Abstract
Several diagnostic tools are available for veterinarians and fish health professionals to evaluate fish health and their abnormalities. However, reference data regarding the character and size of fish blood cells are limited. The purpose of this study was to analyze the morphology and morphometry of normal blood cells in zebrafish (Danio rerio), common carp (Cyprinus carpio carpio), and tilapia (Oreochromis niloticus). To get a representative sample, we took blood from ten 6-mo old healthy fish from each species. Fish were purchased from an ornamental fish market and the local government fish breeding center in Yogyakarta, Indonesia. A total of 100 erythrocytes and a maximum of 30 leukocytes (neutrophils, eosinophils, basophils, lymphocytes and monocytes) were randomly sampled and analyzed qualitatively and quantitatively, including morphometric analysis of both the long axis (LA) and short axis (SA) of these cells. All data obtained were analyzed using ANOVA statistical tests to compare the blood cells in each species with SPSS software. The findings revealed distinct differences in both morphology and morphometry of the blood cells among the species. Basic knowledge obtained from this research will aid in the development of biomarkers and other ancillary diagnostic tools for further hematology research, conservation, and clinical diagnosis in these 3 fish species.
Abbreviations: LA, Long Axis; SA, Short Axis
Fish are widely used as an animal model in biomedical research. Zebrafish (Danio rerio) provide an excellent animal model for the study of vertebrate biology and genetics, embryology and pathology and for preclinical drug screening for both human and veterinary medicine.18,33,38 However, the potential of zebrafish and other fish species as animal models in veterinary medicine is still not fully explored. While zebrafish are the most popular animal model in the study of genetics, pathogenesis and metabolic diseases, the posttreatment evaluation of health status in zebrafish is still lacking due to the dearth of established hematologic data.
Prior studies show that hematologic parameters may reveal important information on fish physiology and could serve as a tool to determine fish health status.12,13 Recent investigations have determined that different feeding habits, lifestyle, and adaptation to the environment may affect the blood parameters in fish species.1,14 Hematologic testing is simple, administered quickly, and delivers a wealth of information about animal health status. Moreover, evaluation of hematologic parameters could be used in quality control of farmed fish species by allowing early detection of disease and changes in health performance.12 However, the basic hematologic parameters, such as cell morphology and morphometry, are not yet fully described in many fish species. Previous research of blood cells in various animal taxa showed a significant difference in the size of blood cells, which may be related to diverse natural activities and behaviors.24 Morphometric studies are defined as quantitative descriptions of a cell's geometric structures in all dimensions.4,45 Cytomorphological classification, staining characteristics, and cytometry analysis of blood cells are sometimes simply neglected in fish hematologic tests although these factors may contribute to the study of fish pathology. For example, the size of erythrocytes could provide information regarding the types of anemia, stress, as well as other biologic, physiologic, and pathologic aspects of fish.24 Knowing the morphology of each type of blood cell is essential to performing counts of erythrocyte or leukocyte counts.
This study aimed to be a broad exploration of the morphology and morphometry of blood cells of zebrafish. We also evaluated the hematologic parameters and blood cell morphometry of 2 other important fish species, the common carp (Cyprinus carpio carpio) and the tilapia (Oreochromis niloticus). Both are popular commercial fish species in Indonesian aquaculture and are frequently studied as agriculturally relevant models of fish disease in Indonesia. As these 3 teleost species are found in different systematic and ecological niches, the blood cells could be different morphologically and morphometrically. Zebrafish and common carp are in the same family (Cyprinidae), while tilapia is in the family of Cichlidae. Therefore, the variations in the morphology and morphometry of blood cells among the 3 species is likely, with the zebrafish and carp likely to be more similar than the tilapia. Furthermore, measuring the morphometric aspects of blood cells from these fish could identify clinical biomarkers and ancillary diagnostic tools to monitor the disease and health status of these fish species, in conjunction with cytologic and histopathologic studies.31,34,39
Materials and Methods
Clearance.
All procedures performed in this research were approved by the Animal Care and Use Committee, Faculty of Veterinary Medicine, Universitas Gadjah Mada (no. 0055/EC-FKH/Int./2019).
Animals and husbandry.
Three groups, consisting of ten 6-mo old zebrafish, ten 6-mo old tilapia, and ten 6-mo old common carp were purchased from ornamental fish market and local governmental fish breeding center in Yogyakarta. Male and female fish were included in this study, with sex identification performed prior to study commencement. Fish health assessment was done weekly based on the morphologic and behavioral examination. All fish were deemed healthy after a 4-mo acclimatization period, showing good appetite and pace of respiration and no physical or behavioral defects. At 6 mo of age, the average (± SD) weight and length of zebrafish, tilapias, and common carps were 0.272 ± 0.043 gram; 356 ± 46 gram; 925 ± 114 gram and 20 ± 4 mm; 161 ± 22 mm; 280 ± 24 mm, respectively.
