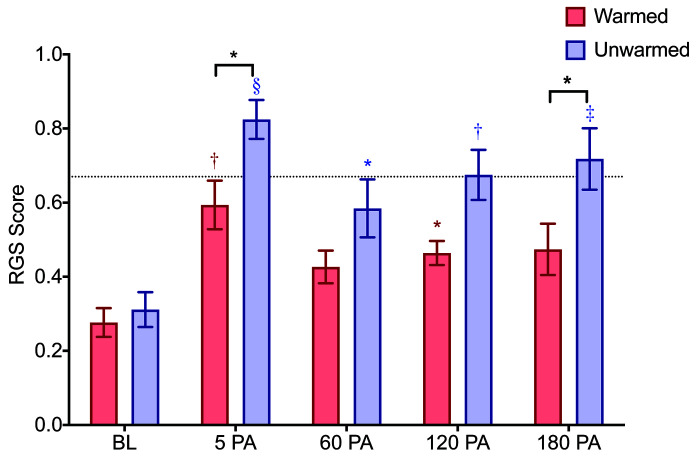
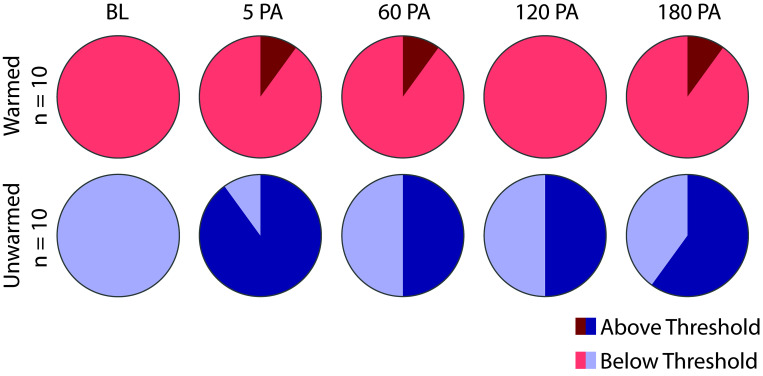
No sex differences were observed in the warmed or unwarmed groups (Table 1). No significant interaction was detected between time and treatment of the rats during anesthesia (F (4, 72) = 1.60, P = 0.184). Significant effects were detected for time (F (4, 72) = 20.8, P < 0.0001) and treatment (F (1, 18) = 8.58, P = 0.009). RGS scores of unwarmed rats were significantly higher than baseline at all timepoints after anesthesia (Figure 2, Table 4). From 60 min after recovery from anesthesia, the mean difference of unwarmed RGS scores increased over time in comparison to baseline (0.27 at 60 min PA, 0.36 at 120 min PA, 0.41 at 180 min PA). In contrast, warmed rats had slightly higher RGS scores from baseline at 5 and 120 min only (Table 4), and from 60 min after recovery, the mean difference from baseline stayed relatively consistent (0.15 at 60 min PA, 0.19 at 120 min PA and 0.2 at 180 min PA). Unwarmed rats had higher RGS scores than warmed rats at 5 and 180 min (Figure 2, Table 5). The values of individual scored action units are shown for each group in Table 6. Scores for the nose action unit were higher in the unwarmed than warmed rats at 5 min PA and 180 min PA. In comparison to the warmed group, a greater proportion of animals in the unwarmed group had RGS scores exceeding a previously derived analgesic intervention threshold (P < 0.0001, Figure 3).9
Figure 2.
RGS scores (on a scale of 0-2) of warmed (n = 10) and unwarmed (n = 10) rats at postanesthesia timepoints. Broken horizontal line indicates the RGS analgesic intervention score (0.679). PA = postanesthesia. Data are mean ± SEM (see Tables 3 and 4 for detailed results). Asterisks above bars (red or blue) indicate within group differences (comparison to baseline). Black asterisks above brackets indicate between group differences. *P < 0.05, †P < 0.01, ‡P < 0.001, §P < 0.0001.
Table 4.
