Abstract
Objectives:
We prospectively evaluated endomyocardial biopsies in heart failure with preserved ejection fraction (HFpEF) patients to identify histopathological phenotypes and their association with clinical characteristics.
Background:
Myocardial tissue analysis from a prospectively defined HFpEF cohort reflecting contemporary comorbidities is lacking.
Methods:
HFpEF patients (EF ≥ 50%) referred to the Johns Hopkins HFpEF Clinic between August 2014 - September 2018 were enrolled for right heart catheterization and endomyocardial biopsy. Clinical features, echocardiography, hemodynamics, and tissue histology were determined and compared to controls (unused donor hearts) and HF with reduced EF (HFrEF).
Results:
Of 108 patients enrolled, median age was 66 (57–74) years, 61% were female, 57% African American, 62% with prior HF hospitalization, median systolic pressure 141 [125–162] mmHg, BMI 37 [32–45] kg/m2, and 97% were on a loop diuretic. Myocardial fibrosis and hypertrophy were often present (93% and 88%, respectively), however mild in 71% with fibrosis and in 52% with hypertrophy. Monocyte infiltration (CD68+ cells/mm2) was greater in HFpEF versus controls (60.4 [36.8–97.8] v. 32.1 [22.3–59.2], p=0.02), and correlated with age and renal disease. Cardiac amyloidosis (CA) was diagnosed in 15 (14%) patients (HFpEF-CA: 7 wild-type transthyretin [ATTR], 4 hereditary ATTR, 3 AL, and 1 AA), of which 7 cases were unsuspected. HFpEF-CA patients were older, with lower BMI, higher LV mass index, and higher NTproBNP and troponin I.
Conclusions:
In this large, prospective myocardial tissue analysis of HFpEF, myocardial fibrosis and hypertrophy were common, CD68+ inflammation was increased, and CA prevalence was 14%. Tissue analysis in HFpEF may improve precision therapies by identifying relevant myocardial mechanisms.
Keywords: HFpEF, inflammation, fibrosis, hypertrophy, amyloidosis, biopsy
Introduction
An estimated 6.5 million American adults suffer from heart failure (1), roughly half of whom have heart failure with preserved ejection fraction (HFpEF) (2). HFpEF represents a heterogeneous population with multiple co-morbidities, portends a poor prognosis following heart failure hospitalization (2–5), and generally lacks effective treatments (6). While clinical phenotyping of HFpEF has been proposed to better target therapies (7,8), this approach is limited by significant overlap in co-morbidities. Over the past 10–15 years, metabolic co-morbidities including obesity and diabetes have become more common than hypertensive heart disease or ischemic heart disease phenotypes in HFpEF, yet tissue assessment in this predominantly metabolic phenotype of HFpEF is lacking (9).
The diagnostic yield of endomyocardial biopsy in establishing an underlying etiology of unexplained dilated cardiomyopathy ranges between 15–37% of patients, with biopsy changing the diagnosis in about one third (10,11). Its application to HFpEF remains limited. Studies to date on myocardial histopathology in HFpEF have mostly originated from autopsy studies (12), HFpEF patients undergoing coronary artery bypass surgery (13,14), or studies of small numbers of patients often with marked systolic hypertension (15–17).
We aimed to assess myocardial tissue histopathology in a large, prospectively enrolled HFpEF cohort with comorbidities representative of contemporary HFpEF (4,18,19). We examined the extent of histopathologic abnormalities to better identify underlying cardiac pathophysiologies in this syndrome.
Methods
Study Population
A total of 108 of 222 patients referred to the Johns Hopkins University HFpEF clinic from August 2014 to September 2018 meeting inclusion/exclusion criteria underwent right heart catheterization and endomyocardial biopsy for etiology assessment. Informed consent was obtained, and the study was approved and overseen by the Johns Hopkins Institutional Review Board. The diagnosis of HFpEF was based on signs and symptoms of clinical HF using Framingham criteria for HF (20), left ventricular ejection fraction (LVEF) ≥ 50% by echocardiography within the prior 12 months, and at least two of the following: 1) structural heart disease (increased left ventricular [LV] wall thickness or left atrial [LA] diameter) or diastolic dysfunction on echocardiography (21); 2) N-terminal pro–B-type natriuretic peptide (NTproBNP) ≥ 100 pg/mL; or 3) hemodynamic evidence of elevated left sided filling pressures (pulmonary artery wedge pressure [PAWP] ≥ 15 mmHg at baseline; or ≥ 25 mmHg with exercise). Exclusion criteria included prior history of reduced LVEF (< 40%), severe valvular disease, known infiltrative or restrictive cardiomyopathy, hypertrophic cardiomyopathy, congenital heart disease, constrictive pericarditis, isolated pulmonary arterial hypertension, or history of heart transplantation.
Demographic and Clinical Characteristics
Detailed history, physical examination, and laboratory assessment were made at the time of initial evaluation. Echocardiographic data closest to this time was utilized. Coronary artery disease was defined as prior myocardial infarction, ≥ 50% stenosis on coronary angiography, or prior coronary revascularization. Obstructive sleep apnea was diagnosed from chart review and documented sleep study, and atrial fibrillation from chart review and electrocardiography. Glomerular filtration rate (GFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation (22). Abnormal serum free kappa/lambda light chain ratio was defined as <0.26 or >1.65.
Echocardiography and Strain Analysis
Comprehensive clinical 2D/Doppler echocardiograms obtained within 12 months prior to enrollment were analyzed (GE Vivid e95, General Electrics, Boston, MA; or Philips iE33, Philips Healthcare, North Ryde, New South Wales, Australia). Patients also underwent limited 2D echocardiography at the time of endomyocardial biopsy, and speckle-tracking strain analysis performed in the apical 4-chamber view (TomTec 2D Cardiac Performance Analysis v.1.2.3.6; Unterschleissheim, Germany). In patients with technically adequate strain images (n=73), peak longitudinal strain for 6 segments of the LV in the apical 4-chamber view was measured and averaged to yield LV longitudinal strain. RV free wall strain was derived by averaging peak longitudinal strain of basal, mid, and apical RV free-wall segments. Relative apical strain was defined as the average of the peak LV longitudinal strain from the 2 apical segments divided by the average of the 4 remaining mid and basal segments (23). The difference between average apical and average basal or mid longitudinal strain was also calculated.
Right Heart Catheterization and Endomyocardial Biopsy
Right heart catheterization and endomyocardial biopsy were performed in the supine position using standard Seldinger technique with echocardiographic and hemodynamic guidance. Biopsies were obtained from the RV septum using a Jawz Endomyocardial Bioptome (Argon Medical, Frisco, TX), and 4 pieces were sent for clinical histology. A thermodilution-equipped, fluid-filled pulmonary artery catheter (Edward Lifesciences Corp, Irvine, CA) was advanced, and right atrial, RV, pulmonary artery (PA), and PAWP pressures were measured at end-expiration with a properly zeroed transducer. Pressure recordings were analyzed off-line at a 50 mm/s paper speed with adjustment of pressure (mmHg) scale as needed. Cardiac output was determined by thermodilution technique from a mean of three consecutive measurements with less than 10% variance.