The zebrafish were placed in an aquarium containing conditioned water at a stocking density of 1 fish/L, as recommended.7,22 The common carp and tilapia were kept in an outdoor pond containing conditioned water. The tap water was conditioned using 1 gram per 960 L of Fraction D (Continuum Aquatics, Fort Payne, AL) to remove chlorine, ammonia, and chloramine. This conditioned water was also used to replace 25% to 33% of water during weekly cleaning. Both the aquarium and pond were equipped with a 3-stage filtration system, including mechanical, chemical, and biologic stage (U1 Underwater Filter, Fluval, Mansfield, MA). The water temperature and pH were maintained at 28 °C and 6.8 to 7.4, respectively. The light and dark cycle was set at a 12:12 h ratio. Water condition was evaluated weekly using Sera Water Test Kit (Heinsberg, Germany), measuring pH (maintained at 6.8 to 7.4), nitrite (maintained at 0 ppm), nitrate (maintained at 0 to 20 ppm), and ammonia (maintained at 0 ppm). Fish were fed with commercial diet twice a day (Takari, CP Pet Food, Jakarta, Indonesia), to meet their daily dietary protein requirements of approximately 40% to 45% of body weight.
Blood collection and blood smear preparation.
The zebrafish were euthanized by hypothermic shock in 2 °C to 4 °C ice water until loss of orientation, the righting reflex and opercular movement (approximately 20 to 60 s), as recommended to lessened the stress experienced by the zebrafish.25,28 Blood was immediately collected using micropipette and heparinized microtip from the cut tail after the zebrafish were euthanized, as described by the previous study.35 The zebrafish were then decapitated as a secondary adjunctive method of euthanasia. Blood samples from common carp and tilapia were collected from the caudal vein using a heparinized syringe after the fish were partially sedated by a gradual decrease of water temperature to 10 °C (reduced 1 °C every 15 min).2,9,24 Heparin was shown to be the best anticoagulant to retain and preserve blood cell morphology for Cyprinidae (zebrafish and common carp).21 To reduce variability, heparin was also used for blood cell preservation in tilapia. The blood smear preparations of zebrafish, common carp, and tilapia were made directly on the slide using a spreader. Blood smears were then fixed using methanol, and stained using Giemsa 10% (Merck, Darmstadt, Germany).
Morphologic and morphometric analysis of blood cells.
Qualitative analysis was to evaluate the blood smear preparations that had been stained with Giemsa 10% under a microscope with a 10 (ocular) × 100 (objective) magnification using immersion oil. Descriptive data were obtained through observing the shape of the cell and its nucleus, the lobulation of the nucleus, the color of the cytoplasm, as well as the color and density of the cytoplasmic granules. Quantitative analysis included measurements of erythrocytes, leukocytes (granular and agranular), as well as comparison of blood cell size among the 3 species we examined. Cell visualization was done using the OptiLab Advance (Miconos, Yogyakarta, Indonesia) followed by cell measurements, which were performed manually using the ImageJ program (NIH, Bethesda, MD). Parameters measured included the long axis (LA) and short axis (SA) of each blood cell. A total of 100 erythrocyte cells and a maximum of 50 cells of each type of leukocyte (neutrophils, eosinophils, basophils, lymphocytes, and monocytes) were obtained randomly from the 10 blood smear preparations taken for each fish species, and were measured according to predetermined measurement parameters. The number of fish in the experimental groups and the number of blood cells examined was twice the number of samples taken in previous study.24 This sample size was chosen to ensure adequate representation of each of the fish species and blood cells.
Statistical analysis.
Comparison of the morphometric of each blood cells among 3 species was executed using One-Way ANOVA. Tukey post hoc analysis was used to evaluate the differences between the 3 fish species when the value F was significant. All data were first analyzed to rule out the outliers using the boxplot analysis. Afterward, the data distribution and the homogeneity of variances of each parameter were examined by Shapiro–Wilk test of normality and Levene test of homogeneity of variances, respectively. Based on the data distribution test results, the range, mean, and standard deviation data from LA and SA were determined. All statistical analyses were performed using SPSS Software (IBM SPSS Statistics, version 24, SPSS, Chicago, IL) and statistical significance was set at P < 0.05.
Results
Blood cells morphology.
The blood cell morphology of zebrafish, tilapia, and common carp are presented in Figures 1, 2, and 3. Zebrafish erythrocytes were oval and disk-shaped with compact and oval nuclei, positioned equivalent to the LA of the erythrocytes (Figure 1 A).30 Unlike tilapia and common carp erythrocytes, the nuclei of the zebrafish erythrocytes were elongated, while the nuclei of tilapia and common carp erythrocytes are almost round. Tilapia (Figure 2 A) and common carp erythrocytes (Figure 3 A) have are similar ovoid shape, which is wider than zebrafish erythrocytes. In Giemsa 10% staining, the erythrocytes cytoplasm of all fishes varied from light blue to lightly basophilic with a dark blue nucleus. Four leukocyte types were identified in peripheral blood of zebrafish and tilapia based on the morphologic characteristics, whereas 5 types of leukocyte were found in the peripheral blood of common carp.
Figure 1.
The micrograph of zebrafish (Danio rerio) blood cells. (A) erythrocytes, (B) neutrophil, (C) eosinophil, (D) vacuolated monocyte, (E) lymphocyte, (F) thrombocytes. Giemsa 10% stain; bar = 10 µm (× 100 objective).