Within group comparisons for RGS scores in the warmed (n = 10) and unwarmed (n = 10) groups during the postanesthetic period.
|
Warmed |
Unwarmed |
|||||
| Timepoints (min) | Mean Dif. | P value | 95% CI | Mean Dif | P value | 95% CI |
| BL compared with 5 PA | 0.318 | 0.0061 | −0.533 to -0.102 | 0.513 | <0.0001 | −0.7104 to -0.322 |
| BL compared with 60 PA | 0.150 | 0.0556 | −0.304 to 0.00357 | 0.273 | 0.0122 | −0.481 to -0.0653 |
| BL compared with 120 PA | 0.188 | 0.0090 | −0.323 to -0.0520 | 0.364 | 0.0021 | −0.572 to -0.155 |
| BL compared with 180 PA | 0.198 | 0.0807 | −0.418 to 0.0231 | 0.407 | 0.0006 | −0.600 to -0.213 |
P values and 95% confidence intervals (95%CI) of mean differences for within group comparisons of rectal temperatures at all experimental timepoints. BL = baseline. PA = postanesthesia. Numbers in bold denote significant differences.
Table 5.
Between group comparisons for Rat Grimace Scale scores in the warmed (n = 10) and unwarmed (n = 10) groups during the postanesthetic period.
| Timepoint (min) | P value | 95% CI |
| Baseline | >0.9999 | −0.259 to 0.189 |
| 5 PA | 0.0402 | −0.454 to -0.00672 |
| 60 PA | 0.3322 | −0.382 to 0.0658 |
| 120 PA | 0.0747 | −0.435 to 0.0128 |
| 180 PA | 0.0256 | −0.468 to -0.0202 |
P values and 95% confidence intervals (95% CI) of mean differences for within group comparisons of rectal temperatures at all experimental timepoints. PA = postanesthesia. Numbers in bold denote significant differences.
Table 6.
Mean ± SD scores of individual RGS action units for warmed (n = 10) and unwarmed (n = 10) groups during all experimental timepoints.
| Orbital tightening | Ear position | Nose/cheek appearance | Whisker appearance | |
| Warmed | ||||
| Baseline | 0.10 ± 0.09 | 0.67 ± 0.27 | 0.13 ± 0.12 | 0.21 ± 0.27 |
| 5 PA | 0.66 ± 0.28†††† | 0.94 ± 0.23†† | 0.23 ± 0.35** | 0.55 ± 0.27†† |
| 60 PA | 0.24 ± 0.24 | 0.64 ± 0.36 | 0.23 ± 0.16 | 0.59 ± 0.25††† |
| 120 PA | 0.41 ± 0.26† | 0.70± | 0.21 ± 0.16 | 0.56 ± 0.29†† |
| 180 PA | 0.53 ± 0.42††† | 0.70± | 0.17 ± 0.19* | 0.52 ± 0.36†† |
| Unwarmed | ||||
| Baseline | 0.14 ± 0.24 | 0.46 ± 0.21 | 0.38 ± 0.34 | 0.27 ± 0.22 |
| 5 PA | 1.04 ± 0.34†††† | 0.96 ± 0.08†††† | 0.7 ± 0.19**†† | 0.59 ± 0.33†† |
| 60 PA | 0.550.47±†† | 0.70 ± 0.26† | 0.48 ± 0.33 | 0.60 ± 0.27†† |
| 120 PA | 0.67 ± 0.29†††† | 0.80 ± 0.32††† | 0.54 ± 0.35 | 0.67 ± 0.26††† |
| 180 PA | 0.77 ± 0.42†††† | 0.76 ± 0.23†† | 0.61 ± 0.36*† | 0.67 ± 0.25††† |
indicates significant differences between treatment groups at the same timepoint.
is P < 0.05,
is P < 0.01,
is P < 0.001.
indicates significant within group differences in comparison to baseline scores.
is P < 0.05,
is P < 0.01,
is P < 0.001,
is P < 0.0001.
Figure 3.
Proportions of rats that crossed the analgesic intervention threshold (> 0.679) for warmed (n = 10) and unwarmed (n = 10) rats at each experimental timepoint. A greater proportion of rats in the unwarmed group had Rat Grimace Scale scores > 0.67 (P < 0.0001).
Recovery.