Clinical Histology
Endomyocardial biopsies were fixed in 10% buffered formalin, analyzed by the Johns Hopkins anatomic pathology laboratory and stained with hematoxylin and eosin, Masson’s trichrome, iron, Congo Red, and CD68. Fibrosis (Masson’s trichrome) and myocyte hypertrophy and were graded using a qualitative scale (mild, moderate, severe) by a cardiovascular pathologist (C.S. or M.K.H.). Cardiac amyloidosis (HFpEF-CA) was based on Congo Red staining with at least moderate interstitial infiltration of amyloid fibrils. Follow-up mass spectrometry was performed at the Mayo Clinic (Rochester, MN) to determine amyloid type, and confirmatory genetic testing was performed for transthyretin cardiac amyloidosis (ATTR).
Quantitative analysis of fibrosis and CD68+ cell number was performed using HALO (Area Quantification FL algorithm, Indica Lab, Albuquerque, NM) using slides digitized at 20X, and areas of endocardial fibrosis, thrombus, or adipose cells were excluded. Percent interstitial fibrosis was quantified in Masson’s trichrome stained slides, with 3 settings due to color variation, excluding HFpEF-CA due to overlap in color between collagen and amyloid. CD68+ monocytes were quantified from immunohistochemical staining, and pixel area of a representative cell was used to determine CD68+ cell number, normalized to tissue area. Myocardial fibrosis and CD68+ analysis employed myocardial tissue from non-failing, explanted, unused donor hearts as controls and explanted hearts prior to cardiac transplantation as HF with reduced ejection fraction (HFrEF), provided by the University of Pennsylvania through an IRB-approved protocol.
Statistical Analysis
Data are reported as median (25th-75th percentile) for continuous variables and numbers (proportions) for categorical variables. Quantitative histologic findings of % fibrosis and CD68+ cell count in non-amyloid HFpEF were compared to controls and HFrEF, respectively, and to HFpEF-CA for CD68+ cell count only, using Wilcoxon tests. Differences in baseline characteristics, echocardiographic and hemodynamic parameters between non-amyloid HFpEF and HFpEF-CA were compared using Wilcoxon tests for continuous variables and Fisher’s exact tests for categorical variables. Multivariable linear regression analyses were used to determine associations between clinical variables and natural log transformed values for % fibrosis and CD68+ cells, respectively. Multivariable logistic regression was used to test associations between clinical variables and myocyte hypertrophy and cardiac amyloidosis. Covariates included age, sex, and race/ethnicity. Regression diagnostics including residual plots, influence, and leverage were run for each model. A p-value <0.05 was considered statistically significant. Statistical analyses were performed with Statistical Analysis Software (Version 9.4 SAS Institute Inc, Cary, NC) and STATA (version 15.1, StataCorp LLC, College Station, TX).
Results
HFpEF Demographics and Clinical Characteristics
The median age of the HFpEF cohort was 66 (57–74) years, 61% were women, 57% were self-reported African American, 93% had hypertension, 54% diabetes, and the median BMI was 36.9 (31.5–45.1) kg/m2 (Table 1). Clinical HF was reflected by: 99% with NYHA class II or more, 97% on a loop diuretic, 62% admitted for HF in the preceding 12 months, and median NTproBNP of 450 (111–1433) pg/mL. Echocardiographic findings (Table 2) were consistent with HFpEF, including median LVEF 65 (60–70) %, interventricular septal wall thickness 1.3 (1.1–1.5) cm, LA diameter 4.2 (3.6–4.6) cm, and E/e’ 16.8 (11.0–21.4). Invasive hemodynamic testing was consistent with HFpEF physiology (Table 2) including median right atrial pressure 10 (6–13) mmHg, PA mean pressure 29 (23–35) mmHg, PAWP 18 (14–22) mmHg, with a median cardiac index of 2.4 (2.0–2.9) L/min/m2. The overall procedural complication rate for endomyocardial biopsies performed at Johns Hopkins over the study enrollment period was 0.17% for sustained arrhythmia events (requiring pharmacologic or electric cardioversion) and 0.06% for pericardial effusion requiring pericardiocentesis (total n=3,495).
Table 1.
Demographic and clinical characteristics of HFpEF stratified by presence or absence of cardiac amyloidosis.
| All patients (n=108) | Non-amyloid HFpEF (n=93) | HFpEF-CA (n=15) | P value | |
|---|---|---|---|---|
| Age (years) | 66 (57–74) | 65 (56–72) | 74 (68–79) | 0.001 |
| Sex, n (%) | 0.09 | |||
| Female | 66 (61.0) | 60 (64.5) | 6 (40.0) | |
| Race, n (%) | 0.23 | |||
| African American | 62 (57.4) | 56 (60.2) | 6 (40.0) | |
| Caucasian | 43 (39.8) | 34 (36.6) | 9 (60.0) | |
| Other | 3 (2.8) | 3 (3.2) | 0 (0) | |
| Hospitalized for Heart Failure in the last 12 months, n (%) | 67 (62.0) | 61 (65.6) | 6 (40.0) | 0.08 |
| NYHA Class, n (%) | 0.14 | |||
| I | 1 (0.9) | 1 (1.1) | 0 (0) | |
| II | 36 (33.6) | 28 (30.4) | 8 (53.3) | |
| III | 67 (62.6) | 61 (66.3) | 6 (40.0) | |
| IV | 3 (2.8) | 2 (2.2) | 1 (6.7) | |
| Systolic BP (mmHg) | 141 (125–162) | 141 (128–163) | 125 (111–139) | 0.01 |
| Diastolic BP (mmHg) | 70 (65–79) | 72 (65–79) | 69 (66–77) | 0.81 |
| Heart Rate (bpm) | 78 (68–88) | 77 (66–87) | 80 (70–92) | 0.16 |
| BMI (kg/m2) | 36.9 (31.5–45.1) | 37.6 (33.2–45.6) | 29.4 (25.1–34.2) | 0.0001 |
| Past Medical History, n (%) | ||||
| Hypertension | 100 (92.6) | 91 (97.8) | 9 (60.0) | <0.0001 |
| Diabetes mellitus | 58 (53.7) | 54 (58.1) | 4 (26.7) | 0.03 |
| Obstructive CAD | 15 (13.9) | 13 (14.0) | 2 (13.3) | 1.00 |
| Atrial fibrillation or flutter | 34 (31.5) | 28 (30.1) | 6 (40.0) | 0.55 |
| Obstructive sleep apnea | 46 (42.6) | 44 (47.3) | 2 (13.3) | 0.02 |
| COPD or asthma | 34 (31.5) | 33 (35.5) | 1 (6.7) | 0.03 |
| Medications, n (%) | ||||
| ACE inhibitor | 31 (28.7) | 30 (32.3) | 1 (6.7) | 0.06 |
| ARB | 33 (30.6) | 29 (31.2) | 4 (26.7) | 1.00 |
| Beta-blocker | 65 (60.2) | 57 (61.3) | 8 (53.3) | 0.58 |
| Aldosterone antagonist | 31 (28.7) | 30 (32.3) | 1 (6.7) | 0.06 |
| Loop Diuretic | 105 (97.2) | 90 (96.8) | 15 (100.0) | 1.00 |
| Insulin | 33 (30.6) | 32 (34.4) | 1 (6.7) | 0.03 |
| Laboratory Studies | ||||
| GFR (CKD-EPI), ml/min/1.73m2 | 49 (34–70) | 49 (34–70) | 55 (35–68) | 0.79 |
| NTproBNP (pg/mL) | 450 (111–1433) | 353 (100–946) | 3245 (1012–4542) | 0.0002 |
| Troponin I (ng/mL, n=83 HFpEF, n=12 HFpEF-CA) | 0 (0–0) | 0 (0–0) | 0.09 (0–0.12) | <0.0001 |
| Troponin I ≥0.04ng/mL (n=83 HFpEF, n=12 HFpEF-CA), n (%) | 18 (18.95) | 10 (12.05) | 8 (66.67) | 0.0001 |
| Abnormal K/L ratio (≤0.26 OR ≥1.65), n (%) | 35 (34.3) | 28 (32.2) | 7 (46.7) | 0.38 |
| 6 minute walk distance (meters, n=55 HFpEF n=6 HFpEF-CA), n (%) | 204 (119–348) | 203 (118–349) | 229 (119–289) | 0.91 |
Data are reported as median (25th–75th percentile), analyzed by Wilcoxon test, or n (%), analyzed by Fisher’s exact test. p<0.05 vs non-amyloid HFpEF (in boldface). ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; BP, blood pressure; BMI, body mass index; CAD, coronary artery disease; CKD-EPI, Chronic Kidney Disease Epidemiology collaboration; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFpEF-CA, HFpEF-cardiac amyloidosis; JVP, jugular venous pressure; K/L ratio, Kappa/Lambda free light chain ratio; NYHA, New York Heart Association symptom class; NTproBNP, N-terminal pro-brain natriuretic peptide.
