Abstract
Background
One of the important factor associated with male infertility is high production of reactive oxygen species (ROS). The main function of Nuclear factor erythroid 2-related factor 2 (NRF2) is to activate the cellular anti- oxidant response by inducing the transcription of a wide array of genes that can combat the harmful effects of factors such as oxidative stress. The purpose of this study was to evaluate the effect of N-acetyl-L-cysteine (NAC), as an antioxidant drug, on NRF2 Gene Expression in Asthenoteratozoospermia Men.
Materials and Methods
In this randomized, blinded clinical trial study, included 50 infertile men with asthenoteratozoo- spermia, who received NAC (600 mg, three times daily). Sperm parameters analyzed according to the world health organiza- tion (WHO; 2010). Sperm DNA fragmentation, relative NRF2 expression, and seminal plasma level of antioxidant enzymes were measured by TUNEL assay, reverse transcription polymerase chain reaction (RT-PCR) and ELISA test, respectively.
Results
After NAC treatment, findings showed a significant increase in sperm concentration and motility compared to pre-treatment status, whereas the percentage of abnormal morphology and DNA fragmentation was significantly decreased (P<0.05). A significant improvement in expression of NRF2 gene and antioxidant enzyme levels were ob- served compared to pre-treatment by NAC (P<0.05). Significant correlations were observed between NRF2 mRNA expression level, specific sperm parameters and level of antioxidant enzymes (P<0.05).
Conclusion
The results demonstrated that NAC oral supplementation protected against oxidative stress by enhancing NRF2 expression. This could improve semen parameters quality parameters in asthenoteratozoospermia men (Regis- tration number: IRCT20170830035998N4).
Keywords: Factor Erythroid 2-Related Factor 2, Nuclear Asthenoteratozoospermia, N-Acetyl-Cysteine, Oxidative Stress
Introduction
One of the main causes of infertility in men is oxidative stress or high production of reactive oxygen species (ROS). It can also be provoked from reduced antioxidant capacity of semen and spermatozoa creating the conditions termed oxidative stress (1). Oxidative stress contributes to damage to various sperm parameters such as sperm morphology, sperm count and sperm DNA fragmentation associated with reducing fertility (2). Although, low amounts of ROS is essential for physiological and functional processes (such as acrosome reaction, capacitation and perm-oocyte penetration), excessive production of ROS can negatively impact the sperm quality and subsequently hampers fertility (3). Naturally, excessive production of ROS is counterbalanced by enzymatic and non-enzymatic antioxidants present in male reproductive tract (4). Production of antioxidant enzymes are regulated by a common regulatory factor-like nuclear factor erythroid 2-related factor 2 (NRF2) (5). NRF2 regulates gene transcriptions containing antioxidant response elements (AREs) (6) like catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX).
In normal conditions, NRF2 is repressed by the negative regulator protein Keap1, largely localized in the cytoplasm. In this condition, NRF2 is targeted by ubiquitination and proteasome degradation. Under oxidative stress condition NRF 2 is phosphorylated. This phenomenon disrupts formation of the Keap1- NRF 2 complex. Subsequently, NRF2 is translocated in the nucleus and the level of enzymes containing this regulatory element is up-regulated (7).
NAC is derived from amino acid L-cysteine containing sulfhydryl groups that has free radical scavenging activ ity (8-10). Therefore, it is supplemented to alleviate glutathione (GSH) depletion during oxidative stress. Despite the well-known antioxidant capacity of NAC in different oxidative stress conditions (including male infertility) the correlation between NAC-induced oxidative protections and signaling transduction pathway remains to be elucidated (11-12).
Therefore, we investigated expression of NRF2 in the sperm of asthenoteratozoospermia individuals treated with NAC. In addition, we studied relationship of NRF2 expression with protein level of antioxidant enzymes, including CAT, SOD and GPX.
Materials and Methods
A randomized, blinded clinical trial was designed for this study. A total of 50 infertile men with idiopathic asthenoteratozoospermia, at the age of 25 to 40 years old, were enrolled. Patients were referred to ACECR Infertility Research Center (Qom, Iran) from July 2018 to November 2018. None of the infertile couples had previously achieved pregnancy.
