Abstract
Corncob is a common bedding material used in laboratory rodents, but little is known about differences in the effects of the 2 available sizes on rodent models and health. This study compared the effects of these 2 corncob bedding sizes on cage ammonia levels, behavior, and respiratory pathology in mice. We hypothesized that the beddings would not differ significantly in their effects on these parameters. Two strains of male mice (C57BL/6 and 129S1/Svlm) were housed in static, filter-top cages containing 1 of the 2 bedding types for the duration of the study (12 wk). Intracage ammonia was measured during 1 wk of the study on days 0, 3, 5, and 7. Behavior was evaluated by using circadian rhythm, open field, and Morris water-maze tests. Animals were euthanized with injectable euthanasia solution to collect respiratory and ocular tissues for histopathologic lesion scoring. Animals that were euthanized immediately upon arrival from the vendor served as negative controls. Bedding size did not significantly affect behavior or ammonia levels. Average intracage ammonia levels on day 7 were 525 ppm for 1/4-in. bedding and 533 ppm for 1/8-in. bedding. Regardless of the bedding size, lesions noted in both strains of mice were of similar incidence and severity, were limited to the nose, and consisted of minimal to mild suppurative rhinitis. The eyes, trachea, and lungs were not affected. In conclusion, 1/4-in. and 1/8-in. corncob beddings have comparable effects on cage ammonia levels and the behavior and respiratory pathology in male mice of the strains tested.
Corncob is a standard bedding material for laboratory rodents, but no studies have compared how the 2 commercially available sizes of pellets (1/4 and 1/8 in.) might differ in their experimental and environmental effects. Corncob is a popular bedding material because of its relatively absorptive capacity and ability to suppress ammonia levels.8,9,11,19,23,42 Anecdotal evidence has suggested that the larger corncob size might be associated with lower flooding rates from automated water valves in some environments; therefore 1/4-in. corncob bedding might be preferred for an animal resources program dealing with this issue. Additional information on the potential effects on research and animals would be useful for an institution considering conversion to corncob bedding or from one pellet size to the other.
Ammonia is a substantial concern regarding the quality of the microenvironment, and the ability of corncob to mitigate ammonia has only been studied under a few conditions.8,27 Ammonia is a known irritant to mucous membranes and respiratory tissues in many species, including rodents.6,7,16,37 In rodents specifically, many studies have shown that exposure to ammonia can have adverse effects.3,6,11,12,23,42 Respiratory tissues are particularly sensitive to ammonia, and exposure can result in inflammation, necrosis, or proliferation of epithelial tissues.3,12 In addition, corneal ulcers and other ocular lesions have been associated with ammonia exposure.40 Although ammonia levels with corncob bedding have been studied relative to other materials, to our knowledge, no studies have directly compared the 2 pellet sizes under the same housing conditions.1,8,9,11,19,23,34,42 In addition, research on the effects of bedding on intracage ammonia levels has been dominated by studies using female mice and ventilated cages. Much less is known about the effects of bedding on ammonia levels in static cages, particularly those containing male mice, which produce more urine than female mice.10
The effect of housing conditions on rodent behavior is important both directly for studies that evaluate behavior and indirectly for how behavior can influence other experimental outcomes.24,30,38 Bedding is a critical component of the housing environment, which is crucial for normal behavior development in mice.13,43 Bedding material has been shown to affect exploratory and spontaneous behavior in male mice, although corncob was not evaluated in that study.35 Other studies have demonstrated that bedding type affects anxiety behaviors and sleep patterns in rats.22,30 Most studies have compared different bedding materials rather than different sizes of the same material. Although the bedding material itself might be expected to have a more significant effect on behavior than bedding size and shape, some studies have shown that mice might prefer larger bedding particles; therefore assessing the effect of corncob pellet size on behavior is informative.2,17,18
The purpose of this study was to compare the effects of 1/4 and 1/8 in. corncob bedding on behavior, intracage ammonia levels, and pathology of the respiratory and ocular tissues of mice. We used male mice in static cages to better determine the effects of bedding under conditions likely to be associated with relatively high ammonia levels. We also used male mice because behavioral research is heavily biased toward male mice to avoid issues related to the estrous cycle. We chose the mouse stains (C57BL/6 and 129S1/Svlm) because they are commonly used background strains in behavioral research.5 We hypothesized that the pellet size of corncob bedding would not significantly alter effects on the measured parameters.
Materials and Methods
Animals and housing conditions.
All animals were housed in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals.16 All animal procedures were reviewed and approved by Emory University's IACUC.
Male mice (n = 36, C57BL/6J JAX stock no. 000664; n = 36, 129S1/Svlm JAX stock no. 002448) were obtained from Jackson Labs (Bar Harbor, ME) at 4 wk of age. All mice came from colonies that were free of ectromelia virus, Theiler virus, Hantaan virus, K virus, LDH elevating virus, lymphocytic choriomeningitis virus, mouse hepatitis virus, mouse minute virus, mouse norovirus, mouse parvovirus, mouse thymic virus, pneumonia virus of mice, polyoma virus, reovirus 3, rotavirus (epizootic diarrhea of infant mice virus), Sendai virus, Bordetella spp., CAR bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium bovis, Corynebacterium kutscheri, Helicobacter spp., Mycoplasma pulmonis, Pasturella spp., Salmonella spp., and Streptobacillus moniliformis. Animals had been housed on a mixture of aspen shavings (Northeastern Products, Warrensburg, NY) and aspen Sani-Chips (PJ Murphy Forest Products, Montville, NJ) at Jackson Labs.
