17-β hydroxysteroid dehydrogenase 13 (HSD17B13) belongs to a 15-member family that is involved in various metabolic processes, including steroid hormones, fatty acids, cholesterol, and bile acids (1). The human HSD17B13 gene is located on chromosome 4 (4q22.1), and its expression is highly restricted to the liver, specifically in hepatocytes but not other cell types in the liver (2, 3). The human HSD17B13 gene encodes a 300-amino-acid protein that is localized on lipid droplet (4). Interestingly, a few single-nucleotide polymorphisms (rs72613567, rs62305723, rs6834314, rs9992651, rs13118664, and rs4607179) of the human HSD17B13 gene have been linked to alcoholic and nonalcoholic fatty liver diseases by genome-wide association studies (5–12). Loss-of-function mutations in the human HSD17B13 gene due to improper splicing, insertion, or nonsynonymous mutations confer a strong protective effect on liver injury, inflammation, fibrosis, cirrhosis, and even hepatocellular carcinoma (5–15). However, the biochemical structure and function of human HSD17B13 protein remains elusive. In this issue of the Journal of Lipid Research, Ma and coauthors report initial biochemical characterization of HSD17B13 domains and specific residues required for subcellular localization and retinol dehydrogenase activity (Fig. 1). This is a significant step forward for better understanding of the functional implication of HSD17B13 in human diseases and for therapeutic development.
Fig. 1.
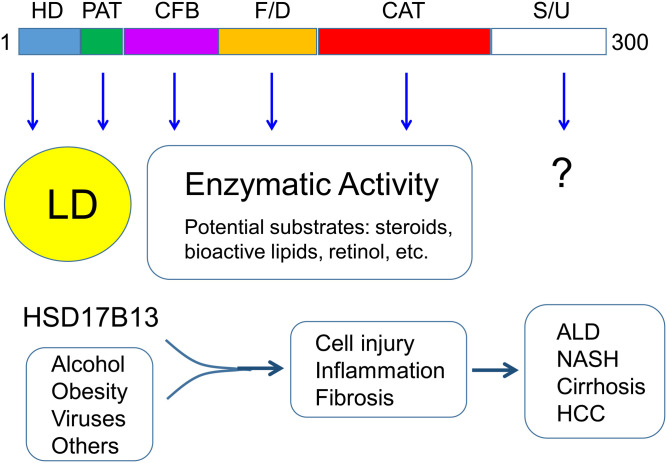
The domain structure and function of the human HSD17B13 gene. The human HSD17B13 gene encodes a 300-amino-acid protein that has been characterized to contain multiple domains, including hydrophobic domain (HD), PAT-like domain (PAT), cofactor-binding domain (CFB), folding/dimerization domain (F/D), catalytic domain (CAT), and stability/unknown domain (S/U). Both HD and PAT are required for targeting to lipid droplet (LD). In addition to CAT, both CFB and F/D are also involved in the enzymatic activity. Although the physiological function of HSD17B13 remains elusive, human genetic data suggest that under conditions like alcoholism, obesity, and viral infection, HSD17B13 enzymatic activity attributes to hepatic cell injury, inflammation, and fibrosis, chronically leading to the development of alcoholic liver disease (ALD), nonalcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma (HCC).
Previous reports have shown that HSD17B13 is associated with lipid droplet (4, 6, 8, 16); however, it is unclear which peptide sequences are involved. The report by Ma et al. has identified three fragments within the N-terminal 106 amino acids, which are required for HSD17B13 targeting to the normal destination; that is, lipid droplets: AA4-16 hydrophobic domain, AA22-28 PAT-like domain, and AA69-106 α-helix/β-sheet/α-helix structure. Interestingly, it was also observed that the α-helix/β-sheet/α-helix structure may play a role in HSD17B13 proper folding in the endoplasmic reticulum (ER) and trafficking from ER to lipid droplet. Deletion of this structure led to ER retention and protein degradation. HSD17B13 was shown to form homodimers in cultured cells.
