Abstract
Mycobacterium abscessus is a rapidly growing, non-tubercular mycobacteria, often associated with skin and soft tissue infections. We report a case of 57-year-old immune-competent woman who suffered recurrent bilateral breast infection for 6 years. She did not benefit from repeated surgical interventions and multiple courses of antibiotics, and one course of empirical antitubercular therapy. Chronicity of the presentation and non-response to varied treatment interventions prompted further microbiological investigations. The patient was diagnosed with M. abscessus and treated with rifabutin, clarithromycin daily for 6 months and injection amikacin for 1 month. Amikacin was replaced with oral levofloxacin due to bilateral sensory-neural hearing loss for higher frequencies after 6 months. Suspicion and identification of NTM are important as the treatment involves long-term combination antibacterial therapy along with surgical debridement for extensive infection or when implants are involved.
Keywords: TB and other respiratory infections, breast surgery, infectious diseases, breast cancer
Background
Mastitis is a common disease with acute, subacute and chronic presentations, most commonly caused by Staphylococcus aureus and Streptococcus sp while Mycobacterium tuberculosis and non-tubercular mycobacteria (NTM) are less often reported to cause breast infections.1–3 Rapidly growing mycobacteria (RGM) such as Mycobacterium abscessus, M. chelonae and M. fortuitum are emerging pathogens and associated with human infections. M. abscessus is a ubiquitous organism which is associated with cutaneous infections, surgical wound infections, pulmonary infections and post-traumatic injuries.4 5 Recurrent mastitis due to M. abscessus and M. fortuitum has been reported. However, these cases remain difficult to diagnose clinically and radiologically. We report a case of recurrent bilateral mastitis with a delayed diagnosis due to lack of suspicion for the causal pathogen.
Case presentation
A 57-year-old woman presented to out-patient department with pain, discharge, redness and itching intermittently in both breasts for 6 years, with radiating pain to axilla. Physical examination revealed palpable tender mobile mass in right breast, which was approximately 2×2 cm in retroareolar region and in left breast about 2×3 cm in the same region. There was erythema, swelling and puckering of skin of both breasts. Nipple discharge was watery in nature but sometimes purulent and blood tinged. It was not associated with axillary lymphadenopathy. There was no history of fever, nipple discharge, tuberculosis, surgery, implant, any injectable drugs, breast carcinoma or immunosuppressive drug intake. There was no family history of breast carcinoma and tuberculosis. Prior to admission, the patient had recurrent breast infections for the last 6 years. She was treated for mastitis two times at an interval of 1 year at a peripheral hospital (with incision and drainage performed and antibiotic coverage for 2 weeks both the times). Thereafter, she remained asymptomatic for 2 years, but again developed bilateral breast mass associated with discharge and redness in both breasts. She was operated for radical duct excision of both breasts on basis of mammography and MRI findings (table 1). Surgical specimen was sent for histopathology and microbiological examination. She was treated with broad-spectrum antibiotics augmentin and metrogyl for the duration of 2 weeks. Thereafter; she remained asymptomtic for 4–6 months and relapsed again with multiple discharging sinuses in the left breast and redness in the right breast. Tissue cytopathology findings were consistent with tuberculosis (figures 1–3) hence empirical antitubercular therapy (ATT) was initiated (rifampicin, isoniazid, pyrazinamide and ethambutol for 2 months, followed by rifampicin and isoniazid for 7 months) but there was minimal response. Microbiological investigations from discharge and biopsy at our institute confirmed it as a case of mastitis due to NTM, identified as M. abscessus (table 1).
Table 1.
Investigations
| Cytopathology report | The FNAC report shows degenerated neutrophils, histiocytes, macrophages and occasional epithelioid cells granuloma and gaint cells suggestive of tuberculosis (figure 1) Ziehl Neelsen stain of FNAC reveals AFB (figure 2) Histopathological examination revealed dilated ducts lined by attenuated epithelium filled with necrotic material, periductal fibrosis and inflammation. At places, breast parenchyma was replaced by extensive lymphoplasmacytic infiltrates suggestive of duct ectasia (figure 3) |
| Radiology findings | Mammogram (2016): bilateral duct ectasia, soft tissue process with increase nodularity on both sides MRI (2018): was suggestive of bilateral breast abscesses with adjacent mastitis with smaller satellite abscesses on left side and sinus formation on right side. |
| Microbiology report | Sample-discharge as well the tissue from the edges of the abscess cavity Ziehl Neelsen’s staining Long slender AFB seen (2+) GeneXpert MTB/RIF: Mycobacterium tuberculosis not detected MGIT 960: growth seen in 5 days, TbcID: negative LJ media with 5% NaCl: growth seen in 6 days MacConkeys agar: growth seen in 4 days Nitrate test: negative Identification: Mycobacterium abscessus Bacterial culture: negative Fungal culture: negative |
| 16S ribosomal gene was sequenced and identified as M. abscessus. Sequence similarities of 16S rRNA genes ranged from 99% to 100% with M. abscessus. Sequences were submitted to the GenBank with accession number MK942689. |
|
| Biochemical report | Erythrocyte sedimentation rate of 27 mm C reactive protein at 32 mg/L. |
AFB, acid fast bacilli; FNAC, fine needle aspiration cytology; LJ, Lowenstein Jensen; MGIT, Mycobacterium growth indicator tube; MTB, Mycobacterium tuberculosis.
