Abstract
Some cases of chylomicronemia are caused by autoantibodies against glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1), an endothelial cell protein that shuttles LPL to the capillary lumen. GPIHBP1 autoantibodies prevent binding and transport of LPL by GPIHBP1, thereby disrupting the lipolytic processing of triglyceride-rich lipoproteins. Here, we review the “GPIHBP1 autoantibody syndrome” and summarize clinical and laboratory findings in 22 patients. All patients had GPIHBP1 autoantibodies and chylomicronemia, but we did not find a correlation between triglyceride levels and autoantibody levels. Many of the patients had a history of pancreatitis, and most had clinical and/or serological evidence of autoimmune disease. IgA autoantibodies were present in all patients, and IgG4 autoantibodies were present in 19 of 22 patients. Patients with GPIHBP1 autoantibodies had low plasma LPL levels, consistent with impaired delivery of LPL into capillaries. Plasma levels of GPIHBP1, measured with a monoclonal antibody–based ELISA, were very low in 17 patients, reflecting the inability of the ELISA to detect GPIHBP1 in the presence of autoantibodies (immunoassay interference). However, GPIHBP1 levels were very high in five patients, indicating little capacity of their autoantibodies to interfere with the ELISA. Recently, several GPIHBP1 autoantibody syndrome patients were treated successfully with rituximab, resulting in the disappearance of GPIHBP1 autoantibodies and normalization of both plasma triglyceride and LPL levels. The GPIHBP1 autoantibody syndrome should be considered in any patient with newly acquired and unexplained chylomicronemia.
Keywords: glycosylphosphatidylinositol-anchored high density lipoprotein binding protein 1, lipoprotein lipase, triglycerides, autoimmune disease, immunoglobulin A, immunoglobulin G4
GPIHBP1 AND LPL, OBLIGATE PARTNERS IN PLASMA TRIGLYCERIDE METABOLISM
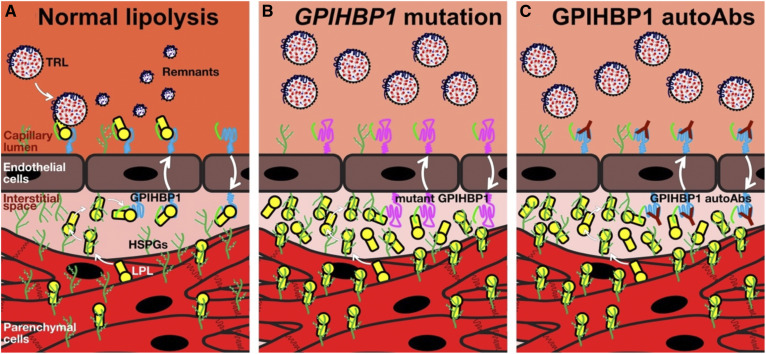
Glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1), a glycosylphosphatidylinositol (GPI)-anchored protein of capillary endothelial cells, is a crucial partner for LPL in plasma triglyceride metabolism (1, 2). GPIHBP1 captures LPL from the subendothelial spaces (where it is secreted by parenchymal cells) and shuttles LPL across endothelial cells to its site of action in the capillary lumen (Fig. 1A) (3). In the absence of GPIHBP1-mediated transport, LPL never reaches the capillary lumen and instead remains trapped within the interstitial spaces, where it is bound to heparan sulfate proteoglycans (HSPGs) (3, 4) (Fig. 1B, C). The LPL that is transported by GPIHBP1 into capillaries is essential for the margination of triglyceride-rich lipoproteins (TRLs) along the surface of capillary endothelial cells, allowing triglyceride hydrolysis to proceed (5). In the absence of a functional GPIHBP1, TRLs do not stop and simply “flow on by” in the bloodstream. The binding of GPIHBP1 also preserves the structural integrity of LPL (6). In the absence of GPIHBP1, the catalytic domain of LPL is inherently unstable and undergoes spontaneous unfolding and loss of catalytic activity (6). The binding of GPIHBP1 blocks this unfolding, even in the setting of physiologic inhibitors of LPL (e.g., ANGPTL4) that function by catalyzing the unfolding of LPL (7). The capacity of GPIHBP1 to stabilize LPL structure and activity focuses active LPL where it needs to be for TRL processing—on the surface of capillary endothelial cells (6–9).
Fig. 1.
Normal intravascular lipolysis (A), lipolysis in the setting of a GPIHBP1 missense mutation in the LU domain (B), or in the presence of GPIHBP1 autoantibodies (autoAbs) (C). Absent GPIHBP1 function, caused either by a GPIHBP1 missense mutation or GPIHBP1 autoAbs, eliminates transport of LPL to the capillary lumen, causing a dramatic reduction in intravascular triglyceride hydrolysis and severe hypertriglyceridemia (chylomicronemia).
GPIHBP1 is a member of the lymphocyte antigen 6/urokinase-type plasminogen activator receptor (LU) family of proteins (10). Mature GPIHBP1 has two functional domains—an N-terminal disordered acidic domain (containing a sulfated tyrosine and multiple aspartates and glutamates) and a three-fingered LU domain containing 10 cysteines, all disulfide linked (10). The LU domain is primarily responsible for the stability of the GPIHBP1–LPL complex, whereas the acidic domain is responsible for enhancing the encounter rate between GPIHBP1 and LPL (6, 8). As judged by surface plasmon resonance studies (8), GPIHBP1’s acidic domain increases the “on-rate” for the binding to LPL by 2,500-fold compared with GPIHBP1’s LU domain alone. From the perspective of plasma triglyceride physiology, GPIHBP1’s acidic domain is likely important for capturing LPL from the subendothelial spaces, detaching it from HSPGs and bringing it into a stable complex with GPIHBP1’s LU domain.
GPIHBP1’s acidic domain is also important for stabilizing LPL structure and activity (6). In the crystal structure for the GPIHBP1–LPL complex [solved recently by Birrane et al. (11)], the concave surface of GPIHBP1’s LU domain covers one side of LPL’s carboxyl-terminal domain, with the binding mediated primarily by hydrophobic contacts. The opposite side of LPL’s carboxyl-terminal domain contains a tryptophan-rich loop that mediates lipid and lipoprotein binding (5, 11, 12). The same tryptophan loop contains the epitope for a widely used LPL-specific monoclonal antibody 5D2 (13, 14). GPIHBP1’s acidic and intrinsically disordered domain was not visualized in the crystal structure (11), but was clearly positioned to extend across a very large basic patch on the surface of LPL (spanning both the N-terminal catalytic domain and the carboxyl-terminal lipid-binding domain) (11). We suspect that electrostatic interactions between GPIHBP1’s acidic domain and LPL’s basic patch form a fuzzy complex, which functions to stabilize LPL and block the propensity of LPL’s catalytic domain to unfold (2, 11).
LPL AND GPIHBP1 MUTATIONS CAUSE THE FAMILIAL CHYLOMICRONEMIA SYNDROME
Soon after LPL was identified and characterized (15, 16), Havel and Gordon (17) reported that a deficiency of LPL was responsible for causing chylomicronemia in three siblings (the first description of an inborn error in lipoprotein metabolism) (17). Since then, numerous LPL mutations have been identified in patients with familial chylomicronemia syndrome (18, 19). Patients with LPL deficiency have lifelong chylomicronemia, which is often uncovered during infancy or childhood (20). Patients with LPL deficiency have a high risk of acute pancreatitis and invariably have low levels of LPL activity in the postheparin plasma (17, 19, 20).
GPIHBP1 deficiency in mice causes severe chylomicronemia (3, 21). For that reason alone, we suspected that, sooner or later, GPIHBP1 mutations would be uncovered as a cause of chylomicronemia in humans. Indeed, over the past 12 years, dozens of GPIHBP1 mutations have been uncovered in patients with familial chylomicronemia syndrome (22–28). GPIHBP1 deficiency causes chylomicronemia by abolishing LPL transport into capillaries (Fig. 1B).
GPIHBP1 deficiency in humans closely resembles LPL deficiency. Patients have severe chylomicronemia (plasma triglyceride levels >1,500 mg/dl), often presenting in childhood, and there is a high risk of pancreatitis (22, 26, 28, 29). An infant with GPIHBP1 deficiency had plasma triglyceride levels >25,000 mg/dl (29), but the reports of extremely high triglyceride levels could reflect selection bias. Several patients with GPIHBP1 deficiency ascertained through family studies had plasma triglyceride levels <1,000 mg/dl (24, 29). Also, an adult female with GPIHBP1 deficiency had a baseline plasma triglyceride level of 234 mg/dl (30) and only manifested severe chylomicronemia during pregnancies. Patients with GPIHBP1 deficiency invariably have low plasma levels of LPL in the pre- and postheparin plasma (22–24, 28), almost certainly reflecting an absence of LPL transport into the intravascular compartment.
The importance of GPIHBP1–LPL interactions has been underscored by human genetics. Missense mutations involving conserved cysteines (or nearby residues) in GPIHBP1’s LU domain cause chylomicronemia by abolishing GPIHBP1’s ability to bind LPL (22–24, 28). In several cases, these mutations have been shown to interfere with the formation of disulfide bonds in the LU domain (24, 31). Also, several LPL missense mutations—all located within the carboxyl-terminal domain—cause disease by abolishing LPL’s ability to bind to GPIHBP1 (11, 32). The recent crystal structure of the GPIHBP1–LPL complex has refined our understanding of how these mutations disrupt GPIHBP1–LPL interactions (11). For example, a p.M404R substitution in LPL abolishes LPL’s ability to bind GPIHBP1 by introducing a charged amino acid (arginine) into the hydrophobic GPIHBP1–LPL binding interface (11). Similarly, mutations in GPIHBP1 Thr-108 (22, 33) or Trp-109 (31) disrupt the GPIHBP1–LPL binding interface (11).
Allan et al. (34) recently generated a Gpihbp1 knock-in mouse harboring a LU domain amino acid substitution (p.C65Y) that had been shown to cause chylomicronemia in humans (23). The mutant GPIHBP1 lacked the ability to bind LPL, and immunohistochemistry studies revealed a complete absence of LPL within the lumen of capillaries (34). All of the LPL in the tissues of knock-in mice was trapped in the interstitial spaces. Identical findings were observed in knockout mice with a deletion of the entire Gpihbp1 gene (3, 21).