Table 2.
Echocardiographic and invasive hemodynamics parameters in HFpEF stratified by presence or absence of cardiac amyloidosis.
| All patients (n=108) | Non-amyloid HFpEF (n=93) | HFpEF-CA (n=15) | P value | |
|---|---|---|---|---|
| Echocardiography | ||||
| LV ejection fraction, % | 65 (60–70) | 65 (60–70) | 60 (60–65) | 0.18 |
| LV end diastolic diameter, cm | 4.5 (4.1–5.0) | 4.6 (4.2–5.0) | 4.2 (3.8–4.9) | 0.07 |
| Interventricular Septum thickness, cm | 1.3 (1.1–1.5) | 1.3 (1.0–1.5) | 1.4 (1.3–2.0) | 0.003 |
| LV posterior wall thickness, cm | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.3 (1.2–1.7) | 0.002 |
| LA diameter, cm | 4.2 (3.6–4.6) | 4.2 (3.6–4.6) | 4.2 (3.8–4.7) | 0.77 |
| LV Mass, g | 206 (157–261) | 199 (155–248) | 240 (201–342) | 0.02 |
| Sex-adjusted LV Mass Index, g/m2 | 110 (91–134) | 105 (90–126) | 133 (104–167) | 0.003 |
| Tricuspid Regurgitant Peak Velocity, cm/s (n=43 HFpEF, n=12 HFpEF-CA) | 276 (252–308) | 276 (245–316) | 273 (254–290) | 0.50 |
| Tissue Doppler e’, cm/s (n=47 HFpEF, n=7 HFpEF-CA) | 5.7 (4.6–8.0) | 6.0 (5.0–8.4) | 4.0 (3.3–5.6) | 0.01 |
| E/e’ (n=53 HFpEF, n=7 HFpEF-CA) | 16.8 (11.0–21.4) | 16.8 (10.1–22.0) | 20.9 (14.3–21.4) | 0.50 |
| E/A (n=74 HFpEF, n=9 HFpEF-CA) | 1.1 (0.8–1.7) | 1.1 (0.8–1.7) | 1.4 (0.8–2.1) | 0.38 |
| Strain (n=64 HFpEF, n=9 HFpEF-CA unless otherwise specified) | ||||
| Global longitudinal % strain, LV (%) | 13.9 (11.5–17.4) | 14.6 (11.7–17.4) | 9.3 (7.1–13.8) | 0.03 |
| Average basal longitudinal strain, LV (%) | 13.9 (9.8–16.3) | 14.5 (11.3–16.9) | 9.2 (7.7–12.6) | 0.009 |
| Average mid longitudinal strain, LV (%) | 12.9 (9.2–15.7) | 13.3 (9.9–15.9) | 7.5 (4.5–13.7) | 0.02 |
| Average apical longitudinal strain, LV (%) | 14.1 (10.1–17.3) | 14.2 (10.2–17.4) | 10.5 (9.5–16.5) | 0.22 |
| Relative apical longitudinal strain, LV (%) | 0.54 (0.35–0.76) | 0.54 (0.35–0.75) | 0.61 (0.46–0.92) | 0.28 |
| Difference in apical vs. basal longitudinal strain, LV (%) | 0.2 (−5.3–5.4) | −0.3 (−5.7–6.1) | 1.1 (0.3–5.4) | 0.43 |
| Difference in apical vs. mid longitudinal strain, LV (%) | 1.5 (−2.7–6.6) | 1.4 (−3.0–6.4) | 3.2 (−2.0–8.1) | 0.60 |
| Relative apical longitudinal strain > 1, LV, n (%) | 5 (6.9) | 3 (4.7) | 2 (22.2) | 0.11 |
| Global longitudinal strain, RV (%, n=46 HFpEF, n=7 HFpEF-CA) | 16.9 (11.9–18.9) | 17.1 (13.1–19.1) | 11.9 (6.9–17.9) | 0.06 |
| Hemodynamics | ||||
| Systolic blood pressure, mmHg | 151 (131–167) | 153 (140–171) | 122 (114–141) | 0.0003 |
| Diastolic blood pressure, mmHg | 76 (70–85) | 76 (71–86) | 72 (67–82) | 0.10 |
| Heart rate, bpm | 75 (65–87) | 75 (65–86) | 82 (74–92) | 0.03 |
| RA pressure, mmHg | 10 (6–13) | 10 (6–13) | 10 (6–11) | 0.73 |
| PA systolic pressure, mmHg | 44 (33–54) | 45 (33–54) | 40 (37–53) | 0.85 |
| PA diastolic pressure, mmHg | 20 (16–25) | 20 (17–25) | 20 (16–24) | 0.76 |
| PA mean, mmHg | 29 (23–35) | 29 (23–35) | 29 (24–34) | 0.94 |
| PAWP, mmHg | 18 (14–22) | 19 (15–22) | 18 (13–22) | 0.71 |
| PA saturation, % | 67.7 (62.6–71.5) | 67.9 (63.6–72.1) | 66.5 (60.9–69.6) | 0.10 |
| Cardiac output, Liters/min | 5.57 (4.38–6.47) | 5.74 (4.49–6.63) | 4.13 (3.30–4.80) | 0.0006 |
| Cardiac index, Liters/min/m2 | 2.43 (2.03–2.92) | 2.48 (2.13–3.00) | 2.19 (1.69–2.57) | 0.02 |
| Systemic vascular resistance index, dynes-sec/cm−5/m2 | 2994 (2387–3519) | 2972 (2386–3499) | 3126 (2537–4188) | 0.26 |
| Pulmonary vascular resistance, Wood units | 1.91 (1.21–2.76) | 1.82 (1.21–2.66) | 2.61 (2.01–4.15) | 0.02 |
| RV stroke work index, g/m2/beat | 8.2 (5.5–10.9) | 8.8 (6.0–12.0) | 5.5 (4.5–8.9) | 0.046 |
| Transpulmonary gradient, mmHg | 10 (7–13) | 10 (7–14) | 11 (8–13) | 0.59 |
| Diastolic pulmonary gradient, mmHg | 2 (0–5) | 2 (0–5) | 2 (1–4) | 0.50 |
| RA/PCWP ratio | 0.54 (0.43–0.63) | 0.55 (0.42–0.63) | 0.50 (0.43–0.65) | 0.68 |
Data are reported as median (25th–75th percentile), analyzed by Wilcoxon test, or n (%), analyzed by Fisher’s exact test. Strain data are reported as absolute values. p<0.05 vs non-amyloid HFpEF (in boldface). E/A, mitral inflow E wave velocity divided by A wave velocity; E/e’, mitral inflow E wave velocity divided by tissue Doppler e’ velocity; LV, left ventricle; LA, left atrium; PA, pulmonary artery; PAWP, pulmonary artery wedge pressure; RV, right ventricle.