Figure 1.
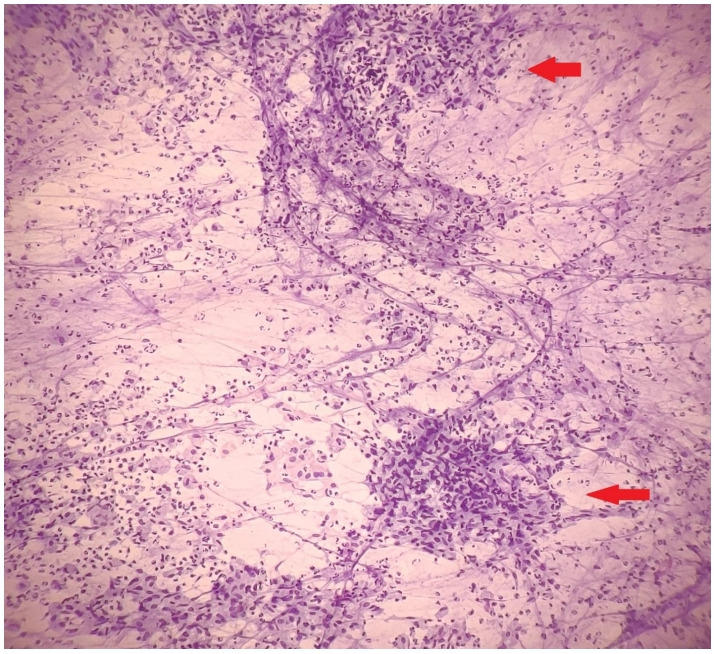
Giemsa stained smears fine needle aspiration cytology revealed epithelioid cell granulomas (arrow) in a background of intact and degenerated neutrophils and foamy histiocytes suggestive of tuberculosis.
Figure 2.
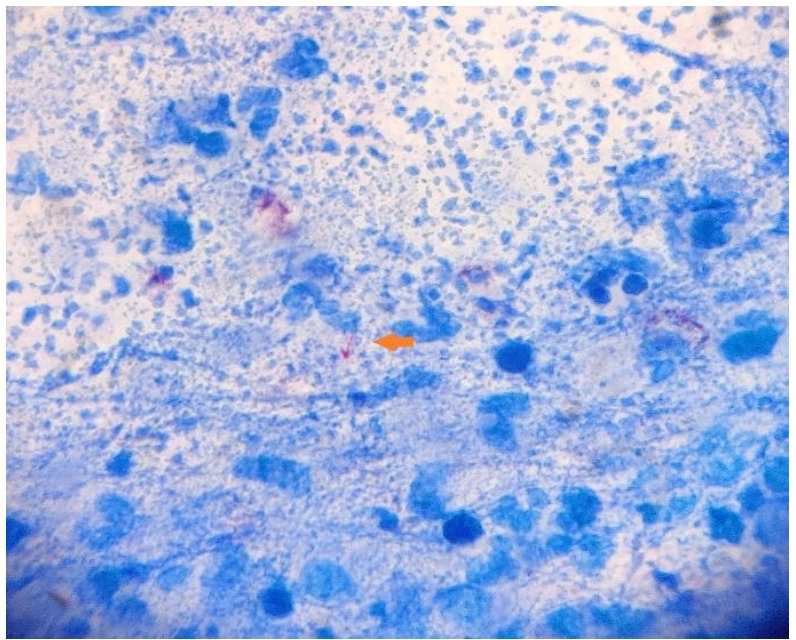
Ziehl Neelsen stained fine needle aspiration cytology smear revealing rod-shaped acid fast bacilli (arrow).
Figure 3.