CHYLOMICRONEMIA FROM GPIHBP1 AUTOANTIBODIES
In 2017, we reported that some acquired forms of chylomicronemia are caused by GPIHBP1 autoantibodies (GPIHBP1 autoantibody syndrome) (35). GPIHBP1 autoantibodies block GPIHBP1’s ability to bind and transport LPL, resulting in impaired TRL processing and markedly elevated plasma triglyceride levels (Fig. 1C). GPIHBP1 appears to be a long-lived protein (36) and has been shown to move bidirectionally across endothelial cells (37). We presume that the GPIHBP1 autoantibodies remain attached to GPIHBP1 as it moves back and forth across endothelial cells, never giving GPIHBP1 a chance to transport LPL (Fig. 1C).
The discovery of GPIHBP1 autoantibodies occurred serendipitously during the characterization of an ELISA for GPIHBP1. We had characterized a panel of GPIHBP1-specific monoclonal antibodies (38) and used two of the antibodies, RF4 [which binds to a short linker between GPIHBP1’s acidic domain and LU domain (8)] and RE3 (which binds to GPIHBP1’s LU domain), to create a sandwich ELISA for GPIHBP1. The ELISA readily detected GPIHBP1 in normal human plasma but not in the plasma from a patient who was homozygous for a deletion of the GPIHBP1 gene. To further assess the validity of the ELISA, we tested whether it was capable of detecting increased levels of GPIHBP1 in samples of human plasma after they had been spiked with recombinant GPIHBP1. In 38 of the 40 plasma samples, the ELISA reliably detected increased levels of GPIHBP1 (the recovery of the recombinant GPIHBP1 averaged 98.8%). However, in two samples, both from chylomicronemia patients with low plasma levels of GPIHBP1, the recovery of the spiked GPIHBP1 was <10% (Fig. 2). The inability of the ELISA to detect the spiked GPIHBP1 indicated “immunoassay interference,” which can be caused by autoantibodies (39–42). Indeed, the plasma of both chylomicronemia patients contained autoantibodies against GPIHBP1 (35). Follow-up studies quickly identified GPIHBP1 autoantibodies in four additional patients with chylomicronemia (35). The presence of GPIHBP1 autoantibodies in the plasma of these patients was confirmed by ELISAs and Western blots (35). In addition, immunofluorescence microscopy studies revealed that the GPIHBP1 autoantibodies bound avidly to GPIHBP1 on the surface of GPIHBP1-transfected cells (Fig. 3) (35). Importantly, the binding of GPIHBP1 autoantibodies to cell-surface GPIHBP1 abolished the ability of GPIHBP1 to bind LPL (Fig. 4) (35).
Fig. 2.
Immunoassay interference—the first clue to the existence of GPIHBP1 autoantibodies. As part of an effort to validate a monoclonal antibody-based sandwich ELISA designed to measure the levels of GPIHBP1 in human plasma, we tested whether the GPIHBP1 ELISA would faithfully detect higher levels of GPIHBP1 in plasma samples that had been “spiked” with recombinant human GPIHBP1 (35). A total of 62.5 pg of human GPIHBP1 was spiked into plasma samples from 40 human patients (31 from patients with hypertriglyceridemia and 9 from normolipidemic controls). This graph shows the ability of the ELISA to recover the 62.5 pg of GPIHBP1 that was spiked into each plasma sample. In 38 out of the 40 samples, the recovery (±SD) of the spiked recombinant human GPIHBP1 was excellent (98.8 ± 3.8%). However, in plasma samples from two patients (both of whom had severe hypertriglyceridemia), the recovery of the spiked GPIHBP1 was negligible (6.8% and 4.4%). The inability of the ELISA to detect GPIHBP1 in these two patients indicated immunoassay interference, which can be caused by autoantibodies. Subsequent studies showed that the plasma from the two patients contained autoantibodies against GPIHBP1. This figure is reproduced with permission from Beigneux et al. (35).
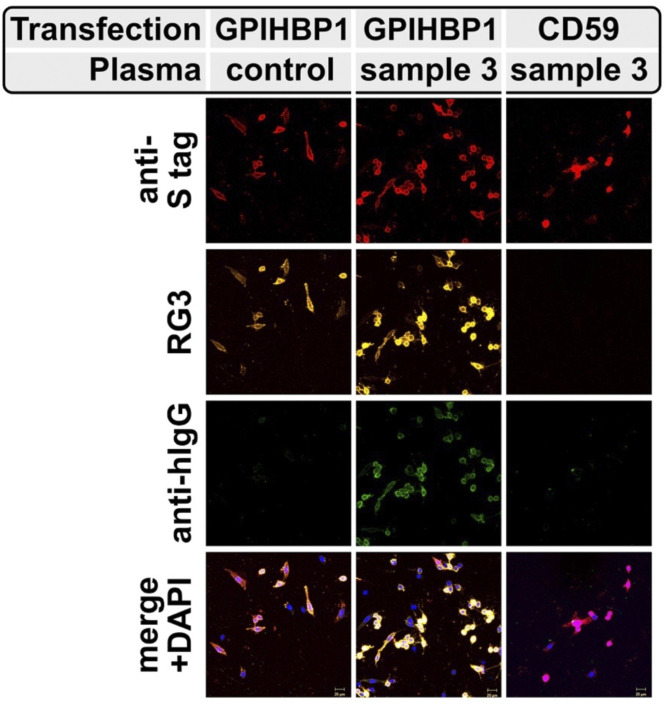
Fig. 3.
Detection, by confocal immunofluorescence microscopy, of GPIHBP1 autoantibodies in the plasma of a patient with chylomicronemia. CHO cells were transfected with expression vectors encoding S-protein tagged versions of human GPIHBP1 or a related LU family member (CD59). After 24 h, the cells were incubated with the plasma from a normolipidemic subject (control) or a patient with chylomicronemia (sample 3 in Table 1). GPIHBP1 and CD59 were both detected with an antibody against the S-protein epitope tag (red); GPIHBP1 was also detected with the human GPIHBP1–specific mAb RG3 (yellow). The presence of GPIHBP1 autoantibodies on the GPIHBP1-transfected cells was detected with an antibody against human IgG (green). DNA was stained with DAPI (blue). This figure is reproduced with permission from Beigneux et al. (35).
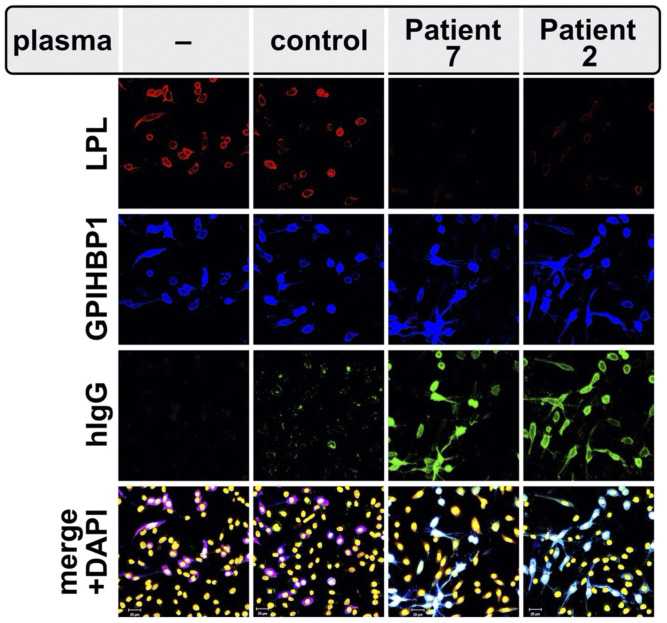
Fig. 4.
GPIHBP1 autoantibodies block the binding of LPL to GPIHBP1 on the surface of GPIHBP1-transfected cells, as judged by confocal fluorescence microscopy. GPIHBP1-transfected cells were preincubated with a plasma sample from a normolipidemic subject (control) or from patients with chylomicronemia from GPIHBP1 autoantibodies (patients 2 and 7 in Table 1) and subsequently incubated with the conditioned media from human LPL–transfected cells. GPIHBP1 autoantibodies (green) in plasma samples from patients 2 and 7 bound avidly to the GPIHBP1 (blue) on the surface of GPIHBP1-transfected cell. The binding of autoantibodies prevented the binding of LPL (red) to GPIHBP1. DNA was stained with DAPI (yellow). This figure is reproduced with permission from Beigneux et al. (35).
In the initial description of the GPIHBP1 autoantibody syndrome (35), three of the six chylomicronemia patients with GPIHBP1 autoantibodies had a history of acute pancreatitis, and three had clinical or serological evidence of autoimmune disease. Interestingly, one of the patients, who had systemic lupus erythematosus (SLE), became pregnant and delivered an infant girl. The infant’s blood contained GPIHBP1 autoantibodies, but at only ∼5% of the level found in the mother’s blood (35). Despite the relatively low levels of autoantibodies, the infant’s plasma triglyceride level on the first day of life was 9,090 mg/dl. Over the next month, the infant’s triglyceride levels gradually returned to normal (35), consistent with the disappearance of maternal immunoglobulins from the infant’s bloodstream.
Patients with GPIHBP1 autoantibodies have low plasma levels of LPL, almost certainly reflecting markedly impaired transport of LPL into capillaries. Thus, chylomicronemia can be caused by both GPIHBP1 deficiency and GPIHBP1 autoantibodies. Dual mechanisms for disease are also observed in thrombotic thrombocytopenic purpura [caused by ADAMTS13 mutations and ADAMTS13 autoantibodies (43, 44)].
We are aware of only one other example of autoantibodies against a GPI-anchored protein (the folate receptor). High-affinity blocking autoantibodies against the folate receptor have been detected in human plasma and have been linked to risk for neural tube defects during development as well as neuropsychiatric disease (45–50). The folate receptor is not a member of the LU protein family but like GPIHBP1 is a cysteine-rich protein with multiple disulfide bonds. In other human diseases caused by autoantibodies (e.g., thrombotic thrombocytopenic purpura, Graves’ disease, membranous nephropathy), the autoantibodies bind to regions of proteins with multiple disulfide bonds (51–54).