HSD17B13 has been reported to catalyze multiple substrates including steroids, bioactive lipids including leukotriene B4 and 12(R)-hydroxyeicosatetraenoic acid, and retinol using bacterial recombinant protein or transfected human cell lines (6, 8, 12). As the protein structure for human HSD17B13 has not been solved, Ma et al. used the structure of HSD17B11 as a template to model the HSD17B13 structure in silico. By doing so, they predicted and verified a number of key residues for the HSD17B13 enzymatic activity in a cell-based assay using retinol as a candidate substrate. The predicted catalytic tetrad Asn144/Ser172/Tyr185/Lys189 is indeed involved in the enzymatic activity, which was demonstrated by site-directed mutagenesis. Several residues, including Lys153, Leu156, Leu199, Glu202, and Lys208, predicted as substrate-binding sites, are also required for the enzymatic activity. In addition, they have shown that the putative homodimer interaction sites Arg97/Tyr101 are also involved in the enzyme activity.
Some significant questions remain unanswered even with this well-performed biochemical characterization. The first question is how HSD17B13 adapts to rather structurally different substrates from steroids (4-ring structure) to retinol (ring plus branch chain) to various bioactive lipids (chain structure). The physiological substrates remain to be defined and verified. The second question is why human and mouse HSD17B13 function differently, although they are structurally similar at the amino acid sequences with 69% identity and 75% similarity, and all the key residues required for dimerization, substrate binding, and catalysis are conserved (according to amino acid sequence alignments). Although human genetic data strongly suggest HSD17B13 loss-of-function mutations have a protective effect against multiple chronic liver diseases, including alcoholic and nonalcoholic fatty liver diseases (5–13), mouse Hsd17b13 gene knockout does not seem to protect the liver from alcoholic liver disease or nonalcoholic steatohepatitis (3, 17). In vitro assays suggest that mouse Hsd17b13 does not show retinol dehydrogenase activity (3). However, this result cannot be simply explained biochemically as the key residues for substrate binding and catalytic domains are well conserved. Humanized animal models and nonhuman primates may be used to study the salutary effects of the loss-of-function mutants or inhibitors of human HSD17B13. The third question is what HSD17B13 actually does on lipid droplets. Some data have shown that HSD17B13 overexpression or deficiency does not seem to have a discernable effect on lipid droplet morphology, whereas other data suggest that hepatic phospholipids, especially phosphatidylcholines (PCs) and phosphatidylethanolamines (PEs), are increased in the livers of the HSD17B13 rs72613567:TA carriers (6, 8, 13). More studies are needed to demonstrate whether those changes in phospholipids are consequences of HSD17B13 loss of function on the lipid droplets and whether the enrichment of PC and PE is a major player in protection from chronic liver disease due to HSD17B13 deficiency.