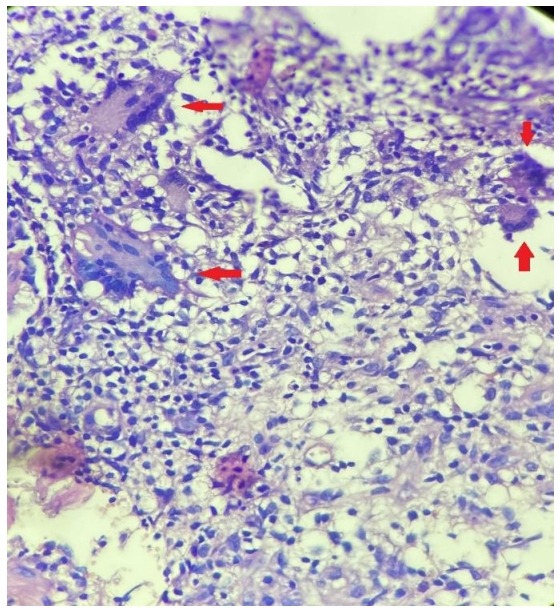
Sections from breast tissue showing multinucleated giant cells (arrow), epithelioid histiocytes and abundant lymphocytic infiltrate (H&E, 40×).
Drug susceptibility test was done by broth microdilution assay as per Clinical Laboratory Standard Institute (CLSI) guidelines.6 The isolate was susceptible to amikacin, clarithromycin, linezolid and rifampicin but resistant to imipenem. Clarithromycin broth microdilution MIC testing was done using the CLSI-recommended broth microdilution Minimum Inhibitory Concentration (MIC) method. The isolate had MIC of ≤2 µg/mL after 14 days.
Differential diagnosis
Few differential diagnosis were considered such as S. aureus breast abscess as it is the most common causative agent of mastitis. M. tuberculosis was suspected due to the chronic nature of the disease. NTM was considered when the patient did not respond to ATT.
Treatment
Patient was started on rifabutin 300 mg, clarithromycin 1000 mg daily for 6 months and injection amikacin 500 mg for 1 month. Amikacin was replaced with oral levofloxacin due to bilateral sensory-neural hearing loss for higher frequencies after 1 month.
Baseline audiometry, renal function tests, ECG and liver function tests were within normal limits. Serial QTc monitoring, renal function, electrolytes and liver enzymes were closely monitored.
Outcome and follow-up
Her lesions were healed at end of 7 months. Routine blood investigations were within normal limits. She has been followed up for one and a half year with no clinical or radiological evidence of recurrence.
Discussion
Recent reports have documented NTM organisms as a causative agent of breast infections.7 8 Interestingly, previous reports mentioned M. fortuitum as most common, followed by M. avium, M. abscessus and less commonly, M. chelonae.7 8 NTM should be considered as a source of infection in any chronic/recurrent infection, with no yield on routine bacterial culture, despite multiple courses of antibiotics and surgical intervention. NTM breast infections are often associated with implants or reconstructive surgery.3 7 However, few case reports have suggested that NTM breast infections can also be present in the absence of implants, could even occur spontaneously,9 10 following nipple piercing.11 12 In chronic and recurrent breast infections M. tuberculosis could be suspected, and ATT may be instituted. A lack of response to ATT or a case of the breast with multiple draining sinuses may require surgical intervention and incline the suspicion towards NTM disease.13
The NTM infection should be treated with targeted combined antibiotic therapy along with surgical intervention whenever required. Removal of implant and foreign body in addition to appropriate antibiotic therapy is recommended for the implant and foreign body-associated NTM infections.3 7 A primary panel of drugs for susceptibility testing may include amikacin, cefoxitin, ciprofloxacin, clarithromycin, doxycycline, imipenem and sulphonamide.14 In vitro susceptibilities of isolated colonies should be evaluated to detect the intrinsic resistance to macrolides. Many pathogenic RGM species are considered susceptible to the newer macrolides, and these agents are traditionally considered important components in the classical treatment regimen of NTM infections, however, few members of the Mycobacterium genus have now been associated with intrinsic resistance to macrolides.15–17 Molecular detection and characterisation of erm gene can be used for identification of M. abscessus complex, and discrimination between the macrolide-susceptible mycobacteria from the macrolide-resistant NTM.18
Our case demonstrated that due to a lack of suspicion for these sinister organisms, the patient had to undergo incision and drainage procedure two times and radical duct excision once, and she did not respond despite using appropriate antibacterial therapy multiple times. She was not immune-compromised and had no history of breast surgery or implant insertion in the past. The patient could have a spontaneous NTM breast infection or the repeated interventions could possibly have been a source of NTM.
In conclusion, it is imperative to suspect and identify such pathogens in any chronic, non-responding infection, especially in light of reports of intrinsic antibiotic resistance in certain species/subspecies of difficult to treat RGM skin and soft tissue involvement.
Patient’s perspective.
I had a breast surgery at Northern Railway Hospital, New Delhi as I had lumps in the breast. After few months I felt itchiness on operated area, I came to know after the fine needle aspiration cytology report that it was tuberculosis in my breast area. I switched to AIIMS New Delhi, Department of Microbiology referred by Dr Randeep Guleria, I really thank and appreciate Dr Urvashi and her team, specially Dr Sanjana Khanna as she treated us so well and suggest us the best. We are thankful to the Microbiology team of AIIMS New Delhi.