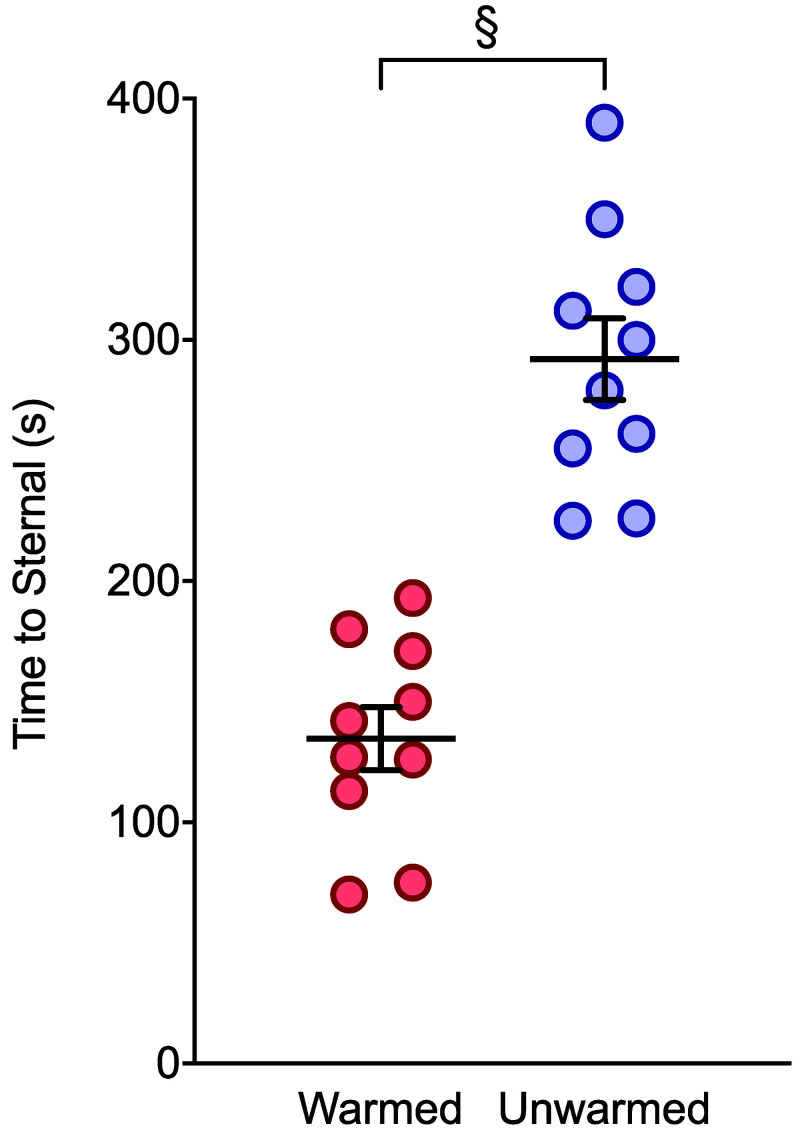
Unwarmed rats took significantly longer to attain sternal recumbency after anesthesia (Figure 4). No significant interaction was detected between time and treatment on the amount of food consumed after anesthesia (F (1, 18) = 0.692, P = 0.416). Further, treatment had no effect on the amount of food consumed (F (1, 18) = 1.106, P = 0.307). However, time did have a significant effect on the amount eaten (F (1, 18) = 28.9), P < 0.0001). Multiple comparisons did not reveal significant differences between groups (60 min: mean difference = 0.166g, P = 0.166, 95% [-0.618 to 0.950], 180 min: mean difference = 0.446g, P = 0.446, 95% CI [[-0.338 to 1.23]).
Figure 4.
Latency to sternal recumbency after termination of isoflurane for warmed and unwarmed rats (n = 10). Unwarmed rats displayed longer latencies to regain sternal recumbency (P < 0.0001 95% CI [112.4 to 202.2]). Data are mean ± SEM.
Discussion
Accurate and timely assessment of ongoing pain is required to maintain the welfare of laboratory rodents and to ensure data accuracy in pain research. The RGS is one of the most promising methods developed in response to this need. It has been validated for use in acute pain models and shows promise in some models of chronic pain.1,3,6,19 The development of real-time RGS scoring has increased the clinical utility of this method.5 In addition, an analgesic intervention threshold of greater than 0.67 has been derived for the RGS to guide pain management.9 This threshold is intended as an aid in analgesic decision making, rather than as a guarantee of the presence/absence of pain. As RGS scores exceed this threshold, the probability of pain being present increases.9 However, the existence of the threshold does not diminish the importance of observed changes in RGS score. For example, a consistent rise in scores should indicate the need for intervention. Effective use of the RGS requires an understanding of limitations and confounding factors that may introduce errors into pain assessments.