ADDITIONAL CASES OF THE GPIHBP1 AUTOANTIBODY SYNDROME
Since the initial report of GPIHBP1 autoantibodies (35), we have documented 22 cases of chylomicronemia caused by GPIHBP1 autoantibodies. Selected clinical findings and clinical chemistry results are summarized in Tables 1 and 2. Eleven of the 22 patients had a history of acute pancreatitis. Several had preexisting autoimmune diseases (e.g., rheumatoid arthritis, Sjögren’s syndrome, Hashimoto’s disease, SLE, anti-phospholipid syndrome, hemolytic anemia, Graves’ disease), and most tested positive for antinuclear antibodies (Table 1). However, GPIHBP1 autoantibodies were the sole manifestation of autoimmune disease in three patients (patients 2, 3, and 12 in Table 1). Levels of GPIHBP1 autoantibodies in the 22 patients varied considerably, and there was no indication that lower levels of GPIHBP1 autoantibodies were associated with lower plasma triglyceride levels. Severe chylomicronemia was observed in all of the patients, including several with low plasma levels of GPIHBP1 autoantibodies (e.g., patients 5 and 19). The plasma levels of LPL were low in all patients with GPIHBP1 autoantibodies (Table 1), consistent with absent LPL transport into capillaries. Plasma levels of endothelial lipase and hepatic triglyceride lipase (lipase family members that do not bind to GPIHBP1) were within the normal range in the majority of patients (Table 2).
TABLE 1.
Biochemical data obtained on archived plasma samples from 22 patients with the GPIHBP1 autoantibody syndrome, along with the available clinical data on the patients
| ID Number | TG (mg/dl) | LPL (ng/ml) | GPIHBP1 (pg/ml) | GPIHBP1 AutoAbs (U/ml) | Gender | Age (years) | ANA | Clinical Features | Response to Rituximab | References |
| 1 | 2,407 | 17 | 156 | 2,612 | F | 26 | + | AP, SLE | Yes | (35) (patient 157) |
| 2 | 1,389 | 45 | 0 | 1,328 | F | 20 | – | AP | (35) (patient 111) | |
| 3 | 2,660 | 11 | 179 | 2,667 | M | 38 | – | AP; diabetes from chronic pancreatitis | (35) (patient 38) | |
| 4 | 1,720 | 5 | 13 | 1,129 | F | 53 | +++ | AP, RA, SS, HD, SLE | (35) (patient 101) | |
| 5 | 2,188 | 26 | 90 | 556 | M | 36 | + | AP | (56) | |
| 6 | 2,265 | 4 | 16 | 2,476 | F | 34 | + | MS | (74) | |
| 7 | 6,500 | 6 | 0 | 29,224 | F | 38 | +++ | SLE, HA | (35) (patient 102) | |
| 8 | 3,277 | 7 | 6 | 29,793 | F | 3 | – | HA | ||
| 9 | 7,971 | 55 | 34 | 3,224 | F | 23 | + | |||
| 10 | 4,740 | 14 | 130,227 | 1,975 | M | 50 | + | HA | ||
| 11 | 5,960 | 9 | 6,660 | 763 | F | 27 | + | AP, APS, GD | Yes | (76) |
| 12 | 1,807 | 13 | 25,622 | 794 | F | 11 | – | AP | ||
| 13 | 7,865 | 14 | 40 | 2,032 | F | 17 | – | AP, suspected HA | Yes | (75) |
| 14 | 1,486 | 12 | 0 | 17,236 | F | 28 | + | |||
| 15 | 1,140 | 24 | 2 | 2,910 | F | 15 | + | SLE, SLE nephritis, APS | Yes | |
| 16 | 1,580 | 15 | 5,074 | 303 | M | 11 | + | HD | ||
| 17 | 2,600 | 7 | 11 | 3,250 | F | 15 | + | AP | ||
| 18 | 2,380 | 6 | 0 | 3,387 | F | 35 | + | HD | ||
| 19 | 1,800 | 9 | 0 | 237 | F | 29 | – | HD | ||
| 20 | 2,591 | 12 | 0 | 3,242 | F | 14 | ++ | AP, SLE | ||
| 21 | 2,120 | 12 | 18 | 2,813 | M | 13 | + | |||
| 22 | 4,440 | 8 | 21,938 | 2,313 | F | 14 | ++ | AP, suspected SLE/RA | Yes | |
| N.R. | 90–150 | 70–140 | 550–1,528 | < 58 | – | (74, 82) |
The normal range (N.R.) for each parameter is provided in the bottom row of this table. ID, identification; AutoAbs, autoantibodies; ANA, antinuclear antibodies; RA, rheumatoid arthritis; SS, Sjögren’s syndrome; HD, Hashimoto disease; MS, multiple sclerosis; APS, anti-phospholipid syndrome; AP, acute pancreatitis; HA, hemolytic anemia; GD, Graves’ disease. F, female; M, male.
TABLE 2.
Lipase levels and immunoglobulin levels in patients with the GPIHBP1 autoantibody syndrome
| Patient Number | EL (ng/ml) | HTGL (ng/ml) | IgA | IgM | IgG1 | IgG2 | IgG3 | IgG4 |
| 1 | 101 | 12 | 2 | 1 | 1.5 | 1.5 | 1.5 | 3 |
| 2 | 196 | 42 | 1.5 | 0 | 1 | 0 | 0 | 3 |
| 3 | 123 | 48 | 2 | 0 | 2 | 0 | 0 | 2 |
| 4 | 23 | 52 | 1.5 | 0 | 1.5 | 0 | 0 | 0 |
| 5 | NA | NA | 1.5 | 0 | 1.5 | 0 | 1 | 1.5 |
| 6 | 54 | 272 | 2 | 0 | 1.5 | 0 | 0 | 4 |
| 7 | 215 | 81 | 3 | 0 | 3 | 3 | 1.5 | 5 |
| 8 | 129 | 19 | 4 | 1 | 5 | 1 | 5 | 5 |
| 9 | 83 | 286 | 2 | 1.5 | 1.5 | 1 | 1.5 | 5 |
| 10 | 152 | 79 | 2 | 1 | 0 | 1 | 0 | 0 |
| 11 | 73 | 7 | 2 | 0 | 1.5 | 0 | 1.5 | 1 |
| 12 | 70 | 15 | 1.5 | 1 | 0 | 0 | 0 | 3 |
| 13 | 237 | 40 | 1.5 | 0 | 1.5 | 0 | 1 | 3 |
| 14 | 75 | 22 | 3 | 1 | 3 | 1.5 | 1.5 | 5 |
| 15 | 287 | 53 | 2 | 1 | 1.5 | 0 | 2 | 3 |
| 16 | 226 | 67 | 1 | 0 | 1 | 0 | 0 | 0 |
| 17 | 70 | 32 | 2 | 0 | 1 | 0 | 0 | 4 |
| 18 | 107 | 25 | 1.5 | 0 | 2 | 0 | 1.5 | 1.5 |
| 19 | 121 | 64 | 1 | 0 | 0 | 0 | 0 | 2 |
| 20 | 94 | 21 | 3 | 1 | 2 | 1 | 0 | 5 |
| 21 | 142 | 38 | 1.5 | 0 | 1 | 0 | 0 | 3 |
| 22 | 107 | 11 | 1.5 | 0 | 1 | 0 | 0 | 2 |
| Normal range | 43–136 | 36–116 | 0 | 0 | 0 | 0 | 0 | 0 |
Levels of GPIHBP1 autoantibodies in different immunoglobulin classes were scored as grades 1–5, based on the optical density in the ELISA (see Fig. 7). EL, endothelial lipase; HTGL, hepatic triglyceride lipase; NA, not available.
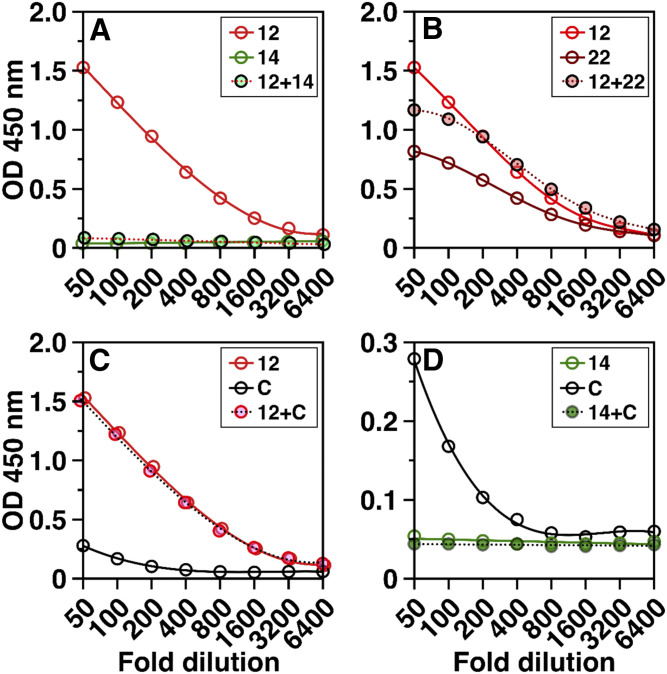
Seventeen of 22 GPIHBP1 autoantibody patients (patients 1–9, 13–15, 17–21) had very low plasma levels of GPIHBP1, as judged by a monoclonal antibody–based ELISA (35, 38, 55). Finding low plasma GPIHBP1 levels did not come as a surprise, given earlier studies demonstrating that GPIHBP1 autoantibodies were capable of interfering with the ELISA (35). To our surprise, five of the 22 patients (patients 10, 11, 12, 16, and 22) had high plasma GPIHBP1 levels (Table 1). We suspected that the autoantibodies in the latter group of patients were functionally distinct, having little or no ability to interfere with the GPIHBP1 ELISA. Mixing studies confirmed this suspicion (Fig. 5). When we mixed a plasma sample from a GPIHBP1 autoantibody patient with high plasma GPIHBP1 levels (e.g., patient 12) with a plasma sample from a GPIHBP1 autoantibody patient with low plasma GPIHBP1 levels (e.g., patient 14), detection of GPIHBP1 by the ELISA was abolished (Fig. 5A). In contrast, when we mixed plasma samples from autoantibody patients with high GPIHBP1 levels (patients 12 and 22), the ability of the ELISA to detect GPIHBP1 was preserved (Fig. 5B). Mixing plasma from a patient with high plasma GPIHBP1 levels with the plasma from a control subject (sample C) did not interfere with detection of GPIHBP1 (Fig. 5C). However, mixing plasma from an autoantibody patient with low GPIHBP1 levels (patient 14) with plasma from the control subject eliminated detection of GPIHBP1 by the ELISA (Fig. 5D).