Inclusion criteria were infertile men with no history of varicocele, obstruction, cancer and chemotherapy as well as abnormal testes, leukospermia, cigarette smoking and alcohol consumption. Infertile patients were considered as male individuals with “asthenoteratozoospermia”, according to the world health organization (WHO) guidelines (13). A normal female partner was defined as a woman with regular menses, normal hormonal profile and hysterosalpigogram. The male individuals were defined as asthenozoospermic, if their total sperm motility was below 40% and/or their progressive motility was below 32%. Most of our participants had absolute asthenozoospermia and both parameters were below the WHO criteria. During this study, the patients received NAC (600 mg daily, for three months). Variables sperm parameters, DNA fragmentation index, NRF2 gene expression and level of the antioxidant enzymes in seminal plasma were measured before and after intervention.
Semen analysis and preparation
Sperm analysis was performed according to the WHO guidelines criteria, 2010 (14). All Semen samples were collected by masturbation after 3-4 days of abstinence and allowed to liquefy for 15-30 minutes at room temperate. Total and progressive motility were analyzed using the computer-aided sperm analysis (CASA) system (LABOMED, SDC313B, and Germany). Sperm morphology was stained with Papanicolaou and 200 sperms were evaluated per slide (15). Sperm number was counted by a sperm counting chamber and expressed as million/ ml. Samples with more than 1 million leukocytes in 1 ml of semen were excluded from the study. Semen samples were washed by Ham’s F-10 solution. The resulting sperm pellet was divided into several aliquot parts and they were kept frozen at -80˚C for subsequent analyses of RNA and biochemical factor levels.
Assessment of DNA fragmentation (TUNEL assay)
Sperm DNA fragmentation analysis was determined using the in-situ cell death detection kit (Roche, Germany) based on the labeling of DNA strand breaks (TUNEL technology) (16). At least 200 stained sperms per field were assessed under an epifluorescent microscope (BX51, Olympus, Japan) at ×100 magnification. Percentage of the sperms with DNA-damaged was considered as number of TUNEL-positive (green fluorescence) and percentage of the sperms with intact DNA was considered as number of TUNEL-negative (red fluorescence).
Assessment of NRF2 by reverse transcription–polymerase chain reaction
After complete liquefaction, the cells in 1 ml of every sample were pelleted by centrifugation (6000 rpm). Total cellular RNA extraction was performed by using RNeasy Plus Micro Kit (Qiagen, Germany) according to the manufacturer’s instruction.
To remove DNA contamination, the extracted RNA samples were treated with DNase I. cDNA was reverse transcribed from 2μg of total RNA using M-MLV reverse transcriptase (Fermentase Corporation, Lithuania) and the corresponding oligonucleotide primers. Polymerase chain reaction (PCR) was carried out using 2μg cDNA specific primers for the both GAPDH and NRF2 genes (Table 1).
Table 1.
Primers used for RT-PCR analysis
| Transcript | Sequence (5'-3') | Length of DNA product (bp) |
|---|---|---|
| GAPDH | F:TGGCTACAGCAACAGGGTG | 104 |
| R: CTCTTGTGCTCTTGCTGGG | ||
| NRF2 | F:AGCACATCCAGTCAGAAACC | 203 |
| R:TAGCCGAAGAAACCTCATTG | ||
Real-time PCR program consisted of enzyme activation at 95°C for 30 seconds, followed by 40 cycles of a twostep program, including template denaturation at 95°C (5 seconds) and annealing/extension at 58°C (30 seconds). The PCR product sizes were 203bp for NRF 2 and 104bp for GAPDH. The 2-∆∆Ct method was calculated to represent the relative quantification of mRNA expression of NRF2 after normalization to that of GAPDH, where ∆CT= (CT, NRF2 antioxidant genes-CT, GAPDH).
Assessment of semen biochemical factors
For the biochemical factors analysis, we separated seminal plasma and stored it at -80°C until use. Total antioxidant capacity (TAC) and Malondialdehyde (MAD) of the plasma for all samples were measured using the commercial kits (Zell Bio GmbH, Wurttemberg and Germany). The level of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX) was assessed by ELIZA kit (Abnova Corporation, Taiwan).