Clinical characteristics of controls (n=13) and HFrEF (n=20) are summarized in Supplemental Table 1, including median age 53 (27–58) years, 46% female, 15% African American, with median BMI of 26.4 (21.9–29.6) kg/m2, and overall normal renal function for controls.
Histologic Analysis of HFpEF
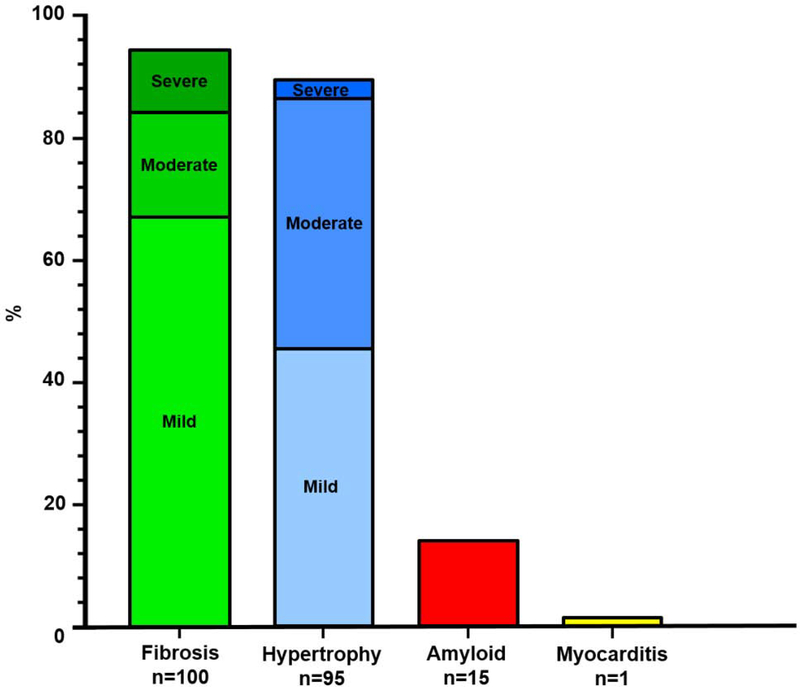
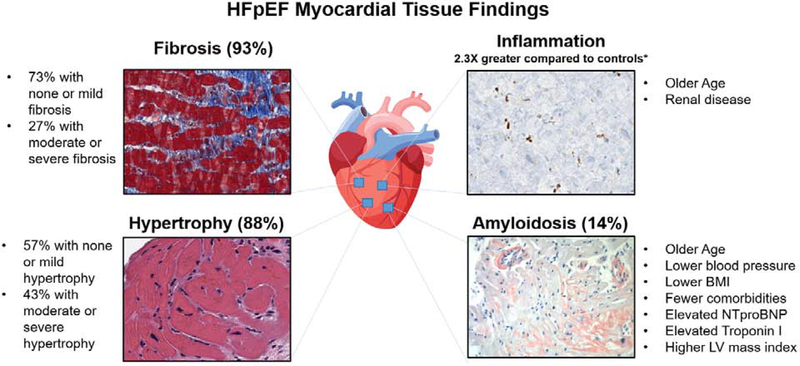
Histopathology revealed (Figure 1): 93% with myocardial fibrosis (7% none, 66% mild or patchy, 17% moderate, 10% severe), 88% with myocyte hypertrophy (12% none, 45% mild, 40% moderate, 3% severe), 14% with cardiac amyloidosis, and one with borderline myocarditis (0.9%). Figure 2 displays representative images.
Figure 1. Frequency of Histologic Findings on Endomyocardial Biopsy in 108 HFpEF Patients.
Qualitative grading of myocardial fibrosis (Masson’s trichrome) and hypertrophy (Hematoxylin & Eosin) by a cardiovascular pathologist. Cardiac amyloidosis (Congo Red stain) was diagnosed in patients with at least moderate infiltration with amyloid fibrils. HFpEF, heart failure with preserved ejection fraction.
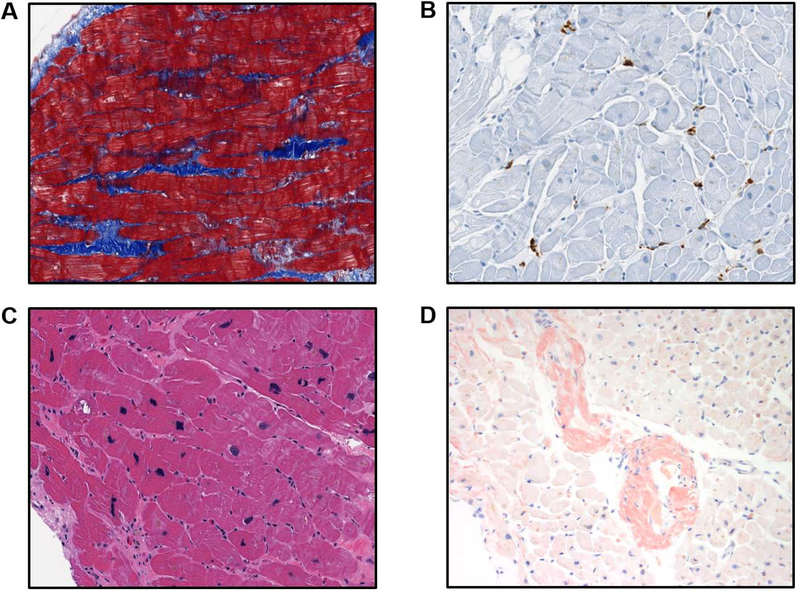
Figure 2. Representative Histologic Findings on Endomyocardial Biopsies in HFpEF.
A) Interstitial fibrosis (blue) on Masson’s Trichrome stain. B) CD68+ cells (brown) on immunohistochemistry. C) Myocyte hypertrophy on Hematoxylin & Eosin stain. D) Cardiac amyloidosis on Congo Red stain. All images captured at 20x magnification.