Figure 2.
The micrograph of tilapia (Oreochromis niloticus) blood cells. (A) erythrocytes, (B) neutrophil, (C) eosinophil, (D) monocyte, (E) lymphocyte, (F) thrombocyte. Giemsa 10% stain; bar = 10 µm (× 100 objective).
Figure 3.
The micrograph of common carp (Cyprinus carpio carpio) blood cells. (A) erythrocytes, (B) white arrow: neutrophil, (C) eosinophil, (D) monocyte, (E) lymphocyte, (F) thrombocyte, (G) basophil. Giemsa 10% stain; bar = 10 µm (× 100 objective).
In light of substantial debate regarding the terminology of heterophil or neutrophil in fish granulocytes,21 we used the term “neutrophil” to identify granulocytes that resembled neutrophils more than heterophils, with a clear to pale blue and grainy appearing cytoplasm and various shapes of nuclei, from round, band-typed to segmented.20 When stained with Giemsa 10%, zebrafish (Figure 1 B) and carp neutrophils (Figure 3 B-white arrow) were similar, with more eosinophilic nuclei as compared with neutrophils from tilapia (Figure 2 B)
The other type of granulocyte found in the peripheral blood of the 3 fish species was the eosinophil. Unlike the other leukocytes, the eosinophils of the zebrafish (Figure 1 C) were different than those of the common carp (Figure 3 C) and tilapia (Figure 2 C). In contrast with eosinophils from the common carp and tilapia, which had eosinophilic cytoplasm, zebrafish eosinophils contained basophilic granules in the cytoplasm and unsegmented peripheral nuclei.
The last type of granulocyte identified based on the morphologic characteristics was common carp basophils (Figure 3 G). These basophils had basophilic and grainy appearing cytoplasm, with small, round, peripheral nuclei. However, granulocytes that resembled basophils were not found in zebrafish and tilapia peripheral blood in this study.
Lymphocytes of zebrafish (Figure 1 E), tilapia (Figure 2 E), and common carp (Figure 3 E) were comparable in shape and size, as they were mainly ovoid to round, with round and unsegmented nuclei that occupied almost all of their cytoplasm. The lymphocyte nuclei stained dark blue, and cytoplasm stained from light blue to deeply basophilic. Fish lymphocytes could be distinguished from fish thrombocytes (Figure 1 F, Figure 2 F, and Figure 3 F), which were smaller in size and had darker nuclei.
Monocytes from zebrafish (Figure 1 D), tilapia (Figure 2 D), and common carp (Figure 3 D) were recognized by their large size, round shape, and their irregular and unsegmented nuclei. The cytoplasm of these cells was basophilic, and their nuclei are eosinophilic. Occasionally, the monocyte cytoplasm contained vacuoles.
Morphometric analysis of blood cells.
Morphometry of blood cells in this study was measured in microns (μm). The descriptive statistics of LA and SA of the blood cells from the 3 fish species are depicted in Table 1. As assessed by the inspection of the boxplot, there were no outliers in all data attained in this study. In addition, the data of LA and SA of the blood cells were all normally distributed (P > 0.05), and there was homogeneity of variances (P > 0.05) in all fish species groups, as assessed by Shapiro–Wilk test of normality and Levene test of homogeneity of variances, respectively.
Table 1.
The size of cells (LA x SA) measured in micron meter (μm).
| Zebrafish (Danio rerio) |
Common Carp (Cyprinus carpio carpio) |
Tilapia (Oreochromis niloticus) |
||||||||
| Cell type | N | Mean | SD | 95% CI | Mean | SD | 95% CI | Mean | SD | 95% CI |
| Erythrocyte* | 300 | 9.75 × 6.42 | 0.58 × 0.60 | 9.63-9.86 × 6.31-6.54 | 12.81 × 8.43 | 0.57 × 0.73 | 12.7-8.31 × 8.28-8.57 | 9.31 × 7.66 | 0.52 × 0.46 | 9.21-9.4 × 7.57-7.75 |
| Erythrocyte nucleus* | 300 | 5.50 × 2.96 | 0.41 × 0.34 | 5.42-5.60 × 2.90-3.04 | 5.13 × 3.51 | 0.42 × 0.35 | 5.04-5.21 × 3.44-3.57 | 4.42 × 3.16 | 0.48 × 0.31 | 4.33-4.52 × 3.09-3.22 |
| Neutrophil* | 150 | 11.17 × 10.16 | 1.70 × 1.51 | 10.69-11.65 × 9.73-10.59 | 12.85 × 11.46 | 1.12 × 1.04 | 12.53-13.16 × 11.17-11.7 | 11.5 × 10.92 | 1.04 × 0.99 | 11.19-11.79 × 10.63-11.2 |
| Eosinophil | 87 | 12.07 × 10.59 | 1.51 × 1.10 | 10.98-13.15 × 9.8-11.34 | 12.16 × 11.19 | 1.28 × 1.03 | 11.69-12.61 × 10.81-11.5 | 12.19 × 11.17 | 1.32 × 1.46 | 11.79-12.59 × 10.73-11.6 |
| Monocyte* | 150 | 11.56 × 9.53 | 2.10 × 1.70 | 10.96-12.16 × 9.05-10.01 | 13.05 × 11.61 | 1.66 × 1.54 | 12.57-13.52 × 11.17-12.1 | 13.44 × 12.12 | 1.76 × 1.56 | 12.93-13.93 × 11.68-12.5 |
| Lymphocyte* | 150 | 6.7 × 5.8 | 0.79 × 0.69 | 6.77-7.22 × 5.61-5.99 | 6.53 × 5.30 | 1.06 × 0.86 | 6.23-6.83 × 5.14-5.46 | 6.25 × 5.42 | 0.86 × 0.86 | 6.01-6.5 × 5.17-5.66 |
Statistically significant different (P < 0.05) between 3 fish species.