Statistical analysis
The statistical software SPSS (Version 20, USA) was used for data analysis. Data are presented as mean ± standard error of the mean (SEM). The paired sample t-test was used for comparison of the samples before and after NAC treatment. Correlation between different variables was studied using the Pearson correlation coefficient. A P<0.05 was considered statistically significant.
Ethical considerations
This clinical trial study was registered in the Iranian Registry of clinical trials (Registration number: IRCT20170830035998N4) and it was approved by the Ethics Committee for Research Involving Human Subjects at Science and Research Branch of Azad Medical University (Tehran, Iran). An informed consent was obtained from each participant and this study was in continuation of previous study (17).
Results
Effect of N-acetyl-L-cysteine treatment on sperm parameters
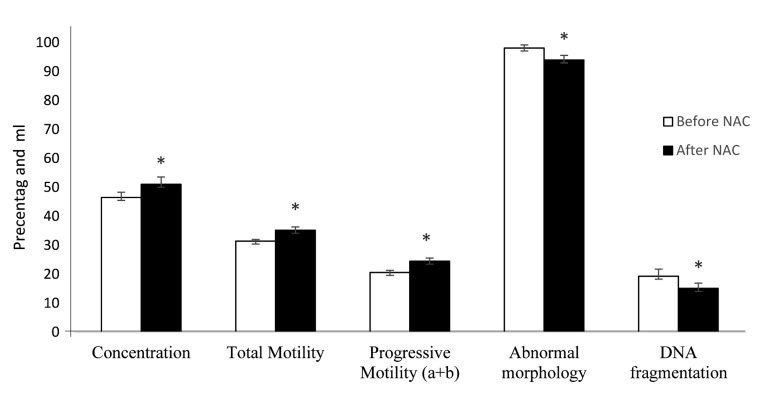
Sperm concentration, sperm motility (total and progressive motilities), sperm morphology were significantly different at end of the study (Fig . 1). After NAC supplementation, mean sperm concentration and percentage of motile sperm were significantly increased compared to the samples before NAC treatment (P<0.05). The results showed significant improvement in the samples with abnormal morphology (P<0.05). Additionally, significant improvement was observed in sperm DNA fragmentation after treatment by NAC (P<0.01).
Fig.1.
Comparison of sperm parameters before and after NAC treatment. *; significant difference before and after treatment, and NAC; N-acetyl-cysteine.
Effect of N-acetyl-L-cysteine treatment on NRF2 gene expression
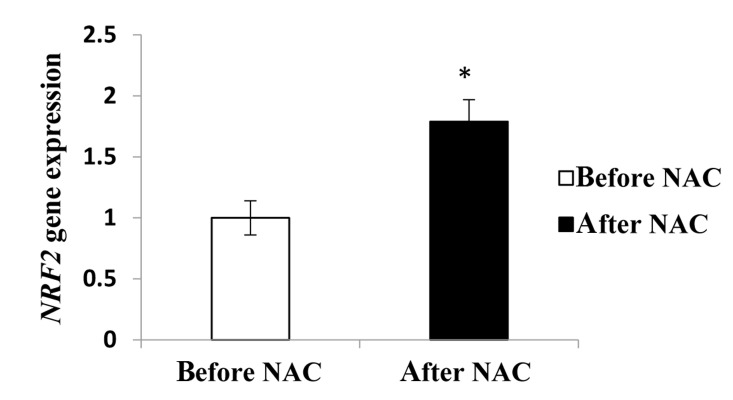
To explore role of NAC in regulating the expressions of NRF2, we analyzed relative expression of NRF2 gene in sperm cells using RT-PCR method. As shown in Figure 2, expression of NRF2 gene after treatment was significantly higher than before treatment. The results indicated that after intervention, NAC significantly increased NRF2 expression level (1.00 ± 0.14 vs. 1.79 ± 0.18 respectively, P=0.01).
Effect of N-acetyl-L-cysteine tr eatment on biochemical factors
A higher level of TAC on seminal plasma was observed after NAC supplementation. Moreover, the level of MDA on seminal plasma was significantly lower in infertile men after treatment with NAC compared to with before treatment with NAC (P<0.05). In addition, the results demonstrated that CAT, GPX and SOD levels were significantly increased in NAC treated group (P<0.05, Table 2).