In summary, the findings from this report pinpoint significant insights into the biochemical nature of human HSD17B13, which lay a foundation for future biological research and therapeutic development. At the same time, this work also raises additional intriguing questions in the field.jlr
REFERENCES
- 1.Su W., Mao Z., Liu Y., Zhang X., Zhang W., Gustafsson J. A., Guan Y.. 2019. Role of HSD17B13 in the liver physiology and pathophysiology. Mol. Cell. Endocrinol. 489: 119–125. [DOI] [PubMed] [Google Scholar]
- 2.Liu S., Huang C., Li D., Ren W., Zhang H., Qi M., Li X., Yu L.. 2007. Molecular cloning and expression analysis of a new gene for short-chain dehydrogenase/reductase 9. Acta Biochim. Pol. 54: 213–218. [PubMed] [Google Scholar]
- 3.Ma Y., Brown P. M., Lin D. D., Ma J., Feng D., Belyaeva O. V., Podszun M. C., Roszik J., Allen J., Umarova R. et al. 2020. Hsd17b13 deficiency does not protect mice from obesogenic diet injury. Hepatology. Epub ahead of print. August 11, 2020; doi: 10.1002/hep.31517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su W., Wang Y., Jia X., Wu W., Li L., Tian X., Li S., Wang C., Xu H., Cao J. et al. 2014. Comparative proteomic study reveals 17beta-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 111: 11437–11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.About F., Abel L., Cobat A.. 2018. HCV-Associated Liver Fibrosis and HSD17B13. N. Engl. J. Med. 379: 1875–1876. [DOI] [PubMed] [Google Scholar]
- 6.Abul-Husn N. S., Cheng X., Li A. H., Xin Y., Schurmann C., Stevis P., Liu Y., Kozlitina J., Stender S., Wood G. C. et al. 2018. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 378: 1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozlitina J., Stender S., Hobbs H. H., Cohen J. C.. 2018. HSD17B13 and chronic liver disease in Blacks and Hispanics. N. Engl. J. Med. 379: 1876–1877. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y., Belyaeva O. V., Brown P. M., Fujita K., Valles K., Karki S., de Boer Y. S., Koh C., Chen Y., Du X. et al. 2019. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 69: 1504–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirola C. J., Garaycoechea M., Flichman D., Arrese M., San Martino J., Gazzi C., Castano G. O., Sookoian S.. 2019. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J. Lipid Res. 60: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stickel F., Lutz P., Buch S., Nischalke H. D., Silva I., Rausch V., Fischer J., Weiss K. H., Gotthardt D., Rosendahl J. et al. 2020. Genetic variation in HSD17B13 reduces the risk of developing cirrhosis and hepatocellular carcinoma in alcohol misusers. Hepatology. 72: 88–102. [DOI] [PubMed] [Google Scholar]
- 11.Yang J., Trepo E., Nahon P., Cao Q., Moreno C., Letouze E., Imbeaud S., Bayard Q., Gustot T., Deviere J. et al. 2019. A 17-beta-hydroxysteroid dehydrogenase 13 variant protects from hepatocellular carcinoma development in alcoholic liver disease. Hepatology. 70: 231–240. [DOI] [PubMed] [Google Scholar]
- 12.Anstee Q. M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D., Burt A. D., Bedossa P., Palmer J., Liu Y. L. et al. 2020. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 73: 505–515. [DOI] [PubMed] [Google Scholar]
- 13.Luukkonen P. K., Tukiainen T., Juuti A., Sammalkorpi H., Haridas P. A. N., Niemela O., Arola J., Orho-Melander M., Hakkarainen A., Kovanen P. T. et al. 2020. Hydroxysteroid 17-beta dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight. 5: e132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gellert-Kristensen H., Nordestgaard B. G., Tybjaerg-Hansen A., Stender S.. 2020. High risk of fatty liver disease amplifies the alanine transaminase-lowering effect of a HSD17B13 variant. Hepatology. 71: 56–66. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Zhuo J. Y., Yang F., Liu Z. K., Zhou L., Xie H. Y., Xu X., Zheng S. S.. 2018. 17-beta-hydroxysteroid dehydrogenase 13 inhibits the progression and recurrence of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 17: 220–226. [DOI] [PubMed] [Google Scholar]
- 16.Horiguchi Y., Araki M., Motojima K.. 2008. 17beta-Hydroxysteroid dehydrogenase type 13 is a liver-specific lipid droplet-associated protein. Biochem. Biophys. Res. Commun. 370: 235–238. [DOI] [PubMed] [Google Scholar]
- 17.Adam M., Heikela H., Sobolewski C., Portius D., Maki-Jouppila J., Mehmood A., Adhikari P., Esposito I., Elo L. L., Zhang F. P. et al. 2018. Hydroxysteroid (17beta) dehydrogenase 13 deficiency triggers hepatic steatosis and inflammation in mice. FASEB J. 32: 3434–3447. [DOI] [PubMed] [Google Scholar]