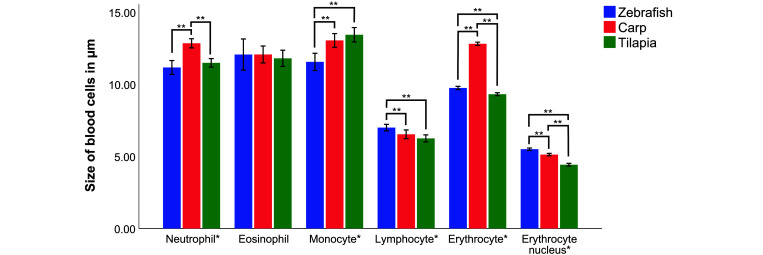
One-way ANOVA was performed to determine the differences in blood cells morphometry between zebrafish, tilapia, and common carp. Figure 4 shows the comparison of the LA of the blood cells, while the comparison of the SA of blood cells is depicted in Figure 5. A statistically significant difference in erythrocyte LA (F(2, 297) = 1172.65, P < 0.001) and SA (F(2, 297) = 275.83, P < 0.001) was detected between the 3 fishes. Tukey post hoc analysis revealed that the LA and SA of common carp erythrocytes was the highest, and that these cells were significantly longer and wider (P < 0.001) than the LA and SA of zebrafish and tilapia erythrocytes. The LA of zebrafish erythrocytes was also significantly longer than tilapia erythrocytes (P < 0.001), whereas zebrafish erythrocyte SA was the narrowest among the 3 fish species (P < 0.001).
Figure 4.
Comparison of the LA of blood cells. Error bars: 95% CI. *Statistically significant different (P < 0.05) between 3 fish species. **Statistically significant different based on Tukey post hoc analysis (P < 0.05).
Figure 5.
Comparison of the SA of blood cells. Error bars: 95% CI. *Statistically significant different (P < 0.05) between 3 fish species. **Statistically significant different based on Tukey post hoc analysis (P < 0.05).
The erythrocyte nucleus analysis found significant differences in the erythrocyte LA (F(2, 297) = 154.86, P < 0.001) and SA (F(2, 297) = 68.94, P < 0.001) of the 3 fish species. Further analysis using the Tukey post hoc test determined that the LA of the erythrocyte nucleus was longest in zebrafish (P < 0.001), followed by common carp (P < 0.001), then tilapia (P < 0.001). Among the 3 species, the SA of the erythrocyte nucleus in common carp was the widest (P < 0.001), followed by tilapia (P < 0.001), then zebrafish (P < 0.001).
One-way ANOVA analysis of each leukocyte between 3 fish species revealed significant differences on LA and SA of neutrophils (F(2, 147) = 22.70, P < 0.001) (F(2, 147) = 14.67, P < 0.001), monocytes (F(2, 147) = 14.31, P < 0.001) (F(2, 147) = 36.54, P < 0.001), and lymphocytes (F(2, 147) = 8.60, P < 0.001) (F(2, 147) = 6.72, P = 0.002), but not of LA and SA of eosinophils (F(2, 84) = 0.04, P = 0.961) (F(2, 84) = 0.94, P = 0.393). Tukey post hoc analysis on LA and SA of each leukocyte revealed differences among the 3 fish species.
The common carp neutrophils had significantly longer LA rather than tilapia (P < 0.001) and zebrafish neutrophils (P < 0.001), but the LA between tilapia and zebrafish neutrophils was not statistically significantly different (P = 0.444). The SA of the common carp neutrophils was significantly longer than those of zebrafish (P < 0.001) but was not significantly different compared with tilapia (P = 0.065). In addition, tilapia had significantly wider neutrophil (SA) rather than zebrafish (P = 0.006).
Monocyte of tilapia had the longest LA and SA among the 3 fish species, and they were statistically significant longer than zebrafish (P < 0.001), but not statistically significantly different than common carp on the LA (P < 0.547) and SA (P = 0.244).
Zebrafish lymphocyte had the longest LA and SA among the 3 fish species based on the Tukey post hoc analysis. Zebrafish lymphocyte was statistically significant longer (LA) than tilapia (P < 0.001) and common carp (P = 0.031). However, the LA of tilapia lymphocytes was not statistically significantly different than common carp lymphocyte (P = 0.268). Zebrafish lymphocytes were significantly wider (SA) than tilapia (P = 0.021) and common carp (P = 0.002), but the SA of tilapia lymphocytes was not significantly different than lymphocytes from common carp (P = 0.702).