Table 2.
Comparison of biochemical factor before and after NAC
| Biochemical factors | Before NAC (n=50) | After NAC (n=50) | P value |
|---|---|---|---|
| TAC(µM) | 1.82 ± 0.11 | 2.51 ± 0.13 | 0.01* |
| MDA(µM) | 2.36 ± 0.10 | 1.97 ± 0.09 | 0.01* |
| CAT(U/ml) | 13.44 ± 2.63 | 18.04 ± 1.79 | 0.005* |
| SOD(U/ml) | 0.14 ± .014 | 0.18 ± .006 | 0.01* |
| GPX(U/ml) | 344 ± 12.68 | 378 ± 13.25 | 0.04* |
Data are shown as mean ± SD, *; Significant differences between before and after NAC treatment, TAC; Total antioxidant capacity, CAT; Catalase, SOD; Superoxide Dismutase, GPX; Glutathione Peroxidase, MDA; Malondialdehyde, and NAC; N-acetylcysteine.
Correlation analysis showed that NRF2 mRNA expression was correlated with sperm parameters (sperm abnormality, total motility and DNA fragmentation). Additionally, NRF2 gene expression was negatively correlated with MDA, while it was positively correlated with seminal plasma TAC and other antioxidant enzymes levels (including CAT, SOD and GPX) were detected both before and after NAC treatment (P<0.05 for all tests, Table 3).
Table 3.
Correlations between NRF2 mRNA level, sperm parameters and level of antioxidant enzymes before and after NAC
| Correlations | NRF2 | |
|---|---|---|
| r | P value | |
| Sperm abnormal morphology (%) | ||
| Before NAC | -0.436 | 0.02 |
| After NAC | -0.473 | 0.01 |
| Total Motility (%) | ||
| Before NAC | 0.399 | 0.04 |
| After NAC | 0.499 | 0.01 |
| DFI (%) | ||
| Before NAC | -0.389 | 0.05 |
| After NAC | -0.430 | 0.03 |
| MDA(µM) | ||
| Before NAC | -0.441 | 0.001 |
| After NAC | -0.438 | 0.001 |
| TAC (µM) | ||
| Before NAC | 0.488 | 0.05 |
| After NAC | 0.408 | 0.02 |
| CAT(U/ml) | ||
| Before NAC | 0.226 | 0.05 |
| After NAC | 0.326 | 0.03 |
| SOD(U/ml) | ||
| Before NAC | 0.664 | 0.01 |
| After NAC | 0.815 | 0.000 |
| GPX(U/ml) | ||
| Before NAC | 0.194 | 0.094 |
| After NAC | 0.255 | 0.05 |
CAT: Catalase; DFI: DNA Fragmentation Index; GPX: Glutathione peroxidase; MDA: Malondialdehyde; NAC: N-acetylcysteine; NRF2: Nuclear factor erythroid 2-related factor 2; SOD: Superoxide dismutase; TAC: Total antioxidant capacity, and significant differences in bold.
Fig.2.
Comparison of relative expression of NRF2 before and after NAC treatment. NAC; N-acetyl-cysteine, NRF2; Nuclear factor erythroid 2-related factor 2, and *; Significant difference before and after NAC treatment.
Discussion
The presence large number of mRNAs in human spermatozoa may effect on the events of spermatogenesis and sperm quality (18). Correlation between sperm quality and mRNA expression has previously been investigated in animals (19). Therefore Analysis of testicular genes may be an essential marker to study the role of antioxidant genes in spermatogenesis and diagnosis of male infertility.
The main results of our study revealed the role of NRF2 gene on sperm quality through NAC supplementation in vivo. Enhancement of NRF 2 gene expression by NAC may account for the improved antioxidant capacity induced by NAC. NAC is a thiol compound which can provide sulfhydryl substance. It should be taken into account that NAC has antioxidant properties. It acts via increasing the intra-cellular concentration of cysteine/GSH and scavenging free radical (20, 21). GSH plays important role in physiological functions and protection against oxidative stress (22, 23). NAC, a known antioxidant drug, can protect cells from oxidative stress through regulating NRF2 signaling pathway by regulating GSH synthesis and maintaining the level of GSH in cells (24, 25).