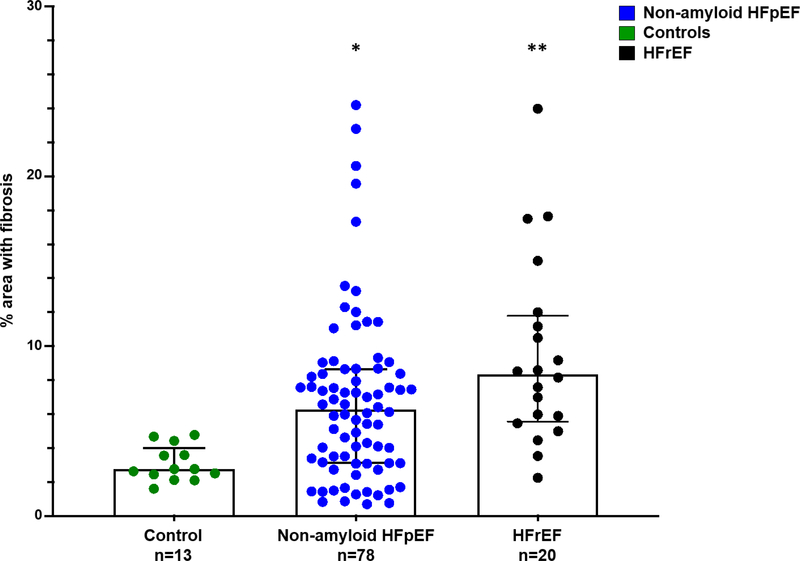
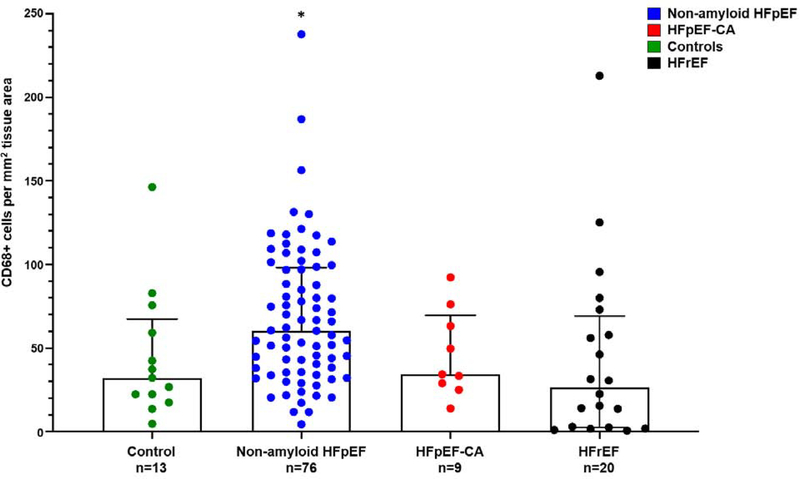
Quantitative histopathology data are provided in Table 3. Non-amyloid HFpEF had more fibrosis compare to controls (6.3% [3.1–8.6] v. 2.8% [2.5–3.6]; p=0.003); but less fibrosis compared to HFrEF (8.3% [5.7–11.6]; p=0.03, Figure 3). Non-amyloid HFpEF had more CD68+ cells per mm2 compared to controls (60.4 cells per mm2 [36.8–97.8] vs 32.1 cells per mm2 [22.3–59.2] cells/mm2; p=0.02) and HFrEF (26.5 cells per mm2 [2.7–65.3]; p=0.003, Figure 4).
Table 3.
Quantitative Histopathologic Results for Myocardial Fibrosis and Inflammation in HFpEF
| Control (n=13) | Non-amyloid HFpEF (n=78) | P value* | HFpEF-CA (n=9) | P value** | HFrEF (n=20) | P value*** | |
|---|---|---|---|---|---|---|---|
| % area with fibrosis | 2.77 (2.45–3.59) | 6.27 (3.12–8.64) | 0.003 | - | - | 8.33 (5.68–11.58) | 0.03 |
| ln (% area with fibrosis) | 1.02 (0.89–1.28) | 1.83 (1.14–2.16) | - | 2.12 (1.74–2.45 | |||
| CD68+ cells per mm2 | 32.1 (22.3–59.2) | 60.4 (36.8–97.8) | 0.02 | 34.3 (29.0–63.1) | 0.09 | 26.5 (2.68–65.3) | 0.003 |
| ln (CD68+ cells per mm2) | 3.47 (3.10–4.08) | 4.10 (3.60–4.58) | 3.53 (3.37–4.15) | 3.27 (0.98–4.17) |
Data are reported as median (25th–75th percentile), analyzed by Wilcoxon test.
Non-amyloid HFpEF vs Control
Non-amyloid HFpEF vs HFpEF-CA
HFrEF vs HFpEF.
HFpEF = Heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis, HFrEF = Heart failure with reduced ejection fraction.
Figure 3. Percent Fibrosis Higher in HFpEF v. Control Tissue, but Lower than HFrEF.
Percent area with fibrosis was analyzed in the Masson’s trichrome stained slides using image analysis platform HALO and slides digitized at 20X. *Non-amyloid HFpEF vs control, p=0.003; **non-amyloid HFpEF vs HFrEF, p=0.03; Wilcoxon test. HFpEF = Heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis, HFrEF = Heart failure with reduced ejection fraction.
Figure 4. CD68+ inflammation greater in HFpEF v. control tissue and HFrEF.
CD68+ inflammation was analyzed using image analysis platform HALO and slides digitized at 20X. CD68+ cell number was calculated as number of cells per tissue area in non-amyloid HFpEF, HFpEF-CA, control, and HFrEF tissue. *Non-amyloid HFpEF vs control, p=0.02; non-amyloid HFpEF vs HFpEF-CA, p=0.09; non-amyloid HFpEF vs HFrEF, p=0.0033, Wilcoxon test. HFpEF = Heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis, HFrEF = Heart failure with reduced ejection fraction.
Clinical correlates of myocardial fibrosis, inflammation, and hypertrophy in HFpEF
In multivariable regression analysis of clinical correlates with histopathologic findings, myocardial fibrosis showed a trend to be greater in African Americans and in patients with higher systolic blood pressure, however not statistically significant, Supplemental Table 2. CD68+ infiltration was associated with increased age (β 1.38, 95% CI [1.02–1.87]; p=0.04) and lower GFR (β 0.99, 95% CI [0.99–1.00]; p=0.02), Supplemental Table 3. Moderate or severe hypertrophy was inversely associated with troponin I ≥ 0.04 ng/mL (OR 0.24, 95% CI [0.06–0.9]; p=0.045), Supplemental Table 4. This result was driven by the HFpEF-CA patients, none of whom had moderate or severe hypertrophy.