Fig. 5.
GPIHBP1 autoantibodies from different patients differ in their capacity to interfere with the detection of GPIHBP1 by a monoclonal antibody–based ELISA. Here, we show the ability of the ELISA to detect GPIHBP1 in plasma samples from three different patients with the GPIHBP1 autoantibody syndrome (patients 12, 14, and 22 in Table 1) and a normolipidemic control “C”. The plasma of patient 14 had undetectable levels of GPIHBP1, reflecting the presence of GPIHBP1 autoantibodies that interfered with the ELISA. Patients 12 and 22 had high levels of GPIHBP1, indicating that their autoantibodies against GPIHBP1 had little capacity to interfere with the ELISA. A: The high GPIHBP1 levels in the plasma of patient 12 were no longer detectable after mixing the plasma of patient 12 with the plasma from patient 14. B: Mixing plasma samples from patients 12 and 22 did not interfere with detection of GPIHBP1. C: Mixing the plasma from patient 12 with a normolipidemic control (sample C) did not alter the ability of the ELISA to detect GPIHBP1. D: Mixing plasma from patient 14 with the plasma from the control subject (subject C) abolished detection of GPIHBP1 by the ELISA. Results show the optical density (OD) in the GPIHBP1 ELISA at each sample dilution (1:50 to 1:6,400).
We suspect (but have not proven) that the high plasma levels of GPIHBP1 in patients 10, 11, 12, 16, and 22 reflect high levels of immune complexes in the plasma (i.e., GPIHBP1 bound to GPIHBP1 autoantibodies). It is also possible that high levels of immune complexes also exist in patients with low plasma levels of GPIHBP1 but are simply not detected, owing to immunoassay interference.
PROPERTIES OF GPIHBP1 AUTOANTIBODIES
GPIHBP1 autoantibodies, purified from the plasma of two patients, bound with high affinity to GPIHBP1’s LU domain and had a very slow “off rate,” as judged by surface plasmon resonance experiments (8). In contrast, there was no binding of GPIHBP1 autoantibodies to a synthetic peptide spanning GPIHBP1’s acidic domain (8). Autoantibodies against GPIHBP1 are specific; they do not bind to structurally related proteins in the LU protein family (35, 56).
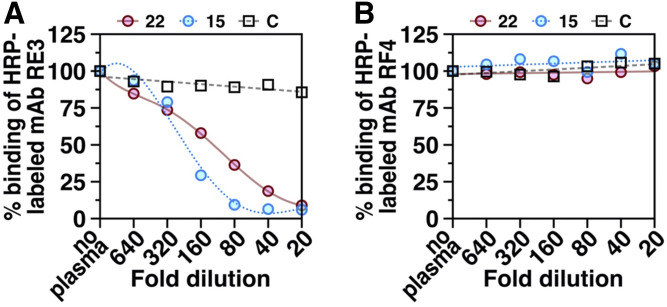
We suspected that GPIHBP1 autoantibodies block LPL binding by binding to the surface of GPIHBP1’s LU domain that interfaces with LPL. In earlier studies, we had identified several monoclonal antibodies against GPIHBP1’s LU domain, including antibody RE3, that also block the binding of LPL (38). We suspected that the epitope for those “blocking monoclonal antibodies” is identical to—or overlaps with—the epitopes of GPIHBP1 autoantibodies that block LPL binding. Consistent with that idea, the plasma from GPIHBP1 autoantibody patients potently blocks monoclonal antibody RE3 binding to recombinant GPIHBP1 (Fig. 6A). In contrast, the same plasma samples had no effect on the binding of monoclonal antibody RF4 to GPIHBP1 (Fig. 6B). The epitope for RF4 is located in a linker region between GPIHBP1’s acidic domain and LU domain (distant from the GPIHBP1–LPL interface) (8).
Fig. 6.
ELISAs testing the ability of dilutions of plasma from a control subject “C” and two GPIHBP1 autoantibody patients (patients 15 and 22 in Table 1) to compete with two different HRP-labeled GPIHBP1-specific monoclonal antibodies (mAb RE3, mAb RF4) for binding to recombinant human GPIHBP1. RE3 binds to GPIHBP1’s LU domain and abolishes GPIHBP1’s ability to bind LPL (38). RF4 binds to an epitope located between GPIHBP1’s acidic domain and LU domain (8). A: Plasma samples from the two GPIHBP1 autoantibody patients, but not the control plasma, inhibited the binding of mAb RE3 to GPIHBP1. B: Neither the control plasma nor the plasma samples from the two GPIHBP1 autoantibody patients inhibited the binding of mAb RF4 to recombinant GPIHBP1. Plasma samples were tested over a range of dilutions (1:20 to 1:640).
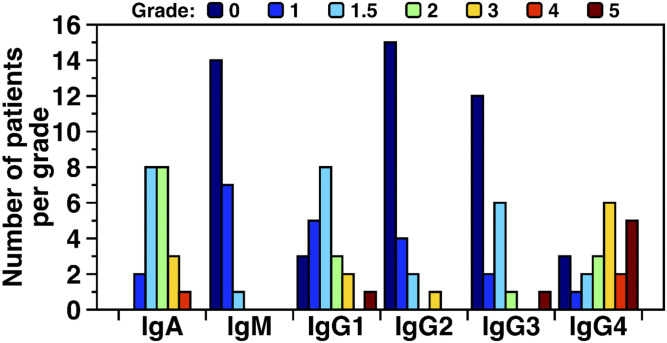
In most patients, IgA class and IgG4 subclass autoantibodies predominated. All 22 GPIHBP1 patients had IgA class GPIHBP1 autoantibodies, with 16 of 22 having an IgA titer grade of 1.5–2 (Table 2). Nineteen of 22 patients had IgG4 GPIHBP1 autoantibodies; 13 had relatively high titer grades (3 or higher); five had a titer grade of 5 (Table 2). IgG1 subclass GPIHBP1 autoantibodies were detectable in 19 patients, with 13 having a relatively low titer grade of 1–1.5 and only one having a titer grade of 5. IgG2 GPIHBP1 autoantibodies were detected in 7 patients, and the titer grade was generally low (grade 1–1.5). IgG3 GPIHBP1 autoantibodies were detected in 10 patients, and all but one had a low titer grade of 1–2. IgM GPIHBP1 autoantibodies were detectable in 8 of 22 samples, but the titer grade was low (grade 1–1.5) (Table 2). Figure 7 depicts the frequency of GPIHBP1 autoantibodies in different immunoglobulin classes, organized according to titer grade, revealing that IgA and IgG4 GPIHBP1 autoantibodies were predominant and tended to have the highest titer grade.
Fig. 7.
IgA and IgG4 autoantibodies are predominant in the GPIHBP1 autoantibody syndrome. Shown here are numbers of GPIHBP1 autoantibody syndrome patients having IgA, IgM, IgG1, IgG2, IgG3, and IgG4 autoantibodies, organized according to “titer grade.” The titer grade for each immunoglobulin class and subclass was assessed for 22 patients with the GPIHBP1 autoantibody syndrome. The titer was assessed by ELISAs in which 1:1,000 dilutions of plasma samples were loaded onto wells coated with recombinant GPIHBP1. After washing the plates, HRP-labeled immunoglobulin class- and subclass-specific antibodies were added to the plates. The binding of those antibodies was graded according to the optical density (OD) in the ELISA. Grades ranged from below detection (grade 0, dark blue bars) to very high levels (grade 5, brown bars). Grade 0, OD < 0.1; grade 1, OD 0.1–0.25; grade 1.5, OD 0. 25–0.5; grade 2, OD 0.5–1.0; grade 3, OD 1.0–2.0; grade 4, OD 2.0–3.0; grade 5, OD > 3.0.
IgA and IgG4 autoantibodies are known to cause human disease. IgA autoantibodies have been observed in SLE (57–60), pemphigus vulgaris (61, 62), and celiac disease (63). IgG4 autoantibodies have been documented in pemphigus vulgaris (61), membranous nephropathy (64), and thrombotic thrombocytopenic purpura (65). IgG4 antibodies are functionally monovalent and do not mediate complement fixation; they are thought to cause disease by disrupting protein–protein interactions rather than by eliciting tissue injury (61, 65–70). Disruption of protein–protein interactions seems consistent with the pathophysiology of the GPIHBP1 autoantibody syndrome, but the possibility that GPIHBP1 autoantibodies triggers tissue injury has not been excluded. No one has examined tissue biopsies from patients with the GPIHBP1 autoantibody syndrome; thus, we do not know whether GPIHBP1 autoantibodies might, at least in some patients, trigger inflammation and endothelial cell injury.
WHEN TO CONSIDER A DIAGNOSIS OF THE GPIHBP1 AUTOANTIBODY SYNDROME
Only a few percent of patients with chylomicronemia (plasma triglycerides >1,000 mg/dl) have a monogenic disorder (e.g., a deficiency of LPL, GPIHBP1, APOC2, LMF1). In the overwhelming majority of cases, the cause of chylomicronemia is multifactorial, influenced by genetic variation in dozens of different genes, diet, alcohol intake, metabolic disease, and prescription drugs (e.g., β-blockers, thiazide diuretics, estrogens, L-asparaginase) (71–73). Undiagnosed or uncontrolled type 2 diabetes and prescription drugs are probably the most common precipitating cause. In a few patients, the hyperlipidemia is responsive to a targeted intervention (e.g., treating diabetes, stopping a drug), but in many cases the cause remains murky, frustrating the patient and physician alike.
GPIHBP1 autoantibodies cause some cases of newly acquired chylomicronemia. Our patients with GPIHBP1 autoantibody syndrome were identified in very small-scale screens and by testing plasma samples mailed to us by clinicians (35, 56, 74–76). The frequency of the GPIHBP1 autoantibodies among patients with chylomicronemia is unknown. We doubt that GPIHBP1 autoantibodies are a common cause of chylomicronemia, but at the same time we suspect that our cases represent the tip of the iceberg. Many clinicians are unaware of the GPIHBP1 autoantibody syndrome. In a recent review of chylomicronemia, authored by experienced clinicians, GPIHBP1 autoantibodies were never considered in the differential diagnosis of chylomicronemia (72).