On arrival at our institution, 12 naïve mice (6 of each strain) were euthanized to serve as negative controls for histopathology. The remaining mice were housed in static isolation caging (model number 75031, 750 Super Mouse Cage, Lab Products, Seaford, DE) for a total of 12 wk with either 1/4 or 1/8 in. corncob bedding (Bed-O-Cobs, The Andersons, Maumee, OH). Cages were bedded with a target of 500 mL per cage, although poststudy evaluation of random cages revealed that the volume could be as large 575 mL. Cages containing bedding were autoclaved before use. We housed 15 mice of each strain on each type of bedding (1/4 or 1/8 in. corncob pellets). Mice were housed 5 per cage, resulting in 3 cages of mice per strain for each of the bedding types, for a total of 12 experimental cages.
Mice were shipped from the vendor in contiguous groups by strain; upon arrival at our facility, they were selected at random to be placed in each cage. The mice were provided rodent chow (Rodent LabDiet 5010, Purina Mills International, St Louis, MO) and water bottles with reverse-osmosis–filtered and UV-light–treated water ad libitum and with compressed cotton squares (catalog no. NES3600, Ancare, Bellmore, NY) for enrichment. Animals were moved into clean cages once weekly. All experimental groups were housed in the same room, and all ammonia measurements were taken in the same week (week 7). For the duration of the experiment, the housing rooms were set to 10 to 15 air changes hourly, a 12:12-h light:dark cycle, a temperature set point of 72 °F (22.2 °C), and a humidity set range of 30% to 70%. The daily average humidity in the housing room for the week of ammonia measurements ranged from 43% to 51%, and the temperature ranged from 70 to 71 °F (21.1 to 21.7 °C). The light intensity in the housing room ranged from 17.9 to 53.0 fc (median, 29.6 fc) when measured 3 ft above the floor in the center of the room. Fluorescent lighting was used in all housing rooms. Cages were opened only to replenish food and water or when a health concern was reported. Only one health concern occurred during the 12 wk study, requiring a single cage to be opened on a noncage change day. Specifically, a single B6 mouse on 1/4-in. bedding was euthanized on day 0 of week 7 because of malocclusion. Therefore, this cage contained 4 animals for the remaining 5 wk of the study. No other clinical abnormalities were noted during the study.
Cage modifications and ammonia measurements.
To measure ammonia levels within the cages, colometric gas detection tubes (range, 0.25 to 3 ppm and 5 to 600 ppm, catalog nos. 8101711 and CH20501, respectively, Drager, Hoogvliet, Netherlands) and a bellows air pump (Drager) were used. A 1-cm–diameter hole was drilled on the long side of each static cage. The hole was drilled 1.5 in. from the bottom of the cage so that it was approximately nose level for mice (Figure 1). A total of 12 mouse cages, 3 cages per experimental group, were used in this study. The holes were fitted with cut-off blood-collection tubes with twist-off caps, similar to previous studies.23 For intracage ammonia measurements, the cap was removed, and the ammonia detection tube was inserted into the cage. The blood collection tubes were a tight fit around the diameter of the ammonia measurement tube to minimize gas leakage. The ammonia tubes were affixed to the bellows air pump, and 10 manual pumps were used for each measurement. Ammonia measurements were taken once daily on days 0, 3, 5, 7 of a 1-wk duration during week 7 (of the 12 wk study). Day 0 was the day that the animals were placed into a new cage with clean bedding, and measurements were taken 2 h after the animals were placed into clean cages. Cages were changed on day 7, after the last ammonia measurement was obtained.
Figure 1.
(A) Cage Modifications. (B) Colorimetric ammonia tubes and Draeger bellows air pump used to measure intracage ammonia.
Behavior studies.
Animals were acclimated to their microenvironment (bedding size) for approximately 8 wk before any behavioral testing, which occurred over 2 wk (weeks 9 through 11). The behavioral tests were performed on different dates. Some of the behavior tests required several days to complete because they involved an acquisition or training period prior to testing. All behavioral testing was performed by a single person in our Rodent Behavioral Core, which has dedicated rooms for behavioral work. The background noise level in the behavior core was 53 db. Light intensity is not routinely measured and was not measured for our study. All tests were performed during regular working hours and started in late morning. For all behavioral studies, animals were maintained in their original housing groups. Ten mice from each group (1/4-in. B6, 1/8-in. B6, 1/4-in. 129, and 1/8-in. 129) were behaviorally tested. Two of the 3 cages from each group were used for behavioral testing; therefore the same animals were tested for all of the behavior studies. Behavioral tests were performed in a pseudorandomized order.
Circadian rhythm.
Mice were placed on clean 1/4 or 1/8 in. corncob bedding in acrylic activity cages that were equipped with infrared photobeams (San Diego Instruments, San Diego, CA). Mice remained in these cages for 23 h, beginning at 1000 with removal the next morning at 0900. While in the activity cages, mice were maintained on a 12:12 h light:dark cycle and therefore experienced approximately equal amounts of light and darkness. A computer calculated ambulation (consecutive beam breaks). Food and water were available ad libitum.