Diagnostic Evaluation of HFpEF-CA
Of the 15 patients diagnosed with HFpEF-CA, 11 had ATTR cardiomyopathy (7 wildtype, 4 mutation positive: 1 Val122Ile, 1 both Val122Ile and Phe44Leu [presumed to be in trans], 2 Leu58His), 3 had amyloidogenic light (AL) chain amyloidosis, and 1 had AA amyloidosis (Table 4). One patient had only mild interstitial infiltration of the myocardium with amyloid and was categorized as non-amyloid HFpEF. At the time of referral, 8 patients (2 AL, 6 ATTR) were suspected to have cardiac amyloidosis based on the following: 2 with unexplained left ventricular hypertrophy, 2 with infiltrative cardiomyopathy on cardiac MRI, 3 with history of amyloid neuropathy, and 1 with very elevated serum free kappa/lambda ratio. Of the 7 unsuspected cases, 1 patient had AL amyloidosis, 1 patient had AA amyloidosis, and 4 patients had ATTR amyloidosis. Two patients with biopsy-confirmed ATTR cardiomyopathy had a prior 99m-technetium pyrophosphate (99mTc-PYP) scan: one positive despite a negative nerve biopsy for amyloid, the other negative despite a history of amyloid neuropathy. Another patient with IgG multiple myeloma was diagnosed with concomitant wild-type ATTR cardiac amyloidosis by biopsy. Of note, 7 of the 15 cases would not have been suspected to have cardiac amyloidosis based on recently published screening guidelines (24).
Table 4.
Summary of cardiac amyloidosis types diagnosed by endomyocardial biopsy from a HFpEF cohort, n=108.
| Type of Cardiac Amyloidosis (n=15) | n (% of HFpEF-CA) |
|---|---|
| Transthyretin amyloidosis (ATTR) | 11 (73.3) |
| Wild type | 7 (46.7) |
| Mutant | 4 (26.7) |
| Val122Ile | 1 (6.7) |
| Leu58His | 2 (13.3) |
| Val122Ile and Phe44Leu (presumed to be in trans) | 1 (6.7) |
| Light chain amyloidosis | 3 (20.0) |
| Serum amyloid A type | 1 (6.7) |
ATTR = Transthyretin amyloidosis; HFpEF = Heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis.
Non-amyloid HFpEF versus HFpEF-CA
Compared to non-amyloid HFpEF, HFpEF-CA patients were older, had lower blood pressures, lower BMI, and less burden of comorbidities such as hypertension, diabetes, obstructive sleep apnea, and obstructive lung disease (Table 1). HFpEF-CA patients had significantly higher NTproBNP (Figure 5A), and higher troponin I compared to non-amyloid HFpEF (Figure 5B). HFpEF-CA had greater wall thickness and LVMI (adjusted for sex), and reduced tissue Doppler e’ velocity compared to non-amyloid HFpEF (Table 2). Global LV longitudinal strain was lower in HFpEF-CA; however, other measures of diastolic function, LV and LA chamber dimensions, and several measures of apical sparing did not differentiate HFpEF-CA from non-amyloid HFpEF (Table 2). Cardiac output and RV stroke work index were lower and pulmonary vascular resistance was higher in HFpEF-CA (Table 2).
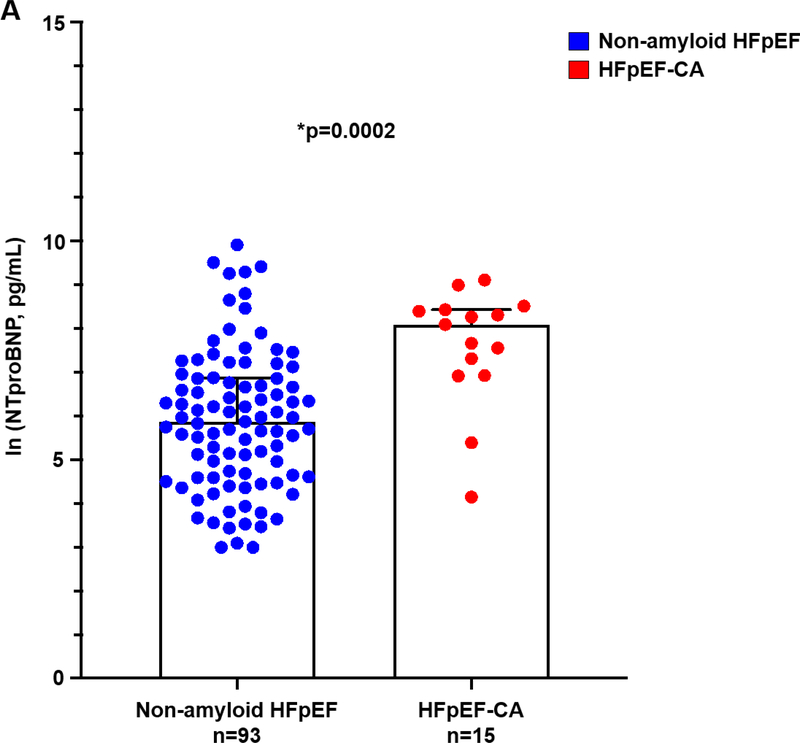
Figure 5A. Serum NTproBNP higher in HFpEF-CA v. non-amyloid HFpEF.
Serum N-terminal pro-B-type natriuretic peptide was significantly higher in HFpEF-CA compared to NTproBNP. Natural log transformed values are shown. *p=0.0002, Wilcoxon test. HFpEF = heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis. NTproBNP = N-terminal pro-B-type natriuretic peptide.
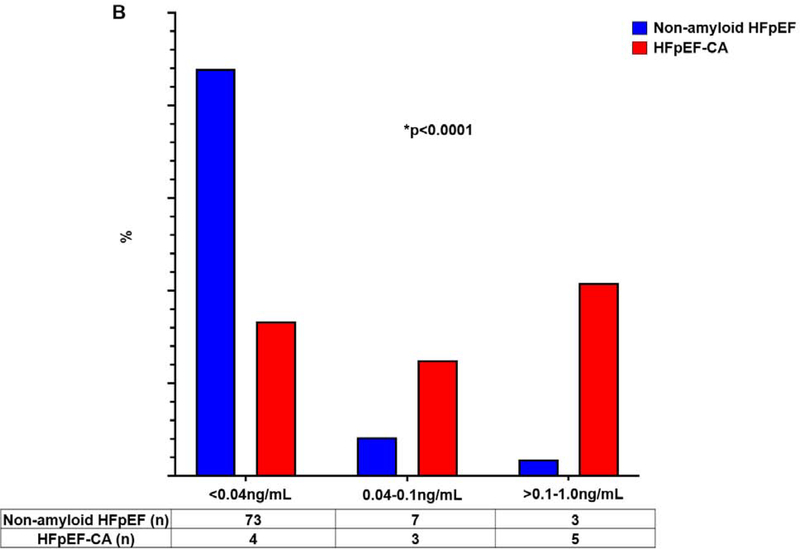
Figure 5B. Troponin I elevated in HFpEF-CA v Non-Amyloid HFpEF.
Frequency of patients within each range of troponin I. *p<0.0001, Fisher’s exact test. HFpEF = Heart failure with preserved ejection fraction; HFpEF-CA = HFpEF-cardiac amyloidosis.
Multivariable logistic regression analysis of clinical correlates of cardiac amyloidosis (Table 5), revealed age ≥ median age of 66 years (OR 4.58 [1.17–17.96], p=0.03), LVMI (OR 1.03 [1.01–1.06] per g/m2, p=0.001), ln (NTproBNP) (OR 1.93 [1.24–2.99], p=0.003) and troponin I ≥ 0.04 ng/mL (OR 17.26 [3.72–80.10], p=0.0003) were positively associated with HFpEF-CA. BMI was inversely associated with HFpEF-CA (OR 0.85 [0.77–0.94], p=0.002).