In patients with acquired chylomicronemia, several clinical signs should prompt the possibility of the GPIHBP1 syndrome. Certainly, it should be considered in any patient positive for ANA antibodies or with an autoimmune disease. Other signs are the abrupt onset of chylomicronemia in the absence of a secondary cause and poor response to diet and lipid-lowering drug therapy. GPIHBP1 autoantibodies should also be considered if chylomicronemia appears during IFN β1α therapy (74). INF β1α has been reported to exacerbate autoimmune disease (77–79). Eguchi et al. (74) recently described a multiple sclerosis patient who developed GPIHBP1 autoantibodies and chylomicronemia during INF β1α treatment. When the INF β1α therapy was stopped, the triglyceride levels normalized; the GPIHBP1 autoantibodies disappeared; and the serum LPL levels increased.
Intermittent episodes of chylomicronemia are perfectly consistent with the GPIHBP1 autoantibody syndrome. GPIHBP1 autoantibodies can disappear spontaneously, with normalization of plasma triglyceride and LPL levels, only to reappear months later in the setting of another episode of chylomicronemia (75). Intermittent disease is not particularly surprising. Autoantibodies against the insulin receptor occasionally disappear, resulting in normalization of glucose levels and insulin sensitivity (80). Also, autoantibodies against the M-type phospholipase A2 receptor occasionally disappear in patients with membranous glomerulopathy, coinciding with remission of disease (81).
ESTABLISHING A DIAGNOSIS OF THE GPIHBP1 AUTOANTIBODY SYNDROME
A diagnosis of the GPIHBP1 antibody syndrome in patients with hypertriglyceridemia fundamentally depends on documenting GPIHBP1 autoantibodies in serum or plasma. GPIHBP1 autoantibodies can be identified quite easily with an ELISA (35, 82). Dilutions of serum or plasma are incubated on 96-well plates coated with recombinant GPIHBP1. After washing the plate, immunoglobulin binding to GPIHBP1 is detected with a peroxidase-labeled anti–human IgG. Currently, there are no FDA-approved tests for GPIHBP1 autoantibodies, but an ELISA for GPIHBP1 autoantibodies is available from Immuno-Biological Laboratories (Fujioka, Japan). Testing the immunoglobulin class and subclass of GPIHBP1 autoantibodies is not essential.
Measuring plasma LPL levels are not absolutely required but documenting very low levels of LPL helps to solidify the diagnosis. Protocols for measuring LPL have been described in detail (83), and ELISA kits to measure human LPL levels are commercially available (e.g., CellBiolabs, Cusabio).
Monoclonal antibody–based ELISAs to measure plasma GPIHBP1 levels have been described in detail (35, 38, 55), and an ELISA kit to measure GPIHBP1 is available from Immuno-Biological Laboratories. Measuring GPIHBP1 levels is not essential. As noted earlier, patients with GPIHBP1 autoantibodies can have either extremely low or very high levels of GPIHBP1, depending on whether the GPIHBP1 autoantibodies interfere with detection of GPIHBP1 in the ELISA.
TREATING PATIENTS WITH GPIHBP1 AUTOANTIBODIES
In the initial report on GPIHBP1 autoantibodies (35), two patients appeared to respond to immunosuppressive drug therapy. In one patient (patient 1 in Table 1), plasma triglyceride levels normalized after infusions of rituximab, a CD20-specific monoclonal antibody that is commonly used to treat other autoimmune diseases (e.g., pemphigus vulgaris, thrombotic thrombocytopenic purpura, SLE) (84–86). In another patient, patient number 164 in the initial report (35), triglycerides normalized after treatment with mycophenolate mofetil, an immunosuppressive agent commonly used to prevent rejection of organ allografts and to treat several autoimmune diseases, including SLE (35). However, in these cases, there was no experimental evidence proving that the remission of disease coincided with disappearance of GPIHBP1 autoantibodies.
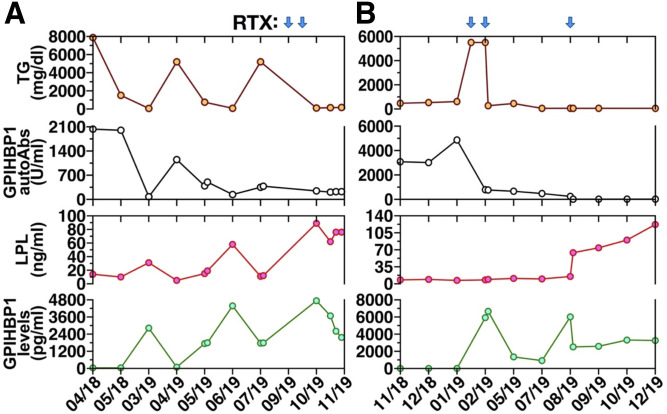
Two recent cases have provided convincing evidence that rituximab is effective for treating chylomicronemia caused by GPIHBP1 autoantibodies (75, 76). The first case involved a 15-year-old girl (patient 13 in Table 1) who presented with chylomicronemia (plasma triglycerides 3,539 mg/dl) complicated by acute pancreatitis (75). GPIHBP1 autoantibodies were easily detectable, and the plasma levels of LPL were low. The patient was treated with mycophenolate mofetil and prednisolone, with normalization of both plasma triglycerides and LPL levels, but compliance with this regimen was poor. After several months, GPIHBP1 autoantibodies reappeared; the plasma triglyceride levels increased to >5,200 mg/dl; and plasma LPL fell to extremely low levels. The patient was then given infusions of rituximab. Within several months, the triglyceride levels normalized (105 mg/dl); the GPIHBP1 autoantibodies disappeared; and the plasma LPL levels returned to normal (Fig. 8A).
Fig. 8.
Plasma triglyceride levels (TG, brown circles), GPIHBP1 autoantibodies (autoAbs, black circles), LPL mass (red circles), and GPIHBP1 mass levels (green circles) in two patients with the GPIHBP1 autoantibody syndrome. Values are shown both before and after treatment with rituximab (RTX); blue arrows indicate timing of RTX infusions. A: TG, GPIHBP1 autoantibodies, LPL mass, and GPIHBP1 mass measurements in a GPIHBP1 autoantibody patient (patient 13 in Table 1) described by Ashrafi et al. (75). Panel A modified, with permission, from the publication by Ashraf et al. (75). B: TG, GPIHBP1 autoantibodies, LPL mass, and GPIHBP1 mass measurements in a GPIHBP1 autoantibody patient (patient 11 in Table 1) described by Lutz et al. (76).
In the second case, a 27-year-old woman with a history of antiphospholipid syndrome and Graves’ disease (patient 11 in Table 1) developed severe chylomicronemia (plasma triglycerides 5,960 mg/dl). Her serum contained GPIHBP1 autoantibodies; the plasma LPL levels were extremely low, and the plasma levels of GPIHBP1 were undetectable. Treatment with fenofibrate, ezetimibe, prednisone, plasma separations, lipid aphereses, and immunoabsorptions were either ineffective or yielded transient decreases in plasma triglyceride levels. Because of persistent chylomicronemia (triglyceride levels >5,500 mg/dl), the patient was given three infusions of rituximab. After several months, the serum triglyceride levels normalized; GPIHBP1 autoantibodies disappeared; and the plasma levels of LPL and GPIHBP1 normalized (Fig. 8B).
Recently, in two other patients with chylomicronemia and GPIHBP1 autoantibodies (patients 15 and 22 in Table 1), plasma triglyceride levels normalized after treatment with rituximab. In these cases, GPIHBP1 autoantibodies were not tested after rituximab treatment, but given the normalization of triglyceride levels it is likely that the autoantibodies disappeared.
REPORTS OF CHYLOMICRONEMIA FROM LPL AUTOANTIBODIES
Several groups have reported cases of chylomicronemia from LPL autoantibodies (87–97). The evidence for LPL autoantibodies has included the ability of the patient’s serum to block LPL catalytic activity, the ability of the immunoglobulins in the serum to bind to LPL on ELISA plates, the ability of immunoglobulins in the serum to bind (in Western blots) to a protein in postheparin plasma with a molecular mass similar to LPL (∼52 kDa), or the ability of immunoglobulins in the serum to bind to LPL purchased from a commercial source. None of the cases reported in the literature have been particularly convincing, for a variety of reasons. Immunoglobulins can bind nonspecifically to many proteins, including proteins with a molecular mass similar to that of LPL (35, 56). In some reports, the fidelity of the reagents was unclear. Commercial sources of LPL can be contaminated with antithrombin III (98), a heparin-binding protein with a molecular mass similar to that of LPL. Polyclonal LPL antibodies used as a control are also known to contain antibodies that bind antithrombin III (98). Chylomicronemia from LPL autoantibodies is an intriguing idea, but at this point more evidence is needed.
At the University of California Los Angeles, we have tested plasma samples from 11 chylomicronemia patients for LPL autoantibodies, including samples from two patients in whom the existence of LPL autoantibodies had been reported or strongly suspected (35). None of the 11 samples that we tested had LPL autoantibodies. The two samples reported to have LPL autoantibodies (35) did not have LPL autoantibodies but did have GPIHBP1 autoantibodies. A third patient reported to have LPL autoantibodies had neither LPL nor GPIHBP1 autoantibodies.
Documenting the existence of an “LPL autoantibody syndrome” would require rigorous and well controlled tests, including Western blots, immunocytochemistry experiments, ELISAs, and LPL activity assays. To test for LPL autoantibodies with Western blots, we recommend preparing cell extracts and medium samples from CHO cells that have been transfected with an expression vector for untagged human LPL, LPL containing an epitope tag, or empty vector. After SDS-PAGE and transfer of the size-fractionated proteins to nitrocellulose, Western blots should be performed with a monoclonal antibody against LPL (e.g., 5D2), an antibody against the epitope tag, and serum samples from normal controls as well as the patient suspected of having LPL autoantibodies. Monoclonal antibody 5D2 should bind avidly to both untagged LPL (52 kDa) and the epitope-tagged LPL (54 kDa), but not to proteins in the CHO cells transfected with empty vector. The epitope tag antibody should bind exclusively to the 54 kDa epitope-tagged LPL. If a patient truly has LPL autoantibodies, then the immunoglobulins in the serum should bind to both tagged and untagged human LPL. Testing normal human plasma samples and testing the fidelity of secondary antibodies is essential. In addition to Western blot studies, it is essential to show that putative LPL autoantibodies bind to LPL in LPL-transfected CHO cells and bind to a recombinant tagged LPL captured on ELISA wells coated with a monoclonal antibody against the epitope tag. Procedures for these types of experiments have been outlined by Beigneux et al. (35, 99).