Open field.
The open-field apparatus is a circular arena (diameter, 96.5 cm) with opaque gray acrylic walls (28 cm high). A circle is inscribed 18 cm from the walls that divide the chamber into a smaller inner circle (area = approximately 3100 cm2) and an outer ring (area = approximately 3800 cm2). Mice were placed individually in the center of the inner circle and allowed to roam freely in the apparatus for 5 min. Their movements were scored via MazeScan (Clever Sys, Reston, VA). Measures include time spent in the inner circle (middle) and the number of entries into the inner circle.
Morris water maze.
Mouse Morris water maze training occurred in a round, water-filled tub (diameter, 52 in.) in an environment rich with extra maze cues and a small platform 1 cm below the surface. White tempera paint (nontoxic) was added to the water to make it opaque so that the mice could not see the platform. Mice were placed at 4 different starting positions (north, south, east, west) in the water maze with their paws touching the wall in water that started at 25 °C. The invisible escape platform was located in the same spatial location at 1 cm below the water surface independent of a subject start position on a particular trial. In this manner, subjects were able to use extra maze cues to determine the platform's location. Each subject was given 4 trials daily for 5 d with a 15-min intertrial interval. The maximum trial length was 60 s; when subjects did not reach the platform in the allotted time, they were manually guided to it. Upon reaching the invisible escape platform, subjects were left on it for an additional 5 s to allow for a survey of the spatial cues in the environment to guide future navigation to the platform. After each trial, subjects were dried and then placed in a dry plastic holding cage filled with paper towels to allow them additional time to dry. The holding cage was placed half on, half off of a heating pad, and mice were monitored closely. After the 5 d of task acquisition, a probe trial was presented, during which the platform was removed, and the amount of time in the quadrant and the distance swam, which previously contained the escape platform during task acquisition, was measured over 60 s. All trials were videotaped, and performance was analyzed by using MazeScan (Clever Sys).
Pathology.
At the end of the study, we randomly selected and euthanized 2 animals from each cage. Negative control animals included 6 mice of each strain that were euthanized upon arrival at our institution. All animals were euthanized by using pentobarbital (200 mg/kg IP; Euthasol, Virbac Animal Health, Westlake, TX). After euthanasia, a complete necropsy was performed, and the heads (with eyes, larynx, trachea, lungs, and hearts left in situ were collected, immersed in neutral-buffered, 10% formalin solution. Histologic examination was performed on tissues from a total of 36 mice. Heads were decalcified in Kirstensen solution. Coronal sections were made through the nose. Tissues were subsequently processed, embedded in paraffin, sectioned at approximately 5 µm, and stained with hematoxylin and eosin. A pathologist who was blind to the group designation and certified by the American College of Veterinary Pathologists microscopically evaluated the stained sections.
Microscopic evaluation consisted of an examination of the eyes, nose (3 levels), trachea, and lung for the presence or absence of significant tissue alterations (lesions), such as, but not limited to, the following: necrosis, inflammation, and hyperplasia. Lesion incidence, severity, and distribution were recorded. Histopathologic scores were assigned as: 0, no significant histopathologic alternations; 1, minimal; 2, mild; 3, moderate; and 4, severe. Lesion distribution was recorded as focal (score, 1), multifocal (score, 2), or diffuse (score, 3; Figure 2). For individual animals, an overall lesion score was calculated for the eyes, nose (3 levels), trachea, and lung by adding individual severity and distribution scores for each lesion. The mean overall lesion score was calculated for each organ by averaging the sum of individual severity and distribution scores for each group.
Figure 2.
Severity and distribution scores from histopathologic analysis. Increase in numbers indicates increase in the severity and distribution of the lesion.
Statistical analysis.
Data are expressed throughout as mean ± SEM. Mixed-effects models were fit to examine whether ammonia levels differed across time (days 0, 3, 5, and 7), by bedding size (1/4 in. and 1/8 in.), or by strain (C57BL/6 and 129S1/Svlm). Interactions terms of these fixed effects were considered in the mixed-effects models. In an effort to better understand the properties of the circadian rhythm test, we considered 3 different time periods: the first 2 h, the dark-phase time (1900 to 0700), and the entire 23 h of testing. For each period, significant associations between bedding size, strain, or ambulations were identified by using the mixed-effects model. For the open-field test, the time spent in the center (middle of open field) and center entry frequencies were compared between bedding sizes and strains by using 2-way ANOVA models. For the Morris water maze, 2-way ANOVA models were fit for the outcome of test results (percentage of time in platform area). In addition, the effects of bedding size, strain, day, and their interactions on latency and distance in the water-maze experiment were examined by using mixed-effects models. Due to the many zero counts for the pathology outcomes, ANOVA models could not be consistently fit. Therefore we performed Fisher exact tests, overall and pairwise, to investigate significant differences by strain or bedding type for each tissue examined. We examined both continuous sum score and binary (mice with at least one lesion compared with no lesions) in these Fisher exact tests. Statistical analyses were conducted by using SAS 9.4 (SAS Institute, Cary, NC). A 2-sided α level of 0.05 was used to determine statistical significance.
Results
Ammonia.