Table 5.
Multivariable logistic regression of correlates of HFpEF-CA
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Age ≥ median (66 years) | 4.58 | 1.17–17.96 | 0.03 |
| Female sex | 0.43 | 0.13–1.43 | 0.17 |
| Black or African-American race | 0.78 | 0.23–2.68 | 0.69 |
| Systolic blood pressure (per mmHg) | 0.97 | 0.94–1.00 | 0.07 |
| Body mass index (per kg/m2) | 0.85 | 0.77–0.94 | 0.002 |
| Diabetes mellitus | 0.32 | 0.09–1.16 | 0.08 |
| Glomerular filtration rate (per mL/min/1.73m2) | 1.00 | 0.98–1.03 | 0.70 |
| Coronary artery disease | 0.54 | 0.10–2.97 | 0.48 |
| LV mass index (per g/m2) | 1.03 | 1.01–1.06 | 0.001 |
| ln(NTproBNP in pg/mL) | 1.93 | 1.24–2.99 | 0.003 |
| Troponin I ≥0.04ng/mL | 17.26 | 3.72–80.10 | 0.0003 |
Age, sex, and race were each adjusted for the other two variables. Each subsequent variable was adjusted for age, sex, and race in a separate model. CI, Confidence Interval. HFpEF-CA, heart failure with preserved ejection fraction-cardiac amyloidosis; LV, left ventricle; NTproBNP, N terminal pro-B type natriuretic peptide.
Discussion
In the largest prospective evaluation of myocardial tissue in HFpEF patients to date, we found myocardial fibrosis and myocyte hypertrophy to be highly prevalent, however relatively mild in the majority of cases. CD68+ monocyte infiltration correlated with advanced age and renal disease in HFpEF. Importantly, we report myocardial biopsy-proven CA prevalence of 14%, with CA often unsuspected. This has major implications, particularly for ATTR disease, given recent advances in treatments (25). Our HFpEF cohort reflects the demographic and comorbidity burden that characterizes HFpEF today: female predominance, African American representation, and a substantial burden of comorbidities including severe obesity. Importantly, the HFpEF patients enrolled in our study had a high burden of HF, with >60% having been previously hospitalized for HF hospitalization, >95% on loop-diuretic therapy, and elevated NTproBNP levels despite obesity.
To place the current histological data set into perspective, in total there have been 3 studies of myocardial tissue assessment in a total of 49 living patients (excluding autopsy studies, those with history of heart transplantation, or those undergoing coronary artery bypass surgery) characterized by patients with predominantly hypertensive heart disease referred for evaluation of etiology of HFpEF (15–17). Additional myocardial studies in HFpEF stem from patients referred for coronary artery bypass surgery (14), representing a less common but important ischemic phenotype of HFpEF, and an autopsy report in HFpEF by Mohammed et al. in which HFpEF subjects had lower microvascular density and greater fibrosis than matched controls.(26)
HFpEF Myocardial Fibrosis and Inflammatory Cell Infiltration
Though prior studies have reported on myocardial fibrosis in HFpEF, tissue obtained at time of coronary artery bypass surgery or at autopsy could influence the extent of fibrosis detected (14–17). We found myocardial fibrosis to be highly prevalent in HFpEF; however, the presence of moderate-severe fibrosis was <30% of subjects. Furthermore, our comparison to HFrEF suggests that fibrosis is not unique to HFpEF. Therapies targeting fibrosis pathways may certainly benefit a subgroup of HFpEF in whom fibrosis is moderate or severe, however the potential benefit in those with mild or less fibrosis is unclear. Our cohort also had a substantial representation of African Americans in whom fibro-proliferative disorders have a higher prevalence; African Americans with HFpEF are younger with greater comorbidities including hypertension, diabetes, chronic kidney disease, and obesity, and this may impact racial differences in myocardial fibrosis (4,27,28).
CD68 is a pan-monocyte marker for macrophages and dendritic cells. In a study of 6 patients referred for bypass surgery, macrophage infiltration was found and suggested to contribute to diastolic dysfunction (13). We found increased CD68+ cells to be associated with increased age and renal dysfunction in HFpEF, which may have implications for age-related increased prevalence of HFpEF. Furthermore, chronic kidney disease has been associated with systemic inflammation (29), with macrophages playing a pathophysiological role (30). Anti-inflammatory therapies are an area of ongoing interest in HFpEF and warrant further study.
Cardiac Amyloidosis: A Distinct Phenotype of HFpEF
The reported prevalence of cardiac amyloidosis in HFpEF ranges from 5–13% (12,31). In an autopsy analysis of patients with HF history and LVEF > 40%, amyloid fibrils were found in 19%; however, only 4.6% had sufficient amyloid deposition to be clinically classified as CA (12). In another study using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), ATTR amyloidosis was diagnosed in 13.3% of HFpEF patients (31), though the cohort was very elderly (mean age 82 years), required the presence of LV hypertrophy, and no confirmatory endomyocardial biopsies were obtained. Importantly, 99mTc-DPD only identifies ATTR amyloid; AL amyloid requires a tissue diagnosis. Until recently, CA diagnosis portended a poor prognosis given the lack of targeted therapies, however new therapies particularly for ATTR that stabilize transthyretin or reduce its gene expression are rapidly changing this landscape (25,32). Survival for patients with AL amyloidosis is also improving, with early detection and advances in light-chain reducing therapies (33).
We report a 14% prevalence of CA, consistent with prior reports, however nearly half of our CA diagnoses were unsuspected or would have been missed by recently proposed screening criteria (24). We suspect CA is significantly underdiagnosed within HFpEF and recommend systematic evaluation for CA in the evaluation of HFpEF patients. Our findings suggest the following clinical clues should raise suspicion for CA in HFpEF: older age, lower blood pressure, lower BMI, increased LV wall thickness, and higher NTproBNP and troponin I levels, some of which have been proposed (31,34). Notably, apical sparing strain pattern did not differentiate non-amyloid HFpEF from HFpEF-CA in our study, thus a normal strain pattern may not rule out CA in a HFpEF patient. Finally, self-identified race was unassociated with HFpEF-CA, though the most common TTR mutation in the US (Val122Ile) is present in ~4% of African Americans (32). This likely is due to the high prevalence of non-amyloid HFpEF in African Americans in our population. While there is growing evidence to support non-invasive diagnostic testing as part of initial screening for CA in HFpEF, our study supports the use of endomyocardial biopsy in select cases where non-invasive testing is equivocal, or clinical suspicion remains high for CA. This overall strategy is supported by a recent expert consensus statement (35).
Study Limitations
Our study has several limitations. This is a single center study from a dedicated HFpEF clinic constituting HFpEF patients with numerous co-morbidities which may limit generalizability. Our control tissue came from unused donor hearts, and therefore myocardial abnormalities associated with the donor’s cause of death cannot be ruled out; however, normal donor heart tissue remains the gold standard for myocardial tissue studies. The clinical characteristics of the control cohort differed from our HFpEF cohort which may further limit comparisons. Furthermore, RV endomyocardial biopsy provides localized sampling of tissue which may limit assessment of non-homogenous processes in the myocardium. Because patients were specifically referred for diagnosis and evaluation of HFpEF, the prevalence of cardiac amyloidosis may be higher from a referral bias. Finally, our patients were not systematically referred for nuclear scans during the study enrollment period, and therefore we cannot definitively conclude whether those patients who were unsuspected for CA would have been identified by nuclear scan to have ATTR amyloidosis.