Our results showed a significant improvement in the sperm parameters after 12 weeks treatment with NAC, compared to the pre-treatment baseline. The results of this study revealed that there was a relationship between NRF2 mRNA levels and specific sperm functional parameters including, (motility, abnormal morphology and DNA fragmentation) after NAC treatment. Excessive oxidative stress directly contributed to the damage of sperm DNA by initiating apoptosis via inducing caspase-mediated enzymatic degradation of sperm DNA (26). Antioxidant administration, such as NAC, may help decrease ROS and improve sperm DNA fragmentation (27,28). A significant correlation was observed with NRF2 mRNA expressions and sperm quality showed that the effect of NAC on sperm parameters might be mediated through NRF2. Several studies determined low sperm quality in humans associated with abnormal mRNA content of the certain gene (29). Yu et al. (30) showed that functional discrepancy in the NRF2 gene promoter was correlated with abnormal spermatogenesis in humans. Previous studies showed that long term cigarette smoking can cause male infertility through inhibiting NRF2 gene expression and sperm DNA fragmentation (31). Therefore, disruption of NRF2 mRNA level might be one of the molecular signaling pathways of disruptive sperm function.
Defect in expression of NRF2 transcription factor is known to be critical in regulating the major determinants of the defense system against oxidative stress leading to harmful effects (32, 33). Results from the recent study demonstrated that mouse testes germ cell and Leydig cell were protected from oxidative stress in the process of heat treated-induced oxidative stress by activation of NRF2 (34). In presence of oxidative stress, NRF2 releases Keap1- mediated repression and is translocated to the nucleus. In addition, it binds to ARE located in the promoter of many antioxidant enzymes and activates the expression of AREdependent genes (35, 36). NAC acts to reduce glutathione (GSH) precursor and increasing of glutathione reductase (GR) levels by up-regulation of NRF2 expression, attenuating the ability to scavenge free radicals and oxidative stress damage (37). In this study, NAC administration increased TAC and decreased MDA levels in seminal plasma. These effects of NAC are consistent with the results obtained from previous study, indicating that NAC could improve lipid metabolism through NRF2 signaling pathway in patients with renal ischemia/reperfusion injury (38).
The obtained negative correlation between NRF2 gene expression and MDA, in addition to the positive correlation of this gene expression with TAC suggests a possible associating effect. Previous studies reported that NRF2-knockout mouse had low total antioxidants levels as well as high testicular and epididymal lipid peroxidation (MDA) levels which resulted in lower sperm motility than normal males 6). According to our results, NAC significantly increased level of the antioxidant enzymes such as CAT, SOD and GPX. It was declared that there is direct correlation between NRF2 gene expression and antioxidant enzyme levels (CAT, SOD and GPX) in seminal plasma. In fact, role of NRF 2 is to maintain homeostasis between oxidative stress and antioxidant system (37).
In contrast to these results, several studies confirmed that NRF2 knockout decreased antioxidant genes expression and increased oxidative injury in mouse, indicating that the NRF2/ARE pathway is a key regulator of the body's redox state. It was reported that activity of many antioxidant enzymes (e.g. SOD and CAT) decreased in NRF2-/- mouse (39). Therefore, men with low sperm quality are likely to decrease NRF2 mRNA and level of antioxidant enzymes. These correlations were further improved after NAC.
Conclusion
In the present study, we observed beneficial effect of NAC, which improves sperm parameters, decreases MDA production and increases antioxidant enzyme levels, in addition to increasing NRF2 levels. Accordingly, normal human spermatogenesis requires an integrated antioxidant capability as reduced antioxidant enzyme levels may be attributed with defective sperm function. Thus, antioxidant therapy, such as NAC, may induce sperm N-Acetyl-Cysteine and NRF2 Gene in Asthenoteratozoospermia Men 175 Int J Fertil Steril, Vol 14, No 3, October-December 2020 function by up-regulating NRF2 expression level.
Acknowledgements
This study was finacially supported by Science and Research Branch, Islamic Azad University (Tehran, Iran). We thank the members of IVF Unit of Infertility Research Center of the ACECR, Qom. The authors declare that they have no competing interests.