Intracage ammonia levels were measured on days 0, 3, 5, and 7 during week 7 of the study (Figure 3). Overall, bedding size did not have a significant effect on ammonia levels (F = 0.05, df1 = 1, df2 = 8, P = 0.836). Ammonia levels did not differ significantly between mouse strains (F = 0.25, df1 = 1, df2 = 8, P = 0.628) but increased significantly (F = 1828, df1 = 3, df2 = 24, P < 0.0001) over time in all cages for both bedding sizes. Overall average ammonia levels were: day 0, 0.7±0.2 ppm; day 3, 134±25 ppm; day 5, 309±10 ppm; and day 7, 529±26 ppm. No statistically significant 2- or 3-way interactions were detected (P ≥ 0.175 for all interactions). Average ammonia concentrations for cages with 1/4 in. bedding were 0.70±0.22 ppm on day 0 and 525±27 ppm on day 7. Average ammonia concentrations for cages with 1/8 in. bedding were 0.80±0.22 ppm on day 0 and 533±26 ppm on day 7.
Figure 3.

Average (mean ± SEM) intracage ammonia over time. Ammonia levels rose over time (days 0, 3, 5, and 7) for mice (n = 3). Day 0 had the lowest levels and day 7 (day of cage change) had the highest levels. There was no significant difference between strain or bedding type.
Behavior.
Circadian rhythm.
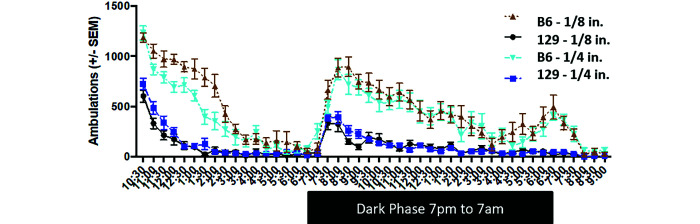
Mice were most active within the initial 2 h and were more active during the dark phase than the light phase (Figure 4). C57BL/6 mice were more active than 129 mice (F = 303.85, df = 1, P < 0.001) when total ambulations were compared over a 23-h period. C57BL/6 and 129 mice had mean ambulation counts of 19,274±1187 and 4852±585, respectively, over 23 h. Bedding size had no significant effect on total ambulations over 23 h (F = 2.5, df = 1, P = 0.123; Figure 4). Strain×bedding interactions were significant during the initial 2 h (F = 8.17, df = 1, P = 0.007) and over 23 h (F = 5.81, DF = 1, P = 0.021) but not during the dark phase (F = 0.90, df = 1, P = 0.350).
Figure 4.
Circadian rhythm test; ambulations of mice (n = 3) over 23h when housed on 1/4 compared with 1/8 in. corncob bedding. There were no significant differences observed between bedding sizes. C57BL/6J mice were more active overall than 129 mice.
Open field activity.
The 2 mouse strains differed significantly in the number of open field entries (F = 28.05, df = 1, P < 0.0001) (Figure 5 A) and time spent in the center (F = 23.34, df = 1, P < 0.0001) (Figure 5 B). The means for number of entries were 12.8±1.3 for C57BL/6 mice and 22.8±2.8 for 129 mice. The means for time spent in the center were 37.4±7.8 s for C57BL/6 mice and 11.3±3.8 s for 129 mice. Neither open field entries (F = 2.10, df = 1, P = 0.155) nor time spent in the center (F = 0, df = 1, P = 0 .960) differed between bedding sizes. Likewise, strain×bedding interactions were not significant for open field entries (F = 0.76, df = 1, P = 0.390) or time spent in the center (F = 0.73, df = 1, P = 0.400).
Figure 5.
Open field data for mice (n = 10). (A) Average (mean ± SEM) number of entries into the center (open field). (B) Average (mean ± SEM) Amount of time spent in the center. There was no significant difference between bedding type. Strains performed these tasks differently.
Morris water maze.
Time spent in the platform quadrant during the probe trial was not different between strains (F = 2.43, df = 1, P = 0.127) or bedding sizes (F = 2.31, df = 1, P = 0.137), but strain×bedding interactions were significant (F = 9.66, df = 1, P = 0.004). According to the probe trial data (Figure 6 A), 129 mice housed on 1/8 in. bedding spent more than 25% of their time in the probe quadrant (>15 s of a 60 s trial), suggesting that they had learned the task, as compared with 129 mice housed on 1/4 in. bedding. However, acquisition data (that is, training latency [Figure 6 B] and distance [Figure 6 C]) showed that 129 mice performed the task similarly on both bedding types and did not learn the task. A single 129 mouse on 1/4-in. bedding group performed the probe trail unusually well, as compared with all other mice. Latency measures showed significant interactions between bedding and day (F = 5.07, df1 = 4, df2 = 36, P = 0.002) and strain and day (F = 2.90, df1 = 4, df2 = 36, P = 0.035). Day 5 had no significant differences between bedding types (SE = 3.97, df = 36, P = 0.5580) but significant differences between strains (SE = 3.97, df = 36, P = 0.0004). For distance, significant interactions were found between bedding and day (F = 4.65, df1 = 4, df2 = 36, P = 0.004) and between strain and day (F = 3.31, df1 = 4, df2 = 36, P = 0.021). Day 5 had no significant differences between bedding types (SE = 597.23, df = 36, P = 0.8282) but a significant difference between strains (SE = 597.23, df = 36, P = 0.0001).
Figure 6.