Discussion
The analysis of the morphologic characteristics of blood cells in zebrafish (Danio rerio), common carp (Cyprinus carpio carpio), and tilapia (Oreochromis niloticus) in this study identified morphologic differences between each of the blood cells. Likewise, the morphometric analysis of normal blood cells in the 3 fish species revealed statistically significant differences between the LA and SA of erythrocytes, erythrocyte nuclei, neutrophils, monocytes, and lymphocytes, but not eosinophils. We believe that the data obtained in this study could help to define blood cell morphometry as a potential biomarker in fish and could be useful for further fish hematology research, conservation, and in establishing clinical application for these cytologic and histopathologic studies.31,34,39
According to our findings, the erythrocytes of the 3 fish species had specific morphologic and morphometric characteristics, which could be used to distinguish the erythrocytes of each fish species. The shape of zebrafish erythrocyte was oval with a compact, oval nucleus, while erythrocytes from tilapia and common carp were wider and had slightly round nuclei. The morphology of erythrocytes in these 3 fish species was consistent with prior studies.8,11,15,23,44 Even though zebrafish and common carp were in the same family of Cyprinidae, the shape and the size of their erythrocytes differed from one to another. This finding supported the preceding study, which explained that the size of erythrocyte dramatically differs from one fish species to another, as determined by an analysis of Clarias batrachus and Anabas testudineus.17 The size differences of the blood cells could determine the species of the animal on the evolutionary scale. Nucleated erythrocytes with larger size occur in the lower vertebrates (poultry and fish), while the higher vertebrates (mammals) have smaller erythrocytes without nuclei.45,46
The different habitats of the 3 fish species could influence their erythrocyte morphology. The morphology of fish erythrocytes is sensitive to various environmental impacts, so it may be a reliable bioindicator of water quality. Water contamination and toxic substances could cause morphologic anomalies in fish erythrocytes, including nuclear fragmentation and deformation, changes in chromatin, cell deformation, mitochondrial anomalies, intracellular inclusions, hemolysis, as well as amitosis.49 An abnormality of fish erythrocyte morphology may indicate the presence of environmental pollutants. Water pollutants are sometimes invisible10 and they can easily contaminate the nearby downstream water reserves and fish farming system. These hidden threats might remain undetected in a very rural aquatic area and contribute to endemic fish conservation issues.6,27 The change of erythrocyte architecture caused by water pollutants potentially compromises the physiology of endemic fish32 and eventually decreases their survival in nature. Previous research reported that waterborne toxic chemicals altered the carp erythrocyte morphology and formation.47,48 Wastewater containing toxic textile dyes were also reported to affect freshwater fish erythrocyte morphology, suggesting poikilocytosis was the sensitive indicator to assess this toxicant in waterway.41 The presence of fish erythrocyte abnormalities, therefore, is a biologic indicator, and strengthens the relevant evidence of water pollution in an aquatic area, when supplemented with water sample analysis. In addition to their primary function as oxygen-delivery vectors, fish erythrocytes are also proven to express immune-related genes and responses.40 Therefore, cellular anomalies and deformation of fish erythrocytes could affect their role in the cellular immune response in fish. Erythrocyte morphometric characteristics in fish are also associated with the age of erythrocyte. Young erythrocytes have a more circular shape, and their membrane appears to be more fluid than the membrane of old erythrocytes.26,43 Age will also affect the erythrocyte organelles, with old erythrocytes having fewer mitochondria and free ribosomes than old erythrocytes.29,36
The terminology of granulocyte types in fish species has been arguable and data regarding the fish granulocytes has been inconsistent, because the identification of fish granulocytes is done based on detailed criteria for mammalian cells, which are more evolved than fish.1 We used the traditional terms neutrophil, eosinophil, and basophil to refer to fish granulocytes, based on cell size, staining, and the shape of the granules and the nucleus. Zebrafish and common carp neutrophils were identical, with eosinophilic granules, various shapes of nuclei and the clear to pale blue and grainy cytoplasm.8,15,30,42,44 However, the size of common carp neutrophils was the largest among the 3 fish species. Tilapia neutrophil had eccentric round-to-segmented nucleus,11 which was more basophilic than zebrafish and tilapia neutrophils with the same staining.
All 3 fish species studied had eosinophils. Common carp and tilapia eosinophils were similar to mammalian eosinophils with the eosinophilic granules on Giemsa 10% staining, but had a segmented nucleus. However, the morphology of the zebrafish eosinophil was not comparable to mammalian eosinophil. Zebrafish eosinophil found in this current study corresponded with the prior studies, which contained basophilic granules in the cytoplasm on Giemsa 10% staining, with round, unsegmented peripheral nuclei.5,19 Morphometric study of eosinophil revealed that there was no significant difference in size of eosinophil among 3 fish species.