Making the diagnosis of an LPL autoantibody syndrome also requires demonstrating that the autoantibodies inhibit LPL catalytic activity. For these studies, we recommend harvesting medium from CHO cells that have been transfected with a human LPL expression vector. LPL activity in the conditioned medium can be verified with a [3H]triolein substrate (99). With this sort of assay, one can test whether the autoantibodies in serum or plasma inhibit LPL catalytic activity. As a control, it is important to show that LPL triglyceride hydrolase activity is blocked by the LPL-specific monoclonal antibody 5D2. We caution against inferring the presence of “LPL autoantibodies” based on the ability of a patient’s serum sample to reduce the LPL activity present in a sample of postheparin plasma.
SUMMARY
We have summarized findings in 22 chylomicronemia patients with GPIHBP1 autoantibodies. GPIHBP1 autoantibodies block the ability of GPIHBP1 to bind LPL and transport LPL into the lumen of capillaries. Patients with the GPIHBP1 autoantibody syndrome have very low plasma levels of LPL, consistent with reduced transport of LPL into capillaries. Many patients with GPIHBP1 autoantibodies had clinical or serologic evidence of autoimmune disease, and many have had one or more bouts of acute pancreatitis. In a few patients, GPIHBP1 autoantibodies were the only manifestation of autoimmune disease. Recent studies have revealed that the GPIHBP1 autoantibody syndrome can be treated with rituximab, resulting in disappearance of the autoantibodies and normalization of both plasma triglyceride and LPL levels. The GPIHBP1 autoantibody syndrome should be considered in unexplained cases of chylomicronemia.
Footnotes
Author ORCIDs—Michael Ploug https://orcid.org/0000-0003-2215-4265; Anne P. Beigneux https://orcid.org/0000-0002-7892-150X
Funding and additional information—This work was supported by National Heart, Lung, and Blood Institute Grants HL090553, HL087228, and HL125335; Fondation Leducq Transatlantic Network Grant 12CVD04; and Novo Nordisk Foundation Grant NNF17OC0026868. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—K,N. holds stock in Immuno-Biological Laboratories (IBL) and serves as a consultant for Skylight and Sysmex. All other authors declare that they have no conflicts of interest with the contents of this article.
Abbreviations—
- GPI
- glycosylphosphatidylinositol
- GPIHBP1
- glycosylphosphatidylinositol-anchored HDL binding protein 1
- HSPG
- heparan sulfate proteoglycan
- LU
- lymphocyte antigen 6/urokinase-type plasminogen activator receptor
- SLE
- systemic lupus erythematosus
- TRL
- triglyceride-rich lipoprotein
Manuscript received September 18, 2020. Published, JLR Papers in Press, September 18, 2020, DOI 10.1194/jlr.R120001116.
REFERENCES
- 1.Fong L. G., Young S. G., Beigneux A. P., Bensadoun A., Oberer M., Jiang H., and Ploug M.. 2016. GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol. Metab. 27: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young S. G., Fong L. G., Beigneux A. P., Allan C. M., He C., Jiang H., Nakajima K., Meiyappan M., Birrane G., and Ploug M.. 2019. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 30: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies B. S. J., Beigneux A. P., Barnes R. H. II, Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I. J., Olivecrona G., et al. 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allan C. M., Larsson M., Jung R. S., Ploug M., Bensadoun A., Beigneux A. P., Fong L. G., and Young S. G.. 2017. Mobility of “HSPG-bound” LPL explains how LPL is able to reach GPIHBP1 on capillaries. J. Lipid Res. 58: 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goulbourne C. N., Gin P., Tatar A., Nobumori C., Hoenger A., Jiang H., Grovenor C. R., Adeyo O., Esko J. D., Goldberg I. J., et al. 2014. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 19: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mysling S., Kristensen K. K., Larsson M., Beigneux A. P., Gardsvoll H., Fong L. G., Bensadouen A., Jorgensen T. J., Young S. G., and Ploug M.. 2016. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 5: e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mysling S., Kristensen K. K., Larsson M., Kovrov O., Bensadouen A., Jorgensen T. J., Olivecrona G., Young S. G., and Ploug M.. 2016. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife. 5: e20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristensen K. K., Midtgaard S. R., Mysling S., Kovrov O., Hansen L. B., Skar-Gislinge N., Beigneux A. P., Kragelund B. B., Olivecrona G., Young S. G., et al. 2018. A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 115: E6020–E6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristensen K. K., Leth-Espensen K. Z., Young S. G., and Ploug M.. 2020. ANGPTL4 inactivates lipoprotein lipase by catalyzing the irreversible unfolding of LPL’s hydrolase domain. J. Lipid Res. 61: 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leth J. M., Leth-Espensen K. Z., Kristensen K. K., Kumari A., Lund Winther A. M., Young S. G., and Ploug M.. 2019. Evolution and medical significance of LU domain-containing proteins. Int. J. Mol. Sci. 20: 2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birrane G., Beigneux A. P., Dwyer B., Strack-Logue B., Kristensen K. K., Francone O. L., Fong L. G., Mertens H. D. T., Pan C. Q., Ploug M., et al. 2019. Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci. USA. 116: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams S. E., Inoue I., Tran H., Fry G. L., Pladet M. W., Iverius P-H., Lalouel J-M., Chappell D. A., and Strickland D. K.. 1994. The carboxyl-terminal domain of lipoprotein lipase binds to the low density lipoprotein receptor-related protein/a2-macroglobulin receptor (LRP) and mediates binding of normal very low density lipoproteins to LRP. J. Biol. Chem. 269: 8653–8658. [PubMed] [Google Scholar]
- 13.Chang S-F., Reich B., Brunzell J. D., and Will H.. 1998. Detailed characterization of the binding site of the lipoprotein lipase-specific monoclonal antibody 5D2. J. Lipid Res. 39: 2350–2359. [PubMed] [Google Scholar]
- 14.Luz J., Beigneux A. P., Asamoto D. K., He C., Song W., Allan C. M., Morales J. E., Tu Y., Kwok A., Cottle T., et al. The structural basis for monoclonal antibody 5D2 binding to the tryptophan-rich loop of lipoprotein lipase. J. Lipid Res. 61: 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn E. D. 1955. Clearing factor, a heparin-activated lipoprotein lipase. II. Substrate specificity and activation of coconut oil. J. Biol. Chem. 215: 15–26. [PubMed] [Google Scholar]
- 16.Korn E. D. 1955. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J. Biol. Chem. 215: 1–14. [PubMed] [Google Scholar]
- 17.Havel R. J., and Gordon R. S. Jr.. 1960. Idiopathic hyperlipemia: metabolic studies in an affected family. J. Clin. Invest. 39: 1777–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mailly F., Palmen J., Muller D. P., Gibbs T., Lloyd J., Brunzell J., Durrington P., Mitropoulos K., Betteridge J., Watts G., et al. 1997. Familial lipoprotein lipase (LPL) deficiency: a catalogue of LPL gene mutations identified in 20 patients from the UK, Sweden, and Italy. Hum. Mutat. 10: 465–473. [DOI] [PubMed] [Google Scholar]
- 19.Peterson J., Ayyobi A. F., Ma Y., Henderson H., Reina M., Deeb S. S., Santamarina-Fojo S., Hayden M. R., and Brunzell J. D.. 2002. Structural and functional consequences of missense mutations in exon 5 of the lipoprotein lipase gene. J. Lipid Res. 43: 398–406. [PubMed] [Google Scholar]
- 20.Brunzell J. D., and Deeb S. S.. 2001. Familial lipoprotein lipase deficiency, apo C–II deficiency, and hepatic lipase deficiency. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, et al., editors. McGraw-Hill, New York. 2789–2816. [Google Scholar]
- 21.Beigneux A. P., Davies B., Gin P., Weinstein M. M., Farber E., Qiao X., Peale P., Bunting S., Walzem R. L., Wong J. S., et al. 2007. Glycosylphosphatidylinositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franssen R., Young S. G., Peelman F., Hertecant J., Sierts J. A., Schimmel A. W. M., Bensadoun A., Kastelein J. J. P., Fong L. G., Dallinga-Thie G. M., et al. 2010. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ. Cardiovasc. Genet. 3: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivecrona G., Ehrenborg E., Semb H., Makoveichuk E., Lindberg A., Hayden M. R., Gin P., Davies B. S., Weinstein M. M., Fong L. G., et al. 2010. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J. Lipid Res. 51: 1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plengpanich W., Young S. G., Khovidhunkit W., Bensadoun A., Karnman H., Ploug M., Gardsvoll H., Leung C. S., Adeyo O., Larsson M., et al. 2014. Multimerization of glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 (GPIHBP1) and familial chylomicronemia from a serine-to-cysteine substitution in GPIHBP1 Ly6 domain. J. Biol. Chem. 289: 19491–19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coca-Prieto I., Kroupa O., Gonzalez-Santos P., Magne J., Olivecrona G., Ehrenborg E., and Valdivielso P.. 2011. Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass: identification of a novel GPIHBP1 mutation. J. Intern. Med. 270: 224–228. [DOI] [PubMed] [Google Scholar]