Learning points.
Non-tubercular mycobacteria (NTM) are an under-reported cause of breast infections.
NTM breast infections are not always associated with implants and breast reconstructive surgery.
Complete species/subspecies identification should be mandatory for such kind of non-resolving infections.
Speciation and antimicrobial susceptibility are important as it aids in the design of treatment regimen, especially for resistant and recurrent cases.
Acknowledgments
We acknowledge Dr Pooja Pandey (Scientist) and the staff of Mycobacteriology laboratory for support in this work.
Footnotes
Contributors: KB has contributed in conception and design, acquisition of data or analysis and interpretation of data. SK has contributed in compilation of all investigations and drafted the article. US contributed in revising it critically for important intellectual content and given the final approval of the version to be published. RG managed the patient and revised the manuscript critically.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin 2015;33:563–77. 10.1016/j.det.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 2.Kamal RM, Hamed ST, Salem DS. Classification of inflammatory breast disorders and step by step diagnosis. Breast J 2009;15:367–80. 10.1111/j.1524-4741.2009.00740.x [DOI] [PubMed] [Google Scholar]
- 3.Macadam SA, Mehling BM, Fanning A, et al. . Nontuberculous mycobacterial breast implant infections. Plast Reconstr Surg 2007;119:337–44. 10.1097/01.prs.0000244924.61968.d2 [DOI] [PubMed] [Google Scholar]
- 4.Villanueva A, Calderon RV, Vargas BA, et al. . Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis 1997;24:1147–53. 10.1086/513656 [DOI] [PubMed] [Google Scholar]
- 5.Uslan DZ, Kowalski TJ, Wengenack NL, et al. . Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol 2006;142:1287–92. 10.1001/archderm.142.10.1287 [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes: Approved standard. 2nd ed CLSI, Wayne, PA.: CLSI document M24-A2, 2011. [PubMed] [Google Scholar]
- 7.Haiavy J, Tobin H. Mycobacterium fortuitum infection in prosthetic breast implants. Plast Reconstr Surg 2002;109:2124–8. 10.1097/00006534-200205000-00051 [DOI] [PubMed] [Google Scholar]
- 8.Achra A, Vispute T, Pandey P, et al. . Mycobacterium fortuitum complicating breast prosthetic implant. Clin Epidemiol Glob Health 2016;4:197–9. 10.1016/j.cegh.2016.03.004 [DOI] [Google Scholar]
- 9.Betal D, Macneill FA. Chronic breast abscess due to Mycobacterium fortuitum: a case report. J Med Case Rep 2011;5:188. 10.1186/1752-1947-5-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke FJ, Friedland JS. Spontaneous breast abscess due to Mycobacterium fortuitum. Clin Infect Dis 1998;26:760–1. 10.1086/517117 [DOI] [PubMed] [Google Scholar]
- 11.Lewis CG, Wells MK, Jennings WC. Mycobacterium fortuitum breast infection following nipple-piercing, mimicking carcinoma. Breast J 2004;10:363–5. 10.1111/j.1075-122X.2004.21393.x [DOI] [PubMed] [Google Scholar]
- 12.Bengualid V, Singh V, Singh H, et al. . Mycobacterium fortuitum and anaerobic breast abscess following nipple piercing: case presentation and review of the literature. J Adolesc Health 2008;42:530–2. 10.1016/j.jadohealth.2007.10.016 [DOI] [PubMed] [Google Scholar]
- 13.Tewari M, Shukla HS. Breast tuberculosis: diagnosis, clinical features & management. Indian J Med Res 2005;122:103–10. [PubMed] [Google Scholar]
- 14.Yasar KK, Sengoz G, Pehlivanoglu F, et al. . Successfully treated Mycobacterium abscessus mastitis: a rare cause of breast masses. Indian J Med Microbiol 2011;29:431–7. 10.4103/0255-0857.90187 [DOI] [PubMed] [Google Scholar]
- 15.Nash KA, Brown-Elliott BA, Wallace RJ, et al. . A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother 2009;53:1367–76. 10.1128/AAC.01275-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash KA. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38). Antimicrob Agents Chemother 2003;47:3053–60. 10.1128/AAC.47.10.3053-3060.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash KA, Andini N, Zhang Y, et al. . Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob Agents Chemother 2006;50:3476–8. 10.1128/AAC.00402-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. . Utility of sequencing the erm(41) gene in isolates of Mycobacterium abscessus subsp. abscessus with low and intermediate clarithromycin MICs. J Clin Microbiol 2015;53:1211–5. 10.1128/JCM.02950-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