In this study, basophils were was only found in the common carp blood smear, consistent with a previous study of fish granulocytes.3 Common carp basophils had basophilic granules in the cytoplasm, with small round peripheral nuclei. An earlier study concerning zebrafish blood cells indicated that it was unknown whether basophils, which were equivalent to basophils in mammals, exists in the zebrafish.5 Basophils are not generally observed in a majority of fish, but common carp has abundant basophils in peripheral blood, along with other granulocytes.3,8,44
Monocytes were similar among the 3 fish species investigated, consistent with descriptions of monocytes in zebrafish, common carp, and tilapia by other authors.17,35,46 Monocytes are the largest blood cell in fish, with the round-shaped cells and irregularly and unsegmented nuclei. When we compared the size of monocytes in the 3 species, we concluded that the tilapia monocyte was the largest. Fish monocytes have the same function as monocytes in mammals, including phagocytosis, antigen presentation, and the production of cytokines.21 Vacuolated cytoplasm was occasionally found in the monocytes of the fish sampled in this study, as is typical for the morphologic alterations in reactive and activated monocytes.21,50
Lymphocytes of the 3 fish species were alike in shape and size, and comparable with the lymphocytes reported in prior studies, which were ovoid to round, with the round dark basophilic nucleus.8,16,19 The zebrafish lymphocyte was the largest among the 3 fish species examined. This finding could be used as a biomarker of zebrafish lymphocytes, compared with lymphocytes from other fish species.
This investigation of morphology and morphometry of blood cells in zebrafish, common carp, and tilapia could be helpful for determining the health status of these fish and for the early detection of clinical pathology and the presence of disturbance in their aquatic habitat. Cellular anomalies and deformation could act as biomarkers for possible pollution in the environment. Furthermore, as the morphology of the blood cells in fish is species-specific, the information regarding the comparison of blood cell morphology and morphometry provided by this study could enhance the identification and characterization of fish species in ecologic studies for conservation purposes. However, some potential limitations of this study should be noted. First, the different housing of the fish groups (indoor-housed for zebrafish and outdoor-housed for common carp and tilapia) could influence the morphology of blood cells. Second, comparing small-bodied fish with 2 larger-bodied fish, where erythrocyte size and shape are evolutionarily tied to capillary diameter, would likely explain the morphology and morphometry differences of blood cells. Third, differences in blood collection methods, both in the sites of blood collection and the chilling methods, could alter the morphology and morphometry of blood cells.37 Likewise, although health was assessed weekly by physical and behavioral examination, the colony fish health status was not monitored through sentinel testing or water culture. Finally, the inability to identify sex of the fish is a potential confounding variable for the blood cell differences. Even though the different blood cell morphology and morphometry in this study provide fundamental information regarding the variation of leukocytes in the 3 fish species, a need for more detailed comparison of the cell structure, description of granule variation, and cytochemistry is still needed for classifying fish blood cells. Future research addressing these topics could benefit determination of the clinical status of fish, add to the existing biomarker information, and supplement the diagnostic tools currently used to examine the health status of fish.
Acknowledgments
This study was supported by the Department Development Grant of the Faculty of Veterinary Medicine-Universitas Gadjah Mada grant no.1000/J01.1.22/HK4/2019. The authors would like to thank Nasser Yazdani and Liz Wilcox from University of Missouri-Columbia for critical evaluation of the manuscript, as well as the laboratory staffs and students in the Department of Clinical Pathology, Faculty of Veterinary Medicine-Universitas Gadjah Mada for providing technical supports during the blood samplings and blood smear preparations.
References
- 1.Acar Ü, Saoca C, Kesbic OS, Yilmaz S, Yiğit M, Inanan BE, Fazio F. 2019. Comparative study on haematological and biochemical parameters of two wild sparid fish species. Cahiers de Biologie Marine 60:51–57. DOI: 10.21411/CBM.A.39A890F1 [Google Scholar]
- 2.Ackerman PA, Morgan JD, Iwama GK. 2005. Anesthetics. In: Supplement to the guidelines on: the care and use of fish in research, teaching, and testing. Ottawa (Canada): Canadian Council on Animal Care (CCAC). [Google Scholar]