Morris water maze data for mice (n = 10). (A) Probe trial; the percent of time the animal spent in the correct quadrant after acquisition (training) phase. (B) Latency (sec), amount of time it took for the animal to find the platform during acquisition phase. (C) Distance (mm), how far the animal swam to find the platform, during acquisition phase.
Pathology.
Lesions were seen in nasal tissues but not in lungs, trachea, or ocular tissues (Table 1). When compared with negative controls and regardless of the bedding size or strain, the lesions found were of similar distribution and severity. Nasal lesions were mainly present in the rostral sections of the nose (nose level 1). Mild suppurative inflammation was the main lesion type, and no lesions affected the most caudal section of the nose (nose level 3). The only significant difference was less nasal inflammation at nose level 1 in negative control mice compared with experimental groups (P = 0.029).
Table 1.
Mean lesion scores (severity and distribution scores combined) for inflammation and necrosis found for nasal tissues in mice.
| Experimental group | Necrosis | Inflammation | |
| Nose Level 1 | Negative Control B6 | 0 | 0.8 |
| 1/4 inch B6 | 0.5 | 2.5 | |
| 1/8 inch B6 | 0 | 1.8 | |
| Negative Control 129 | 0 | 1 | |
| 1/4 inch 129 | 0 | 2.2 | |
| 1/8 inch 129 | 0 | 2.7 | |
| Nose Level 2 | Negative Control B6 | 0 | 0.5 |
| 1/4 inch B6 | 0 | 1 | |
| 1/8 inch B6 | 0 | 0.7 | |
| Negative Control 129 | 0 | 2.5 | |
| 1/4 inch 129 | 0 | 0 | |
| 1/8 inch 129 | 0 | 0 | |
| Nose Level 3 | Negative Control B6 | 0 | 0 |
| 1/4 inch B6 | 0 | 0 | |
| 1/8 inch B6 | 0 | 0 | |
| Negative Control 129 | 0 | 0 | |
| 1/4 inch 129 | 0 | 0 | |
| 1/8 inch 129 | 0 | 0 |
Discussion
Intracage cage ammonia levels did not differ significantly between pellet sizes of corncob bedding or between strains, supporting our hypothesis that ammonia levels would not differ between the 2 bedding sizes because although pellet size differed, the bedding material is the same. The ammonia levels rose with each measurement, and by day 7, the average ammonia level was 525±27 ppm for 1/4 in. bedding and 533±26 ppm for 1/8 in. bedding. The sample size was relatively small (3 cages per experimental group, 12 cages total). Regardless, the variance between cages was low (Figure 3) and we therefore expect results would be similar for a larger sample size. One of the 3 cages for the B6 mice on 1/4 in. bedding group contained 4 mice instead of 5 for days 3, 5, and 7 of ammonia measurements; however, ammonia levels were not different in this cage as compared with the others in this group. The ammonia levels we measured are higher than those previously reported under similar conditions in mouse cages, as ammonia levels in static mouse cages with corncob bedding at similar housing densities have varied from less than 25 ppm to 400 ppm by day 7 for an individual cage.9,11,15,23,34 The sex of the mice is likely a contributing factor to the higher ammonia levels in our study, given that 4 of these 5 previous studies used female mice. Male mice excrete as much as twice as much urine as female mice, and a previous study evaluating ammonia levels in ventilated cages found much higher intracage ammonia levels in male mice than females.10,42 One study used 4 male mice per static cage with corncob bedding and still found very low ammonia levels; the reason for the difference from our study is not clear.34,35 The cage and filter lid types differed with our study, and others have suggested that static cages could vary in their containment of gases.23 In addition, the preponderance of urease-producing bacteria as part of the normal fecal flora could vary between institutions and contribute to differing ammonia levels. Other potential factors to consider whether and how they affect ammonia levels include the age of animals, bedding vendor, volume of bedding, method of bedding sterilization or lack thereof, and relative humidity—although no obvious differences in these parameters explain the large variation in ammonia values noted between studies. Our study shows the importance of evaluating ammonia levels under institution-specific conditions if the institution is targeting particular levels.
The ammonia levels in our study were higher than those acceptable for human exposure, but the relevance of these levels to rodents is unknown. For humans, the permissible exposure limit set by the Occupational Safety and Health Administration is 50 ppm and the recommended 8 to 10 h time-weighted average is 25 ppm according to the National Institute for Occupational Safety and Health and the American Conference of Governmental Industrial Hygienists.26 In our study, we found that ammonia levels rose above the 25-ppm threshold by day 3. The average ammonia levels on day 3 were 138±33 ppm for 1/4 in. bedding and 130±13 ppm for 1/8 in. bedding. Ammonia has no regulatory-established upper limit for laboratory rodents, and wild rodents naturally live in crowded underground burrows with little airflow and are likely exposed to relatively high ammonia levels, although to our knowledge, the levels under natural conditions have not been studied.23 The Guide for the Care and Use of Laboratory Rodents recommends maintaining air pollutants at low concentrations but does not provide definitive values.16 Although a safe threshold is unknown, ammonia is a known irritant, and studies have shown that airborne ammonia at or below levels found in our study can have negative effects on the respiratory system in rodents.3,6,12,16,19,23,28,41,42 Pathologic changes in the nose such as inflammation and necrosis have been noted in mice exposed to ammonia levels of 300 ppm.7 Ammonia levels of 93 ppm and 181 ppm have been associated with rhinitis and necrosis, respectively, in mice, and mild to moderate nasal pathology was noted in another study with intracage ammonia levels of approximately 100 to 400 ppm.23,42 Although time-weighted averages and chronic exposure are more relevant than an ammonia level at a particular time point, the intracage levels in our study were over 100 ppm by day 3 and therefore could be expected to cause pathologic changes in light of previous research.