Conclusions
In this study of myocardial histopathology findings from a large-scale, prospectively enrolled HFpEF cohort, we found myocardial fibrosis and myocyte hypertrophy to be highly prevalent, however often mild in severity in HFpEF. Myocardial monocyte infiltration was greater in HFpEF compared to control tissue, and further studies are needed to clarify its contribution to disease pathobiology. We found HFpEF-CA prevalence to be 14%, with ATTR amyloidosis most commonly diagnosed, and often unsuspected. Given advances in non-invasive diagnostic testing and new therapies available, cardiac amyloidosis should be routinely considered and evaluated for as an etiology of HFpEF in patients with characteristic features. Further studies in tissue-based phenotyping in HFpEF may not only provide important insights into mechanisms of disease, but also potentially identify key treatable HFpEF phenotypes.
Supplementary Material
Central Illustration. Myocardial Histopathologic Findings and Prevalence in Heart Failure with Preserved Ejection Fraction and Associated Clinical Comorbidities.
Representative histologic images and frequency of findings from 108 HFpEF patients studied are shown for fibrosis (Masson’s trichrome), hypertrophy (Hematoxylin & Eosin), inflammation (CD68 immunohistochemistry), and cardiac amyloidosis (Congo Red stain). CD68+ inflammation was compared to control tissue in n=76 non-amyloid HFpEF patients. *p=0.02 for comparison between CD68+ staining cells in HFpEF v. controls. HFpEF, heart failure with preserved ejection fraction.
Clinical Perspectives.
Competency in Medical Knowledge:
In a contemporary HFpEF population, myocardial fibrosis, myocyte hypertrophy, and monocyte infiltration were common, however often mild in severity. Cardiac amyloidosis was prevalent in 14% of HFpEF and was most commonly ATTR amyloidosis. Older age, lower blood pressure, lower BMI, increased wall thickness, and higher NTproBNP and troponin I correlated with amyloidosis in HFpEF.
Translational Outlook:
Myocardial tissue analysis is rare in HFpEF. Data from a large HFpEF cohort reflecting the prominence of metabolic syndrome and morbid obesity as co-morbidities show myocardial abnormalities including fibrosis, hypertrophy, and inflammation are highly prevalent, however with substantial variance. Furthermore, the findings also highlight that cardiac amyloidosis is likely underdiagnosed in HFpEF, and efforts to assess this in HFpEF are warranted.
Acknowledgements:
We thank Dr. Kenneth Margulies and Kenneth Bedi at the Perelman School of Medicine at the University of Pennsylvania in Philadelphia, PA for providing the control and heart failure with reduced ejection fraction myocardial tissue.
Funding: This project was partly funded by an American Heart Association Go Red for Women Network Grant (AHA #16SFRN28780016; K.S), Dallas, TX.
Abbreviations list:
- BMI
body mass index
- GFR
glomerular filtration rate
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFpEF-CA
HFpEF-cardiac amyloidosis
- HFrEF
heart failure with reduced ejection fraction
- LA
left atrium
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- LVMI
left ventricular mass index
- NTproBNP
N-terminal pro-B-type natriuretic peptide
- NYHA
New York Heart Association
- PA
pulmonary artery
- PAWP
pulmonary artery wedge pressure
- RV
right ventricle
Footnotes
Disclosures: No relationships with industry relevant to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Zhao X, Heidenreich PA et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 4.Sharma K, Hill T, Grams M et al. Outcomes and Worsening Renal Function in Patients Hospitalized With Heart Failure With Preserved Ejection Fraction. The American journal of cardiology 2015;116:1534–40. [DOI] [PubMed] [Google Scholar]
- 5.Sharma K, Vaishnav J, Kalathiya R et al. Randomized Evaluation of Heart Failure With Preserved Ejection Fraction Patients With Acute Heart Failure and Dopamine: The ROPA-DOP Trial. JACC Heart failure 2018;6:859–870. [DOI] [PubMed] [Google Scholar]
- 6.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circulation research 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah SJ, Kitzman DW, Borlaug BA et al. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SJ, Katz DH, Selvaraj S et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parikh KS, Sharma K, Fiuzat M et al. Heart Failure With Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC Heart failure 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 10.Ardehali H, Qasim A, Cappola T et al. Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. American heart journal 2004;147:919–23. [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Thompson RE, Hare JM et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England journal of medicine 2000;342:1077–84. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed SF, Mirzoyev SA, Edwards WD et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart failure 2014;2:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulsmans M, Sager HB, Roh JD et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zile MR, Baicu CF, Ikonomidis JS et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borbely A, van der Velden J, Papp Z et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 2005;111:774–81. [DOI] [PubMed] [Google Scholar]
- 16.van Heerebeek L, Borbely A, Niessen HW et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 2006;113:1966–73. [DOI] [PubMed] [Google Scholar]
- 17.Westermann D, Lindner D, Kasner M et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circulation Heart failure 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 18.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. The New England journal of medicine 1971;285:1441–6. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phelan D, Collier P, Thavendiranathan P et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–8. [DOI] [PubMed] [Google Scholar]
- 24.Witteles RM, Bokhari S, Damy T et al. Screening for Transthyretin Amyloid Cardiomyopathy in Everyday Practice. JACC Heart failure 2019;7:709–716. [DOI] [PubMed] [Google Scholar]
- 25.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. Journal of the American College of Cardiology 2019;73:2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. . Circulation 2015;131:550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellwege JN, Torstenson ES, Russell SB, Edwards TL, Velez Edwards DR. Evidence of selection as a cause for racial disparities in fibroproliferative disease. PloS one 2017;12:e0182791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell SB, Smith JC, Huang M, Trupin JS, Williams SM. Pleiotropic Effects of Immune Responses Explain Variation in the Prevalence of Fibroproliferative Diseases. PLoS Genet 2015;11:e1005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin Dial 2002;15:329–37. [DOI] [PubMed] [Google Scholar]
- 30.Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int 2011;80:915–925. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. European heart journal 2015;36:2585–94. [DOI] [PubMed] [Google Scholar]
- 32.Lane T, Fontana M, Martinez-Naharro A et al. Natural History, Quality of Life, and Outcome in Cardiac Transthyretin Amyloidosis. Circulation 2019;140:16–26. [DOI] [PubMed] [Google Scholar]
- 33.Barrett CD, Dobos K, Liedtke M et al. A Changing Landscape of Mortality for Systemic Light Chain Amyloidosis. JACC Heart failure 2019;7:958–966. [DOI] [PubMed] [Google Scholar]
- 34.Ton VK, Bhonsale A, Gilotra NA et al. Baseline Characteristics Predict the Presence of Amyloid on Endomyocardial Biopsy. Journal of cardiac failure 2017;23:340–344. [DOI] [PubMed] [Google Scholar]
- 35.Maurer MS, Bokhari S, Damy T et al. Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circulation Heart failure 2019;12:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.