- 3.Ainsworth AJ. 1992. Fish granulocytes: morphology, distribution, and function. Annu Rev Fish Dis 2:123–148. 10.1016/0959-8030(92)90060-B. [DOI] [Google Scholar]
- 4.Baak JP. 1987. The principles and advances of quantitative pathology. Anal Quant Cytol Histol 9:89–95. [PubMed] [Google Scholar]
- 5.Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, Banuelos K, Romo-Fewell O, Aroian RV, Traver D. 2010. Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116:3944–3954. 10.1182/blood-2010-03-267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassem SM. 2020. Water pollution and aquatic biodiversity. Biodiversity International Journal 4:10–16. [Google Scholar]
- 7.Castranova D, Lawton A, Lawrence C, Baumann DP, Best J, Coscolla J, Doherty A, Ramos J, Hakkesteeg J, Wang C, Wilson C, Malley J, Weinstein BM. 2011. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 8:141–146. 10.1089/zeb.2011.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chernyavskikh SD, Kuet DH, Trikula LN, Buslovskaya LK, Kovtunenko AY, Makarova YA. 2017. Hematologic profile for Cyprinus carpio. Indo American Journal of Pharmaceutical Sciences 4:3155–3161. [Google Scholar]
- 9.Coyle SD, Durborow RM, Tidwell, JH. 2004. Anesthetics in aquaculture. SRAC Publication 3900. [Cited 12 July 2019]. Available at: http://fisheries.tamu.edu/files/2013/09/SRAC-Publication-No.-3900-Anesthetics-in-Aquaculture.pdf
- 10.da Silva Antunes de Souza MC, Antunes de Souza GK. 2019. Invisible pollutants: environmental, economic and social impacts as threats to water quality. Sustainability economic social and environmental 1:1–12. 10.14198/Sostenibilidad2019.1.01 [DOI] [Google Scholar]
- 11.da Silva Corrêa SA, de Souza Abessa DM, dos Santos LG, da Silva EB, Seriani R. 2017. Differential blood counting in fish as a non-destructive biomarker of water contamination exposure. Toxicol Environ Chem 99:482–491. 10.1080/02772248.2016.1189554 [DOI] [Google Scholar]
- 12.Fazio F. 2019. Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 500:237–242. 10.1016/j.aquaculture.2018.10.030. [DOI] [Google Scholar]
- 13.Fazio F, Saoca C, Costa G, Zumbo A, Piccione G, Parrino V. 2019. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 513:1–5. 10.1016/j.aquaculture.2019.734398. [DOI] [Google Scholar]
- 14.Fazio F, Saoca C, Acar Ü, Tezel R, Çelik M, Yilmaz S, Kesbic OS, Yalgiin F, Yiğit M. 2020. A comparative evaluation of hematological and biochemical parameters between the Italian mullet Mugil cephalus (Linnaeus 1758) and the Turkish mullet Chelon auratus (Risso 1810). Turkish Journal of Zoology 44:22–30. 10.3906/zoo-1907-37. [DOI] [Google Scholar]
- 15.Franco Z, Streisinger M, Corbo CP, Raths LA, Fulop ZL. 2011. Morphophysiological characterization of the peripheral blood of adult zebrafish (Danio rerio). In Vivo 32:56–66. [Google Scholar]
- 16.Galindo-Peña L, Pérez-Cuervo F, Poche-Ceballos A, Martinez-Moyano E, Hernandez-Sanchez J, Murcia-Ordoñez B, Chaves-Moreno L. 2019. Hematological parameters in Oreochromis niloticus cultivated in tropical conditions in experimental farm Santo Domingo of University of Amazonia, Florencia, Caquetá, Colombia. Scientia et Technica 24:125–139. [Google Scholar]
- 17.Gayatri A, Prafulla M. 2014. The morphometrical characterisation of normal blood cells of two airbreathing fishes, Clarias batrachus and Anabas testudineus. International Research Journal of Biological Sciences 3:37–41. [Google Scholar]
- 18.Goldsmith JR, Jobin C. 2012. Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol 2012:1–12. 10.1155/2012/817341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grzelak AK, Davis DJ, Caraker SM, Crim MJ, Spitsbergen JM, Wiedmeyer CE. 2017. Stress leukogram induced by acute and chronic stress in zebrafish (Danio rerio). Comp Med 67:263–269. [PMC free article] [PubMed] [Google Scholar]
- 20.Hrubec TC, Cardinale JL, Smith SA. 2000. Haematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet Clin Pathol 29:7–12. 10.1111/j.1939-165X.2000.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 21.Hrubec TC, Smith SA. 2000. Hematology of fish, p 1120–1125. Feldman BF, Zinkl JG, Jain NC, editors. Schalm's veterinary hematology, 5th ed. Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- 22.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 23.Kulkeaw K, Sugiyama D. 2012. Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Res Ther 3:1–11. 10.1186/scrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar MV. 2016. Morphometric studies of blood cells in Cyprinus carpio, Ctenopharyngodan idella and Hypophthalmichthys molitrix cultured fish in west Godavari region of Andhra Pradesh. International Journal of Fisheries and Aquatic Studies 5:489–493. [Google Scholar]
- 25.Leary S, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Turner T, Yanong R. 2020. AVMA guidelines for the euthanasia of animals: 2020 ed. Scaumburg (IL): American Veterinary Medical Association. [Google Scholar]