Histopathology of respiratory and ocular tissues was used to evaluate potential adverse effects associated with the bedding. We evaluated tissues that were most likely to be affected by high ammonia levels. The lesions in experimental mice were limited to mild inflammation in the rostral nasal cavity; these lesions were significantly different from controls but were not affected by bedding or strain. No animals exhibited clinical signs of illness during the study. Approximately 75% of mice had lesions at nose level 1, and 33% had lesions at nose level 2. These findings support our hypothesis that the corncob bedding sizes would not differ in their effects on respiratory pathology in laboratory mice. We statistically analyzed the histopathologic data, but statistics are generally not used to interpret subjective parameters like severity and distribution scores.31
The histopathologic changes observed in the nasal cavities of our mice are most likely due to urine ammonia exposure. The nasal pathology changes found in our study were similar to those found in many publications analyzing exposure to inhalant ammonia in rodents, as described above.11,23,29,42 Although some studies have failed to find pathologic changes associated with similar ammonia levels,33,34 our observation period was relatively long (12 wk), which might have resulted in the detection of histologic changes. We cannot definitively rule out that the pathologic changes we saw might be due to other factors related to the bedding or environment, such as dust levels and bacterial loads. One study found that corncob bedding and hardwood bedding contained the higher levels of endotoxins and coliform bacteria as compared with other commercial rodent beddings, but the study did not evaluate respiratory pathology.44 Another study found average dust levels to be below 0.15% in all commercial bedding types tested, which included the 1/4 and 1/8 in. corncob bedding that we used in the current study.44 The strains in our study are immunocompetent, but opportunistic bacteria possibly could have contributed to the nasal pathology. A weakness of the negative controls used in our study is that they were not the same age as the experimental animals, and possibly the animals would have developed these lesions even under their originating housing conditions if given enough time.
Bedding size did not affect the behavioral tests, whereas strain did have an effect. As expected, all animals were initially very active when placed in the circadian rhythm caging because it was a novel environment. As previously reported, B6 mice were more active than 129 mice in the circadian rhythm test.4 In the open field test, 129 mice had significantly more entries into the center, but B6 mice spent more time in the center. In the Morris water maze, significant differences were detected due to bedding size or in overall strain performance. However, strain×bedding interaction statistics for the probe trial suggested that 129 mice on 1/4-in. bedding did not learn the task but those on 1/8 in. bedding did. We believe this result is unlikely to be repeatable, because our acquisition (training) data over 5 d failed to show that 129 mice learned the task, given the similarities between day 1 and day 5 for latency and distance and the presence of a single outlier in the 129 1/4-in. bedding group that affected the probe trial data. Conversely, B6 mice learned the task, as reflected by their probe trial performance data and the distance and latency data, which show decreasing times and distances for finding the platform over the 5-d training period. These data support the conclusion that the learning of B6 mice was not affected by bedding type, which is consistent with previous work.40 These tests would be have to be repeated to determine whether the probe trial for 129 mice on the 2 beddings is a consistent finding. A previous study has suggested that 129 mice have differences in motor learning as compared with other strains, such as C57BL/6J and FBN/NTac.5 Because 129 mice might take longer to learn than B6 mice, repeating the test with a longer training period would determine the reproducibility of this result. These findings support our hypothesis that size of corncob bedding would not have an effect on rodent behavior. Although behavior had not been previously studied with corncob bedding, our findings are consistent with a study that found no effect of aspen formulation on rodent behavior.17 The differences that we found were between stains and were consistent with previous findings.5,14,20
Our study was limited to male mice of 2 strains. Bedding size could potentially affect other research outcomes for female mice or other strains. We used male B6 and 129 mice because of the preponderance of behavioral studies that use these mice either as wildtype or background strains. Corncob bedding size could affect female but not male behavior, and possibly other strains would be affected differently than the 2 strains in our study. Previous studies have found sex- and strain-associated differences in the effects of housing conditions on behavior.24,39 Bedding size of other materials has been shown to affect the development of neuropathic pain models, and the type of bedding material could affect behavior or other phenotypes.1,21,25,39
This study shows that although the pellet size of corncob bedding is unlikely to affect ammonia levels and respiratory pathology, intracage ammonia levels can be higher than those measured in static cages of most previous studies.11,23 We expect female mice to have lower cage ammonia levels than males and therefore their associated respiratory pathology likely will be similar to or less pronounced than that in males. More frequent cage changes for high-density cages, particularly of males, should be considered to reduce the exposure to high ammonia levels. Ventilated caging can reduce pollutants also, although static caging will sometimes be required for scientific reasons or preferred for husbandry-related reasons.9,11,15,19,23,24,28,32,36,42 An established standard has not been set for ammonia levels in rodent cages, but this and similar studies can help guide cage-changing frequency and housing condition decisions to best control and minimize the effects of ammonia levels.