The current study showed that hypothermia induced by isoflurane anesthesia increases RGS scores and confirmed that exposure to isoflurane elicits an acute increase in RGS scores. During the PA period in which unwarmed rats were hypothermic (5 min PA), their RGS scores were significantly higher than those of warmed rats. Even after unwarmed rats returned to normothermia at 15 min PA, their RGS scores remained elevated above baseline for at least 180 min. The RGS scores of unwarmed rats showed an upward pattern between 60 and 180 min PA, at which time these scores were significantly higher than the RGS scores of warmed rats. This gradual increase was in contrast to the stable RGS scores of the warmed group. In contrast to warmed rats, the elevated RGS scores of unwarmed rats resulted in a substantially higher proportion of these rats crossing the analgesic intervention threshold (>0.67). At each of the postanesthetic timepoints, there was never more than one warmed rat that crossed the threshold; however, at least 50% of unwarmed rats crossed the analgesic intervention threshold at any given postanesthetic timepoint. Thus, the effects of hypothermia on RGS scoring could translate to a meaningful difference in pain management.
When analyzed according to specific RGS components, the nose action unit score differed significantly between groups. However, all action units increased above baseline. Because the RGS has been validated as a composite score of these action units, we do not recommend excluding or weighting any action units when using the RGS.
While warmed rats did not show increased RGS scores at all PA timepoints, their scores were significantly higher than baseline at 5 min PA and 120 min PA. Isoflurane anesthesia is the probable contributor to the increase at 5 min PA, as this was the factor common to both treatment groups. Previous work investigated the effects of isoflurane anesthesia exposure on RGS assessment when performed 15 min after the end of anesthesia.8 This study reported an association between 12 min of isoflurane anesthesia and elevated RGS scores; however, temperature management was not described. Our data support their finding that isoflurane alone causes an acute increase in RGS score. We further show that this increase is exacerbated and prolonged by concurrent hypothermia. This observation may also explain why the previous report found increased RGS scores after 12 min of isoflurane anesthesia but not after 4 min.8 Hypothermia was unlikely to have occurred during the shorter anesthetic, and performing RGS scoring 15 min after anesthesia may have missed the acute increase that we observed at 5 min PA in the warmed group.
Our findings underline the importance of maintaining normothermia during rodent anesthesia. In addition to confounding pain assessment, anesthetic-induced hypothermia extended recovery time by approximately 2.5 min. This agrees with previous reports in multiple species showing that recovery is prolonged after hypothermia.4,10,16 The mechanism for prolonged recovery from volatile anesthesia in the presence of hypothermia could include differences in anesthetic depth. Isoflurane requirements show a linear relationship with body temperature, with a reducing requirement as temperature decreases.21 Therefore, when the concentration of delivered isoflurane is constant (as in this study), hypothermic animals would be at a greater depth of anesthesia. Although not well studied in rodents, mild hypothermia reportedly produces thermal discomfort and increases the risk for surgical complications in humans.14 Based on our findings and those of others, we also recommend that pain assessment based solely on the RGS should not be conducted within 5 to 15 min of recovery from anesthesia and that researchers should ensure animals have returned to a normothermic state before RGS measurements are taken.8 These steps will reduce the artificial inflation of RGS scores in the postanesthetic period.
A limitation of this study is that it was not possible to capture the full length of time that RGS scores remain elevated in unwarmed rats. Further investigation is required to better characterize the duration of effect of hypothermia in disrupting pain scoring and the minimum amount of time that hypothermia must be present to produce this effect. A consequence of hypothermia in the unwarmed group was to slightly prolong the duration of anesthesia. This difference between groups was reflected in the longer recovery time in the unwarmed group (approximately 2.5 min; less than 10% of the total anesthesia time). This was accounted for by standardizing RGS scoring times to the time of return of sternal recumbency for each rat. Finally, it would have been ideal to have both scorers blinded to the treatment, but one scorer in this study was not blinded to the treatment groups, as they were required to deliver the anesthetic.
We have identified isoflurane-induced hypothermia as a confound to RGS scoring, demonstrating that RGS scores from hypothermic rats during surgery should be interpreted with caution and that the maintenance of normothermia is important for accurate RGS assessments.