Authors’ Contributions
R.J., M.N., K.P., N.H.; Contributed to prepare concept, design and draft the manuscript. Registration in IRCT, ethical committee approval, data collection and statistical analysis was carried out by R.J. All authors approved the final version of the manuscript.
References
- 1.Cardoso JP, Cocuzza M, Elterman D. Optimizing male fertility: oxidative stress and the use of antioxidants. World J Urol. 2019;37(6):1029–1034. doi: 10.1007/s00345-019-02656-3. [DOI] [PubMed] [Google Scholar]
- 2.Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14(8):470–485. doi: 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 3.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J Urol. 2018;16(1):35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal A, Anandh Prabakaran SJIJoRB. Oxidative stress and antioxidants in male infertility: a difficult balance. Int J Reprod Biomed. 2005;3(1):1–0. [Google Scholar]
- 5.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura BN, Lawson G, Chan JY, Banuelos J, Cortés MM, Hoang YD, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49(9):1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38(1-2):171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- 8.Dhouib IE, Jallouli M, Annabi A, Gharbi N, Elfazaa S, Lasram MM. A minireview on N-acetylcysteine: an old drug with new approaches. Life Sci. 2016;151:359–363. doi: 10.1016/j.lfs.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Mokhtari V, Afsharian P, Shahhoseini M, Kalantar SM, Moini A. A review on various uses of N-acetyl cysteine. Cell J. 2017;19(1):11–17. doi: 10.22074/cellj.2016.4872. 10 Goldstein GAN-acetylcysteine amide (nac amide) for treatment of oxidative stress associated with infertilityGoogle Patents; 2010Available from: https://patentsgooglecom/patent/EP1877044A2/ en(14 Apr 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein GA. N-acetylcysteine amide (nac amide) for treatment of oxidative stress associated with infertility. Google Patents. 2010. Available from: https://patents.google.com/patent/EP1877044A2/ en . (14 Apr 2020)
- 11.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on semen parameters and oxidative/antioxidant status. Urology. 2009;74(1):73–76. doi: 10.1016/j.urology.2009.02.034. 12 Baetas J, Rabaça A, Gonçalves A, Barros A, Sousa M, Sá RProtective role of N-acetylcysteine (NAC) on human sperm exposed to etoposideBasic Clin Androl2019; 29: 3. [DOI] [PubMed] [Google Scholar]
- 12.Baetas J, Rabaça A, Gonçalves A, Barros A, Sousa M, Sá R. Protective role of N-acetylcysteine (NAC) on human sperm exposed to etoposide. Basic Clin Androl. 2019;29(9):3–3. doi: 10.1186/s12610-018-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. WHO; 2010. [Google Scholar]
- 14.Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava N, Pande M. Protocols in semen biology (comparing assays) Springer. 2017:89–107. [Google Scholar]
- 16.Kheirollahi-Kouhestani M, Razavi S, Tavalaee M, Deemeh MR, Mardani M, Moshtaghian J, et al. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum Reprod. 2009;24(10):2409–2416. doi: 10.1093/humrep/dep088. [DOI] [PubMed] [Google Scholar]
- 17.Jannatifar R, Parivar K, Roodbari NH, Nasr-Esfahani MH. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod Biol Endocrin. 2019;17(1):24–24. doi: 10.1186/s12958-019-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushima W, Brink K, Schroeder J, Miska EA, Gapp K. Mature sperm small-RNA profile in the sparrow: implications for transgenerational effects of age on fitness. Environ Epigenet. 2019;5(2):dvz007–dvz007. doi: 10.1093/eep/dvz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arangasamy A, Kasimanickam V, DeJarnette JM, Kasimanickam R. Association of CRISP2, CCT8, PEBP1 mRNA abundance in sperm and sire conception rate in Holstein bulls. Theriogenology. 2011;76(3):570–577. doi: 10.1016/j.theriogenology.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Zhitkovich A. N-acetylcysteine: antioxidant, aldehyde scavenger, and more. Chem Res Toxicol. 2019;32(7):1318–1319. doi: 10.1021/acs.chemrestox.9b00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein GA. N-Acetylcysteine Amide (Nac Amide) for Treatment of Oxidative Stress Associated with Infertility. Google Patents. 2008. Available from: https://patentsgooglecom/patent/US8354449B2/en 14 Apr 2020)