Acknowledgments
We thank Neville Whitehead, Derrick McCalla, Leela Geeter, Niya Xiong, and Julia Gallini for their technical assistance with this project. Support for this study was provided by the Division of Animal Resources, School of Medicine, Emory University.
References
- 1.Ambery AG, Tackett L, Penque BA, Hickman DL, Elmendorf JS. 2014. Effect of Corncob bedding on feed conversion efficiency in a high-fat diet-induced prediabetic model in C57Bl/6J mice. J Am Assoc Lab Anim Sci 53:449–451. [PMC free article] [PubMed] [Google Scholar]
- 2.Blom HJ, Van Tintelen G, Van Vorstenbosch CJ, Baumans V, Beynen AC. 1996. Preferences of mice and rats for types of bedding material. Lab Anim 30:234–244. 10.1258/002367796780684890. [DOI] [PubMed] [Google Scholar]
- 3.Bolon B, Bonnefoi MS, Roberts KC, Marshall MW, Morgan KT. 1991. Toxic interactions in the rat nose: pollutants from soiled bedding and methyl bromide. Toxicol Pathol 19:571–579. 10.1177/019262339101900402. [DOI] [PubMed] [Google Scholar]
- 4.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. 2004. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav 3:149–157. 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- 5.Bothe GWM, Bolivar VJ, Vedder MJ, Geistfeld JG. 2005. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med 55:326–334. [PubMed] [Google Scholar]
- 6.Broderson JR, Lindsey JR, Crawford JE. 1976. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol 85:115–130. [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley LA, Jiang XZ, James RA, Morgan KT, Barrow CS. 1984. Respiratory-tract lesions induced by sensory irritants at the Rd50 concentration. Toxicol Appl Pharmacol 74:417–429. 10.1016/0041-008X(84)90295-3. [DOI] [PubMed] [Google Scholar]
- 8.Burn CC, Mason GJ. 2005. Absorbencies of six different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74. 10.1258/0023677052886592. [DOI] [PubMed] [Google Scholar]
- 9.Domer DA, Erickson RL, Petty JM, Bergdall VK, Hickman-Davis JM. 2012. Processing and treatment of corncob bedding affects cage-change frequency for C57BL/6 mice. J Am Assoc Lab Anim Sci 51:162–169. [PMC free article] [PubMed] [Google Scholar]
- 10.Drickamer LC. 1995. Rates of urine excretion by house mouse (Mus domesticus): Differences by age, sex, social status, and reproductive condition. J Chem Ecol 21:1481–1493. 10.1007/BF02035147. [DOI] [PubMed] [Google Scholar]
- 11.Ferrecchia CE, Jensen K, Van Andel R. 2014. Intracage ammonia levels in static and individually ventilated cages housing C57BL/6 mice on 4 bedding substrates. J Am Assoc Lab Anim Sci 53:146–151. [PMC free article] [PubMed] [Google Scholar]
- 12.Gamble MR, Clough G. 1976. Ammonia build-up in animal boxes and its effect on rat tracheal epithelium. Lab Anim 10:93–104. 10.1258/002367776781071477. [DOI] [PubMed] [Google Scholar]
- 13.Goodwill HL, Manzano-Nieves G, LaChance P, Teramoto S, Lin S, Lopez C, Stevenson RJ, Theyel BB, Moore CI, Connors BW, Bath KG. 2018. Early life stress drives sex-selective impairment in reversal learning by affecting parvalbumin interneurons in orbitofrontal cortex of mice. Cell Rep 25:2299 –2307.e4 10.1016/j.celrep.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helder R, Desor D, Toniolo AM. 1995. Potential stock differences in the social behavior of rats in a situation of restricted access to food. Behav Genet 25:483–487. 10.1007/BF02253377. [DOI] [PubMed] [Google Scholar]
- 15.Huerkamp MJ, Lehner ND. 1994. Comparative effects of forced-air, individual cage ventilation or an absorbent bedding additive on mouse isolator cage microenvironment. Contemp Top Lab Anim Sci 33:58–61. [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Jackson E, Demarest K, Eckert WJ, Cates-Gatto C, Nadav T, Cates LN, Howard H, Roberts AJ. 2015. Aspen shaving versus chip bedding: effects on breeding and behavior. Lab Anim 49:46–56. 10.1177/0023677214553320. [DOI] [PubMed] [Google Scholar]
- 18.Kirchner J, Hackbarth H, Stelzer HD, Tsai PP. 2012. Preferences of group-housed female mice regarding structure of softwood bedding. Lab Anim 46:95–100. 10.1258/la.2011.010173. [DOI] [PubMed] [Google Scholar]
- 19.Koontz JM, Kumsher DM, Kelly R, 3rd, Stallings JD. 2016. Effect of 2 bedding materials on ammonia levels in individually ventilated cages. J Am Assoc Lab Anim Sci 55:25–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Ku KM, Weir RK, Silverman JL, Berman RF, Bauman MD. 2016. Behavioral phenotyping of juvenile Long–Evans and Sprague–Dawley rats: Implications for preclinical models of autism spectrum disorders. PLoS One 11:1–25. 10.1371/journal.pone.0158150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landeros RV, Morisseau C, Yoo HJ, Fu SH, Hammock BD, Trainor BC. 2012. Corncob bedding alters the effects of estrogens on aggressive behavior and reduces estrogen receptor-α expression in the brain. Endocrinology 153:949–953. 10.1210/en.2011-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leys LJ, McGaraughty S, Radek RJ. 2012. Rats housed on corncob bedding show less slow-wave sleep. J Am Assoc Lab Anim Sci 51:764–768. [PMC free article] [PubMed] [Google Scholar]
- 23.Mexas AM, Brice AK, Caro AC, Hillanbrand TS, Gaertner DJ. 2015. Nasal histopathology and intracage ammonia levels in female groups and breeding mice housed in static isolation cages. J Am Assoc Lab Anim Sci 54:478–486. [PMC free article] [PubMed] [Google Scholar]
- 24.Mineur YS, Crusio WE. 2009. Behavioral effects of ventilated micro-environment housing in three inbred mouse strains. Physiol Behav 97:334–340. 10.1016/j.physbeh.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Moehring F, O'Hara CL, Stucky CL. 2016. Bedding material affects mechanical thresholds, heat thresholds, and texture preference. J Pain 17:50–64. 10.1016/j.jpain.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.OSHA. [Internet]. 2020. Ammonia. OSHA Annotated Table Z-1. [Cited 10 November 2016]. Available at: https://www.osha.gov/dsg/annotated-pels/tablez-1.html.