Acknowledgments
Funding was provided by the Faculty of Veterinary Medicine of the University of Calgary through an Investigative Medicine rotation (HNKR), and a Neuroscience Summer Studentship (CBK) along with an NSERC Discovery Grant (DSJP). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Akintola T, Raver C, Studlack P, Uddin O, Masri R, Kellar A. 2017. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol Pain 2:13–17. 10.1016/j.ynpai.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Council on Animal Care. [Internet]. 1993. Guide to the care and use of experimental animals, Vol. 1, 2nd ed. [Cited 28 August 2020]. Available at: https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf
- 3.De Rantere D, Schuster CJ, Reimer JN, Pang DSJ. 2015. The relationship between the Rat Grimace Scale and mechanical hypersensitivity testing in three experimental pain models. Eur J Pain 20:417–426. 10.1002/ejp.742. [DOI] [PubMed] [Google Scholar]
- 4.Lenhardt R, Marker E, Goll V, Tschernich H, Kurz A, Sessler DI, Narzt E, Lackner F. 1997. Mild intraoperative hypothermia prolongs postanesthetic recovery. Anesthesiology 87:1318–1323. 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Leung V, Zhang E, Pang DS. 2016. Real-time application of the Rat Grimace Scale as a welfare refinement in laboratory rats. Sci Rep 6:1–12. 10.1038/srep31667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung VS, Benoit-Biancamano MO, Pang DS. 2019. Performance of behavioral assays: the Rat Grimace Scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. Pain Rep 4:1–8. 10.1097/PR9.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long H, Liao L, Gao M, Ma W, Zhou Y, Jian F, Wang Y, Lai W. 2015. Periodontal CGRP contributes to orofacial pain following experimental tooth movement in rats. Neuropeptides 52:31–37. 10.1016/j.npep.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Miller AL, Golledge HD, Leach MC. 2016. The influence of isoflurane anaesthesia on the Rat Grimace Scale. PLoS One 11:1–8. 10.1371/journal.pone.0166652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliver V, De Rantere D, Ritchie R, Chisholm J, Hecker KG, Pang DS. 2014. Psychometric assessment of the Rat Grimace Scale and development of an analgesic intervention score. PLoS One 9:1–7. 10.1371/journal.pone.0097882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pottie RG, Dart CM, Perkins NR, Hodgson DR. 2007. Effect of hypothermia on recovery from general anaesthesia in the dog. Aust Vet J 85:158–162. 10.1111/j.1751-0813.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 11.Redondo JI, Suesta P, Gil L, Soler G, Serra I, Soler C. 2012. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 170:206 10.1136/vr.100184. [DOI] [PubMed] [Google Scholar]
- 12.Redondo JI, Suesta P, Serra I, Soler C, Soler G, Gil L, Gómez-Villamandos RJ. 2012. Retrospective study of the prevalence of postanaesthetic hypothermia in dogs. Vet Rec 171:374 10.1136/vr.100476. [DOI] [PubMed] [Google Scholar]
- 13.Rice ASC, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil J, Stöhr T. 2008. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain 139:243–247. 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Rose N, Kwong GP, Pang DS. 2016. A clinical audit cycle of post-operative hypothermia in dogs. J Small Anim Pract 57:447–452. 10.1111/jsap.12547. [DOI] [PubMed] [Google Scholar]
- 15.Schubert A. 1995. Side effects of mild hypothermia. J Neurosurg Anesthesiol 7:139–147. 10.1097/00008506-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Schuster CJ, Pang DSJ. 2017. Forced-air pre-warming prevents peri-anaesthetic hypothermia and shortens recovery in adult rats. Lab Anim 52:142–151. 10.1177/0023677217712539. [DOI] [PubMed] [Google Scholar]
- 17.Sessler DI. 1997. Mild perioperative hypothermia. N Engl J Med 336:1730–1737. 10.1056/NEJM199706123362407. [DOI] [PubMed] [Google Scholar]
- 18.Sessler DI. 2001. Complications and treatment of mild hypothermia. Anesthesiology 95:531–543. 10.1097/00000542-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 19.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JCS, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. 2011. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 7:1–10. 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tappe-Theodor A, Kuner R. 2014. Studying ongoing and spontaneous pain in rodents - challenges and opportunities. Eur J Neurosci 39:1881–1890. 10.1111/ejn.12643 [DOI] [PubMed] [Google Scholar]
- 21.Vitez TS, White P, Eger EI., 2nd 1974. Effects of hypothermia on halothane MAC and isoflurane MAC in the rat. Anesthesiology 41:80–81. 10.1097/00000542-197407000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Waite ME, Tomkovich A, Quinn TL, Schumann AP, Dewberry LS, Totsch SK, Sorge RE. 2015. Efficacy of common analgesics for postsurgical pain in rats. J Am Assoc Lab Anim Sci 54:420–425. [PMC free article] [PubMed] [Google Scholar]
- 23.Waterman A. 1975. Accidental hypothermia during anaesthesia in dogs and cats. Vet Rec 96:308–313. 10.1136/vr.96.14.308. [DOI] [PubMed] [Google Scholar]