- 26.Ariza M. J., Martinez-Hernandez P. L., Ibarretxe D., Rabacchi C., Rioja J., Grande-Aragon C., Plana N., Tarugi P., Olivecrona G., Calandra S., et al. 2016. Novel mutations in the GPIHBP1 gene identified in 2 patients with recurrent acute pancreatitis. J. Clin. Lipidol. 10: 92–100.e1. [DOI] [PubMed] [Google Scholar]
- 27.Rabacchi C., D’Addato S., Palmisano S., Lucchi T., Bertolini S., Calandra S., and Tarugi P.. 2016. Clinical and genetic features of three patients with familial chylomicronemia due to mutations in GPIHBP1 gene. J. Clin. Lipidol. 10: 915–921.e4. [DOI] [PubMed] [Google Scholar]
- 28.Beigneux A. P., Franssen R., Bensadoun A., Gin P., Melford K., Peter J., Walzem R. L., Weinstein M. M., Davies B. S., Kuivenhoven J. A., et al. 2009. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler. Thromb. Vasc. Biol. 29: 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rios J. J., Shastry S., Jasso J., Hauser N., Garg A., Bensadoun A., Cohen J. C., and Hobbs H. H.. 2012. Deletion of GPIHBP1 causing severe chylomicronemia. J. Inherit. Metab. Dis. 35: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin M. H., Tian X. H., Hao X. L., Fei H., Yin J. L., Yan D. D., and Li T.. 2020. Management of a pregnant patient with chylomicronemia from a novel mutation in GPIHBP1: a case report. BMC Pregnancy Childbirth. 20: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beigneux A. P., Fong L. G., Bensadoun A., Davies B. S., Oberer M., Gardsvoll H., Ploug M., and Young S. G.. 2015. GPIHBP1 missense mutations often cause multimerization of GPIHBP1 and thereby prevent lipoprotein lipase binding. Circ. Res. 116: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voss C. V., Davies B. S., Tat S., Gin P., Fong L. G., Pelletier C., Mottler C. D., Bensadoun A., Beigneux A. P., and Young S. G.. 2011. Mutations in lipoprotein lipase that block binding to the endothelial cell transporter GPIHBP1. Proc. Natl. Acad. Sci. USA. 108: 7980–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chyzhyk V., Kozmic S., Brown A. S., Hudgins L. C., Starc T. J., Davila A. D., Blevins T. C., Diffenderfer M. R., He L., Geller A. S., et al. 2019. Extreme hypertriglyceridemia: genetic diversity, pancreatitis, pregnancy, and prevalence. J. Clin. Lipidol. 13: 89–99. [DOI] [PubMed] [Google Scholar]
- 34.Allan C. M., Jung C. J., Larsson M., Heizer P. J., Tu Y., Sandoval N. P., Dang T. L. P., Jung R. S., Beigneux A. P., de Jong P. J., et al. 2017. Mutating a conserved cysteine in GPIHBP1 reduces amounts of GPIHBP1 in capillaries and abolishes LPL binding. J. Lipid Res. 58: 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beigneux A. P., Miyashita K., Ploug M., Blom D. J., Ai M., Linton M. F., Khovidhunkit W., Dufour R., Garg A., McMahon M. A., et al. 2017. Autoantibodies against GPIHBP1 as a cause of hypertriglyceridemia. N. Engl. J. Med. 376: 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olafsen T., Young S. G., Davies B. S., Beigneux A. P., Kenanova V. E., Voss C., Young G., Wong K. P., Barnes R. H. 2nd, Tu Y., et al. 2010. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) in mouse tissues revealed by positron emission tomography scanning. J. Biol. Chem. 285: 39239–39248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies B. S., Goulbourne C. N., Barnes R. H. 2nd, Turlo K. A., Gin P., Vaughan S., Vaux D. J., Bensadoun A., Beigneux A. P., Fong L. G., et al. 2012. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J. Lipid Res. 53: 2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X., Sleeman M. W., Miyashita K., Linton M. F., Allan C. M., He C., Larsson M., Tu Y., Sandoval N. P., Jung R. S., et al. 2017. Monoclonal antibodies that bind to the Ly6 domain of GPIHBP1 abolish the binding of LPL. J. Lipid Res. 58: 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward G., Simpson A., Boscato L., and Hickman P. E.. 2017. The investigation of interferences in immunoassay. Clin. Biochem. 50: 1306–1311. [DOI] [PubMed] [Google Scholar]
- 40.Tate J., and Ward G.. 2004. Interferences in immunoassay. Clin. Biochem. Rev. 25: 105–120. [PMC free article] [PubMed] [Google Scholar]
- 41.Tang G., Wu Y., Zhao W., and Shen Q.. 2012. Multiple immunoassay systems are negatively interfered by circulating cardiac troponin I autoantibodies. Clin. Exp. Med. 12: 47–53. [DOI] [PubMed] [Google Scholar]
- 42.Eriksson S., Hellman J., and Pettersson K.. 2005. Autoantibodies against cardiac troponins. N. Engl. J. Med. 352: 98–100. [DOI] [PubMed] [Google Scholar]
- 43.Peyvandi F., Ferrari S., Lavoretano S., Canciani M. T., and Mannucci P. M.. 2004. von Willebrand factor cleaving protease (ADAMTS-13) and ADAMTS-13 neutralizing autoantibodies in 100 patients with thrombotic thrombocytopenic purpura. Br. J. Haematol. 127: 433–439. [DOI] [PubMed] [Google Scholar]
- 44.Levy G. G., Nichols W. C., Lian E. C., Foroud T., McClintick J. N., McGee B. M., Yang A. Y., Siemieniak D. R., Stark K. R., Gruppo R., et al. 2001. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 413: 488–494. [DOI] [PubMed] [Google Scholar]
- 45.Ramaekers V. T., Rothenberg S. P., Sequeira J. M., Opladen T., Blau N., Quadros E. V., and Selhub J.. 2005. Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N. Engl. J. Med. 352: 1985–1991. [DOI] [PubMed] [Google Scholar]
- 46.Molloy A. M., Quadros E. V., Sequeira J. M., Troendle J. F., Scott J. M., Kirke P. N., and Mills J. L.. 2009. Lack of association between folate-receptor autoantibodies and neural-tube defects. N. Engl. J. Med. 361: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramaekers V. T., Blau N., Sequeira J. M., Nassogne M. C., and Quadros E. V.. 2007. Folate receptor autoimmunity and cerebral folate deficiency in low-functioning autism with neurological deficits. Neuropediatrics. 38: 276–281. [DOI] [PubMed] [Google Scholar]
- 48.Cabrera R. M., Shaw G. M., Ballard J. L., Carmichael S. L., Yang W., Lammer E. J., and Finnell R. H.. 2008. Autoantibodies to folate receptor during pregnancy and neural tube defect risk. J. Reprod. Immunol. 79: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou J., Liu A., He F., Jin Y., Zhou S., Xu R., Guo H., Zhou W., Wei Q., and Wang M.. 2018. High prevalence of serum folate receptor autoantibodies in children with autism spectrum disorders. Biomarkers. 23: 622–624. [DOI] [PubMed] [Google Scholar]
- 50.Yang N., Wang L., Finnell R. H., Li Z., Jin L., Zhang L., Cabrera R. M., Ye R., and Ren A.. 2016. Levels of folate receptor autoantibodies in maternal and cord blood and risk of neural tube defects in a Chinese population. Birth Defects Res. A Clin. Mol. Teratol. 106: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamidi S., Chen C. R., Murali R., McLachlan S. M., and Rapoport B.. 2013. Probing structural variability at the N terminus of the TSH receptor with a murine monoclonal antibody that distinguishes between two receptor conformational forms. Endocrinology. 154: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz-Lauer L., Pichurin P. N., Chen C. R., Nagayama Y., Paras C., Morris J. C., Rapoport B., and McLachlan S. M.. 2003. The cysteine-rich amino terminus of the thyrotropin receptor is the immunodominant linear antibody epitope in mice immunized using naked deoxyribonucleic acid or adenovirus vectors. Endocrinology. 144: 1718–1725. [DOI] [PubMed] [Google Scholar]
- 53.Chen C. R., Tanaka K., Chazenbalk G. D., McLachlan S. M., and Rapoport B.. 2001. A full biological response to autoantibodies in Graves’ disease requires a disulfide-bonded loop in the thyrotropin receptor N terminus homologous to a laminin epidermal growth factor-like domain. J. Biol. Chem. 276: 14767–14772. [DOI] [PubMed] [Google Scholar]
- 54.Ostertag E. M., Kacir S., Thiboutot M., Gulendran G., Zheng X. L., Cines D. B., and Siegel D. L.. 2016. ADAMTS13 autoantibodies cloned from patients with acquired thrombotic thrombocytopenic purpura: 1. Structural and functional characterization in vitro. Transfusion. 56: 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyashita K., Fukamachi I., Nagao M., Ishida T., Kobayashi J., Machida T., Nakajima K., Murakami M., Ploug M., Beigneux A. P., et al. 2018. An enzyme-linked immunosorbent assay for measuring GPIHBP1 levels in human plasma or serum. J. Clin. Lipidol. 12: 203–210.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu X., Dallinga-Thie G. M., Hovingh G. K., Chang S. Y., Sandoval N. P., Dang T. L. P., Fukamachi I., Miyashita K., Nakajima K., Murakami M., et al. 2017. GPIHBP1 autoantibodies in a patient with unexplained chylomicronemia. J. Clin. Lipidol. 11: 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Markey A. C., and MacDonald D. M.. 1988. Systemic lupus erythematosus with complement deficiency and IgA anti-cardiolipin antibody. Br. J. Dermatol. 119: 633–637. [DOI] [PubMed] [Google Scholar]
- 58.Tsutsumi A., Matsuura E., Ichikawa K., Fujisaku A., Mukai M., and Koike T.. 1998. IgA class anti-beta2-glycoprotein I in patients with systemic lupus erythematosus. J. Rheumatol. 25: 74–78. [PubMed] [Google Scholar]
- 59.Gould T., Tikly M., Asherson R., Loizou S., and Singh S.. 2006. Prevalence and clinical correlates of anti-phospholipid antibodies in South Africans with systemic lupus erythematosus. Scand. J. Rheumatol. 35: 29–34. [DOI] [PubMed] [Google Scholar]