- 27.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98. [PubMed] [Google Scholar]
- 28.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73. 10.1258/0023677011911381. [DOI] [PubMed] [Google Scholar]
- 29.Renne RA, Gideon KM, Harbo SJ, Staska LM, Grumbein SL. 2007. Upper respiratory tract lesions in inhalation toxicology. Toxicol Pathol 35:163–169. 10.1080/01926230601052667. [DOI] [PubMed] [Google Scholar]
- 30.Sakhai SA, Preslik J, Francis DD. 2013. Influence of housing variables on the development of stress—sensitive behaviors in the rat. Physiol Behav 120:156–163. 10.1016/j.physbeh.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Schafer KA, Eighmy J, Fikes JD, Halpern WG, Hukkanen RR, Long GG, Meseck EK, Patrick DJ, Thibodeau MS, Wood CE, Francke S. 2018. Use of severity grades to characterize histopathologic changes. Toxicol Pathol 46:256–265. 10.1177/0192623318761348. [DOI] [PubMed] [Google Scholar]
- 32.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62. [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AL, Mabus SL, Stockwell JD, Muir C. 2004. Effects of housing density and cage floor space on C57BL/6J mice. Comp Med 54:656–663. [PubMed] [Google Scholar]
- 34.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage micro-environment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17. [PubMed] [Google Scholar]
- 35.Tanaka T, Ogata A, Inomata A, Nakae D. 2014. Effects of different types of bedding materials on behavioral development in laboratory CD1 mice (Mus musculus). Birth Defects Res B Dev Reprod Toxicol 101:393–401. 10.1002/bdrb.21129. [DOI] [PubMed] [Google Scholar]
- 36.Teixeira MA, Chaguri LC, Carissimi AS, Souza NL, Mori CM, Saldiva PH, Lemos M, Macchione M, Guimaraes ET, King M, Merusse JL. 2006. Effects of an individually ventilated cage system on the airway integrity of rats (Rattus norvegicus) in a laboratory in Brazil. Lab Anim 40:419–431. 10.1258/002367706778476398. [DOI] [PubMed] [Google Scholar]
- 37.Tepper JS, Weiss B, Wood RW. 1985. Alternations in behavior produced by inhaled ozone or ammonia. Fundam Appl Toxicol 5:1110–1118. 10.1016/0272-0590(85)90147-2. [DOI] [PubMed] [Google Scholar]
- 38.Toth LA. 2015. The influence of the cage environment on rodent physiology and behavior: Implications for reproducibility of pre-clinical rodent research. Exp Neurol 270:72–77. 10.1016/j.expneurol.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. 2013. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav 63:543–550. 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upchurch M, Wehner JM. 1988. Differences between inbred strains of mice in Morris water maze performance. Behav Genet 18:55–68. 10.1007/BF01067075. [DOI] [PubMed] [Google Scholar]
- 41.Van Winkle TJ, Balk MW. 1986. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci 36:248–255. [PubMed] [Google Scholar]
- 42.Vogelweid CM, Zapien KA, Honigford MJ, Li L, Li H, Marshall H. 2011. Effects of a 28-day cage-change interval on intracage ammonia levels, nasal histology, and perceived welfare of CD1 mice. J Am Assoc Lab Anim Sci 50:868–878. [PMC free article] [PubMed] [Google Scholar]
- 43.Wearick-Silva LE, Orso R, Martins LA, Creutzberg KC, Centeno-Silva A, Xavier LL, Grassi-Oliveira R, Mestriner RG. 2019. Dual influences of early life stress induced by limited bedding on walking adaptability and Bdnf/TrkB and Drd1/Drd2 gene expression in different mouse brain regions. Behav Brain Res 359:66–72. 10.1016/j.bbr.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Whiteside TE, Thigpen JE, Kissling GE, Grant MG, Forsythe D. 2010. Endotoxin, coliform, and dust levels in various types of rodent bedding. J Am Assoc Lab Anim Sci 49:184–189. [PMC free article] [PubMed] [Google Scholar]