- 22.Baetas J, Rabaça A, Gonçalves A, Barros A, Sousa M, Sá RJB, et al. Protective role of N-acetylcysteine (NAC) on human sperm exposed to etoposide. Basic Clin Androl. 2019;29:3–3. doi: 10.1186/s12610-018-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammadi-Sardoo M, Mandegary A, Nabiuni M, Nematollahi-Mahani S-N, Amirheidari B. Mancozeb induces testicular dysfunction through oxidative stress and apoptosis: protective role of N-acetylcysteine antioxidant. Toxicol Ind Health. 2018;34(11):798–811. doi: 10.1177/0748233718778397. [DOI] [PubMed] [Google Scholar]
- 24.Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, et al. N-acetylcysteine attenuates phosgene-induced acute lung injury via upregulation of Nrf2 expression. Inhal Toxicol. 2010;22(7):535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- 25.Vomund S, Schäfer A, Parnham M, Brüne B, von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci. 2017;18(12):2772–2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bansal AK, Kaushik KJTI-AIJoRiaA. Role of oxidative stress, reactive oxygen species & antioxidants in male reproductive functions. Theriogenology Insight. 2019;9(1):35–45. [Google Scholar]
- 27.Miner SA, Robins S, Zhu YJ, Keeren K, Gu V, Read SC, et al. Evidence for the use of complementary and alternative medicines during fertility treatment: a scoping review. BMC Complement Altern Med. 2018;18(1):158–158. doi: 10.1186/s12906-018-2224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barekat F, Tavalaee M, Deemeh MR, Bahreinian M, Azadi L, Abbasi H, et al. A preliminary study: N-acetyl-L-cysteine improves semen quality following varicocelectomy. Int J Fertil Steril. 2016;10(1):120–126. doi: 10.22074/ijfs.2016.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B, Wang P, Wang Z, Jia Y, Niu X, Wang W, et al. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J Assist Reprod Genet. 2010;27(12):719–724. doi: 10.1007/s10815-010-9466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu B, Lin H, Yang L, Chen K, Luo H, Liu J, et al. Genetic variation in the Nrf2 promoter associates with defective spermatogenesis in humans. J Mol Med (Berl) 2012;90(11):1333–1342. doi: 10.1007/s00109-012-0914-z. [DOI] [PubMed] [Google Scholar]
- 31.Elsamanoudy AZ, Shaalan D, Abo El-khair SM, Gaballah MA, State A, Helaly A. NRF2 gene expression and DNA fragmentation markers as possible predictors of chronic smoking induced spermatozoa dysfunction in infertility with normal seminogram. Human Andrology. 2017;7(4):127–135. [Google Scholar]
- 32.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.David JA, Rifkin WJ, Rabbani PS, Ceradini DJ. The Nrf2/Keap1/ ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res. 2017;2017:4826724–4826724. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Huang Y, Piao Y, Nagaoka K, Watanabe G, Taya K, et al. Protective effects of nuclear factor erythroid 2-related factor 2 on whole body heat stress-induced oxidative damage in the mouse testis. Reprod Biol Endocrin. 2013;11:23–23. doi: 10.1186/1477-7827-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egea J, González Rodríguez Á, Gómez-Guerrero C, Moreno JAJFip. Role of Nrf2 in disease: Novel molecular mechanisms and therapeutic approaches. Front Pharmacol. 2019;10:1149–1149. doi: 10.3389/fphar.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keleku-Lukwete N, Suzuki M, Yamamoto M. An overview of the advantages of KEAP1-NRF2 system activation during inflammatory disease treatment. Antioxid Redox Signal. 2018;29(17):1746–1755. doi: 10.1089/ars.2017.7358. [DOI] [PubMed] [Google Scholar]
- 37.Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, et al. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med. 2009;46(4):443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Zhu Z, Liu J, Zhu Z, Hu Z. Protective effect of N-acetylcysteine (NAC) on renal ischemia/reperfusion injury through Nrf2 signaling pathway. J Recept Signal Transduct Res. 2014;34(5):396–400. doi: 10.3109/10799893.2014.908916. [DOI] [PubMed] [Google Scholar]
- 39.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96(22):12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]