- 26.Lecklin T, Tuominen A, Nikinmaa M. 2000. The adrenergic volume changes of immature and mature rainbow trout (Oncorhynchus mykiss) erythrocytes. J Exp Biol 203:3025–3031. [DOI] [PubMed] [Google Scholar]
- 27.Maitland PS. 1995. The conservation of freshwater fish: past and present experience. Biol Conserv 72:259–270. 10.1016/0006-3207(94)00088-8. [DOI] [Google Scholar]
- 28.Matthews M, Varga ZM. 2012. Anesthesia and euthanasia in zebrafish. ILAR J 53:192–204. 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- 29.Moyes CD, Sharma ML, Lyons C, Leary SC, Leon M, Petrie A, Lund S, Tufts BL. 2002. Origins and consequences of mitochondrial decline in nucleated erythrocytes. Biochim Biophys Acta 1591:11–20. 10.1016/S0167-4889(02)00224-0. [DOI] [PubMed] [Google Scholar]
- 30.Murtha JM, Qi W, Keller ET. 2003. Hematologic and serum biochemical values for zebrafish (Danio rerio). Comp Med 53:37–41. [PubMed] [Google Scholar]
- 31.Nafe R. 1991. Planimetry in pathology—a method in its own right besides stereology and automatic image analysis. Exp Pathol 43:239–246. 10.1016/S0232-1513(11)80125-8. [DOI] [PubMed] [Google Scholar]
- 32.Nikinmaa M. 1992. How does environmental pollution affect red cell function in fish? Aquat Toxicol 22:227–238. 10.1016/0166-445X(92)90042-L. [DOI] [Google Scholar]
- 33.Nowik N, Podlasz P, Jakimiuk A, Kasica N, Sienkiewicz W, Kaleczyc J. 2015. Zebrafish: an animal model for research in veterinary medicine. Pol J Vet Sci 18:663–674. 10.1515/pjvs-2015-0086. [DOI] [PubMed] [Google Scholar]
- 34.Oberholzer M, Christen H, Ettlin R, Buser M, Oestreicher M, Gschwind R. 1991. Some fundamental aspects of morphometry in clinical pathology, demonstrated on a simple, multipurpose analysis system. Anal Quant Cytol Histol 13:316–320. [PubMed] [Google Scholar]
- 35.Pedroso GL, Hammes TO, Escobar TDC, Fracasso LB, Forgiarini LF, da Silveira TR. 2012. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp 63:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips MC, Moyes CD, Tufts BL. 2000. The effects of cell ageing on metabolism in rainbow trout (Oncorhynchus mykiss) red blood cells. J Exp Biol 203:1039–1045. [DOI] [PubMed] [Google Scholar]
- 37.Rahman MM, Kim HB, Baek HJ. 2019. Changes in blood cell morphology and number of red spotted grouper, Epinephelus akaara in response to thermal stress. Dev Reprod 23:139–148. 10.12717/DR.2019.23.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rissone A, Burgess SM. 2018. Rare genetic blood disease modeling in zebrafish. Front Genet 9:1–14. 10.3389/fgene.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russack VA, Artymyshyn RL. 1994. Image cytometry: current applications and future trends. Crit Rev Clin Lab Sci 31:1–34. 10.3109/10408369409084672. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Wang D, Zhao J, Chen X. 2018. Fish red blood cells express immune genes and responses. Aquac Fish 3:14–21. 10.1016/j.aaf.2018.01.001. [DOI] [Google Scholar]
- 41.Soni P, Sharma S, Kumar S, Sharma KP. 2006. A comparative study on the toxic effects of textile dye wastewaters (untreated and treated) on mortality and RBC of a freshwater fish Gambusia affinis (Baird and Herard). J Environ Biol 27:623 –628. [PubMed] [Google Scholar]
- 42.Suljević D, Martinović-Jukić A, Fočak M, Alijagić A, Rukavina D, Zahirović A. 2017. Hematological importance of pseudoeosinophilic granulocytes in acclimation of common carp (Cyprinus carpio Linnaeus, 1758). Maced Vet Rev 40:1–7. [Google Scholar]
- 43.Tavares-Dias M. 2006. A morphological and cytochemical study of erythrocytes, thrombocytes, and leukocytes in four freshwater teleosts. J Fish Biol 68:1822–1833. 10.1111/j.1095-8649.2006.01089.x. [DOI] [Google Scholar]
- 44.Tripathi NK, Latimer KS, Burnley VV. 2004. Hematologic reference intervals for koi (Cyprinus carpio), including blood cell morphology, cytochemistry, and ultrastructure. Vet Clin Pathol 33:74–83. 10.1111/j.1939-165X.2004.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 45.Van Diest P, Baak JP. 1991. Morphometry, p 946–964. In: Bibbo M, editor, Comprehensive cytology. Philadelphia (PA): WB Saunders Company. [Google Scholar]
- 46.Wintrobe MM. 1933. Variations in the size and haemoglobin content of erythrocytes in the blood of various vertebrates. Folia Haematol (Frankf) 51:32–49. [Google Scholar]
- 47.Witeska M. 2004. The effect of toxic chemicals on blood cell morphology in fish. Fresenius Environ Bull 13:1379–1384. [Google Scholar]
- 48.Witeska M, Kondera E, Szczygielska K. 2011. The effects of cadmium on common carp erythrocyte morphology. Pol J Environ Stud 20:783–788. [Google Scholar]
- 49.Witeska M. 2013. Erythrocytes in teleost fishes: a review. Zoology and Ecology 23:275–281. 10.1080/21658005.2013.846963. [DOI] [Google Scholar]
- 50.Zhang F, Feng R, Fang W, Shi Y, An L, Yang G. 2018. Cytochemical characterization of peripheral blood cell populations of two Cyprinidae, Carassius auratus and Ctenopharyngodon idellus. Anat Histol Embryol 48:22–32. 10.1111/ahe.12407. [DOI] [PubMed] [Google Scholar]