- 60.Villalta D., Bizzaro N., Bassi N., Zen M., Gatto M., Ghirardello A., Iaccarino L., Punzi L., and Doria A.. 2013. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS One. 8: e71458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellebrecht C. T., Mukherjee E. M., Zheng Q., Choi E. J., Reddy S. G., Mao X., and Payne A. S.. 2018. Autoreactive IgG and IgA B cells evolve through distinct subclass switch pathways in the autoimmune disease pemphigus vulgaris. Cell Rep. 24: 2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kridin K., Patel P. M., Jones V. A., Cordova A., and Amber K. T.. 2020. IgA pemphigus: A systematic review. J. Am. Acad. Dermatol. 82: 1386–1392. [DOI] [PubMed] [Google Scholar]
- 63.Lindfors K., Koskinen O., Laurila K., Collin P., Saavalainen P., Haimila K., Partanen J., Maki M., and Kaukinen K.. 2011. IgA-class autoantibodies against neuronal transglutaminase, TG6 in celiac disease: no evidence for gluten dependency. Clin. Chim. Acta. 412: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 64.Salant D. J. 2019. Unmet challenges in membranous nephropathy. Curr. Opin. Nephrol. Hypertens. 28: 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huijbers M. G., Plomp J. J., van der Maarel S. M., and Verschuuren J. J.. 2018. IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders. Ann. N. Y. Acad. Sci. 1413: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y., Oomen R., and Klein M. H.. 1994. Residue at position 331 in the IgG1 and IgG4 CH2 domains contributes to their differential ability to bind and activate complement. J. Biol. Chem. 269: 3469–3474. [PubMed] [Google Scholar]
- 67.Wilkinson I. C., Fowler S. B., Machiesky L., Miller K., Hayes D. B., Adib M., Her C., Borrok M. J., Tsui P., Burrell M., et al. 2013. Monovalent IgG4 molecules: immunoglobulin Fc mutations that result in a monomeric structure. MAbs. 5: 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Zee J. S., van Swieten P., and Aalberse R. C.. 1986. Inhibition of complement activation by IgG4 antibodies. Clin. Exp. Immunol. 64: 415–422. [PMC free article] [PubMed] [Google Scholar]
- 69.Schuurman J., Van Ree R., Perdok G. J., Van Doorn H. R., Tan K. Y., and Aalberse R. C.. 1999. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology. 97: 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aalberse R. C., and Schuurman J.. 2002. IgG4 breaking the rules. Immunology. 105: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johansen C. T., Wang J., McIntyre A. D., Martins R. A., Ban M. R., Lanktree M. B., Huff M. W., Peterfy M., Mehrabian M., Lusis A. J., et al. 2012. Excess of rare variants in non-genome-wide association study candidate genes in patients with hypertriglyceridemia. Circ. Cardiovasc. Genet. 5: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chait A., and Eckel R. H.. 2019. The chylomicronemia syndrome is most often multifactorial: a narrative review of causes and treatment. Ann. Intern. Med. 170: 626–634. [DOI] [PubMed] [Google Scholar]
- 73.Dron J. S., and Hegele R. A.. 2020. Genetics of Hypertriglyceridemia. Front. Endocrinol. (Lausanne). 11: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eguchi J., Miyashita K., Fukamachi I., Nakajima K., Murakami M., Kawahara Y., Yamashita T., Ohta Y., Abe K., Nakatsuka A., et al. 2019. GPIHBP1 autoantibody syndrome during interferon beta1a treatment. J. Clin. Lipidol. 13: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashraf A. P., Miyashita K., Nakajima K., Murakami M., Hegele R. A., Ploug M., Fong L. G., Young S. G., and Beigneux A. P.. 2020. Intermittent chylomicronemia caused by intermittent GPIHBP1 autoantibodies. J. Clin. Lipidol. 14: 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutz J., Dunaj-Kazmierowska M., Arcan S., Kassner U., Miyashita K., Murakami M., Ploug M., Fong L. G., Young S. G., Nakajima K., et al. Chylomicronemia from GPIHBP1 autoantibodies successfully treated with rituximab: a case report. Ann. Intern. Med. Epub ahead of print. August 11, 2020; doi:10.7326/L20-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banchereau J., and Pascual V.. 2006. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 25: 383–392. [DOI] [PubMed] [Google Scholar]
- 78.Silva M. O. 2012. Risk of autoimmune complications associated with interferon therapy. Gastroenterol. Hepatol. (N Y). 8: 540–542. [PMC free article] [PubMed] [Google Scholar]
- 79.Di Domizio J., and Cao W.. 2013. Fueling autoimmunity: type I interferon in autoimmune diseases. Expert Rev. Clin. Immunol. 9: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flier J. S., Bar R. S., Muggeo M., Kahn C. R., Roth J., and Gorden P.. 1978. The evolving clinical course of patients with insulin receptor autoantibodies: spontaneous remission or receptor proliferation with hypoglycemia. J. Clin. Endocrinol. Metab. 47: 985–995. [DOI] [PubMed] [Google Scholar]
- 81.Diaz M., Agraz I., and Soler M. J.. 2019. Anti-phospholipase A2 receptor antibody and spontaneous remission in membranous nephropathy. Clin. Kidney J. 12: 33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyashita K., Fukamachi I., Machida T., Nakajima K., Young S. G., Murakami M., Beigneux A. P., and Nakajima K.. 2018. An ELISA for quantifying GPIHBP1 autoantibodies and making a diagnosis of the GPIHBP1 autoantibody syndrome. Clin. Chim. Acta. 487: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Machida T., Miyashita K., Sone T., Tanaka S., Nakajima K., Saito M., Stanhope K., Havel P., Sumino H., and Murakami M.. 2015. Determination of serum lipoprotein lipase using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin. Chim. Acta. 442: 130–135. [DOI] [PubMed] [Google Scholar]
- 84.Bylsma L. C., Fryzek J. P., Cetin K., Callaghan F., Bezold C., Mehta B., and Wasser J. S.. 2019. Systematic literature review of treatments used for adult immune thrombocytopenia in the second-line setting. Am. J. Hematol. 94: 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacIsaac J., Siddiqui R., Jamula E., Li N., Baker S., Webert K. E., Evanovitch D., Heddle N. M., and Arnold D. M.. 2018. Systematic review of rituximab for autoimmune diseases: a potential alternative to intravenous immune globulin. Transfusion. 58: 2729–2735. [DOI] [PubMed] [Google Scholar]
- 86.Schmidt E., Kasperkiewicz M., and Joly P.. 2019. Pemphigus. Lancet. 394: 882–894. [DOI] [PubMed] [Google Scholar]
- 87.Kihara S., Matsuzawa Y., Kubo M., Nozaki S., Funahashi T., Yamashita S., Sho N., and Tarui S.. 1989. Autoimmune hyperchylomicronemia. N. Engl. J. Med. 320: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 88.de Carvalho J. F., Viana V. S., Neto E. F., Santos R. D., and Bonfa E.. 2011. Anti-lipoprotein lipase antibodies in patients with hypertriglyceridemia without associated autoimmune disease. Isr. Med. Assoc. J. 13: 350–353. [PubMed] [Google Scholar]
- 89.Ashraf A. P., Beukelman T., Pruneta-Deloche V., Kelly D. R., and Garg A.. 2011. Type 1 hyperlipoproteinemia and recurrent acute pancreatitis due to lipoprotein lipase antibody in a young girl with Sjogren’s syndrome. J. Clin. Endocrinol. Metab. 96: 3302–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kodera M., Hayakawa I., Komura K., Yanaba K., Hasegawa M., Takehara K., and Sato S.. 2005. Anti-lipoprotein lipase antibody in systemic sclerosis: association with elevated serum triglyceride concentrations. J. Rheumatol. 32: 629–636. [PubMed] [Google Scholar]
- 91.Lilley J. S., Linton M. F., Kelley J. C., Graham T. B., Fazio S., and Tavori H.. 2017. A case of severe acquired hypertriglyceridemia in a 7-year-old girl. J. Clin. Lipidol. 11: 1480–1484. [DOI] [PubMed] [Google Scholar]
- 92.Moret M., Pruneta-Deloche V., Sassolas A., Marcais C., and Moulin P.. 2010. Prevalence and function of anti-lipoprotein lipase auto-antibodies in type V hyperchylomicronemia. Atherosclerosis. 208: 324–327. [DOI] [PubMed] [Google Scholar]
- 93.Okamoto Y., Tominaga K., Uemura S., Matsuoka H., Tsujii T., and Nakano H.. 1995. Hypertriglyceridemia caused by the autoantibody to lipases for plasma lipoproteins: a case report. J. Atheroscler. Thromb. 2: 66–69. [DOI] [PubMed] [Google Scholar]
- 94.Pruneta V., Moulin P., Labrousse F., Bondon P. J., Ponsin G., and Berthezene F.. 1997. Characterization of a new case of autoimmune type I hyperlipidemia: long-term remission under immunosuppressive therapy. J. Clin. Endocrinol. Metab. 82: 791–796. [DOI] [PubMed] [Google Scholar]
- 95.Pruneta-Deloche V., Marcais C., Perrot L., Sassolas A., Delay M., Estour B., Lagarde M., and Moulin P.. 2005. Combination of circulating antilipoprotein lipase (Anti-LPL) antibody and heterozygous S172 fsX179 mutation of LPL gene leading to chronic hyperchylomicronemia. J. Clin. Endocrinol. Metab. 90: 3995–3998. [DOI] [PubMed] [Google Scholar]
- 96.Reichlin M., Fesmire J., Quintero-Del-Rio A. I., and Wolfson-Reichlin M.. 2002. Autoantibodies to lipoprotein lipase and dyslipidemia in systemic lupus erythematosus. Arthritis Rheum. 46: 2957–2963. [DOI] [PubMed] [Google Scholar]
- 97.Rodrigues C. E., Bonfa E., and Carvalho J. F.. 2010. Review on anti-lipoprotein lipase antibodies. Clin. Chim. Acta. 411: 1603–1605. [DOI] [PubMed] [Google Scholar]
- 98.Casanovas A., Carrascal M., Abian J., Lopez-Tejero M. D., and Llobera M.. 2008. Application of proteomic tools to detect the nonspecificity of a polyclonal antibody against lipoprotein lipase. J. Proteome Res. 7: 4173–4177. [DOI] [PubMed] [Google Scholar]
- 99.Beigneux A. P., Allan C. M., Sandoval N. P., Cho G. W., Heizer P. J., Jung R. S., Stanhope K. L., Havel P. J., Birrane G., Meiyappan M., et al. 2019. Lipoprotein lipase is active as a monomer. Proc. Natl. Acad. Sci. USA. 116: 6319–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]