Abstract
Introduction
The Afghanistan war (2003–2014) was a unique period in military medicine. Many service personnel survived injuries of a severity that would have been fatal at any other time in history; the long-term health outcomes of such injuries are unknown. The ArmeD SerVices TrAuma and RehabilitatioN OutComE (ADVANCE) study aims to determine the long-term effects on both medical and psychosocial health of servicemen surviving this severe combat related trauma.
Methods and analysis
ADVANCE is a prospective cohort study. 1200 Afghanistan-deployed male UK military personnel and veterans will be recruited and will be studied at 0, 3, 6, 10, 15 and 20 years. Half are personnel who sustained combat trauma; a comparison group of the same size has been frequency matched based on deployment to Afghanistan, age, sex, service, rank and role. Participants undergo a series of physical health tests and questionnaires through which information is collected on cardiovascular disease (CVD), CVD risk factors, musculoskeletal disease, mental health, functional and social outcomes, quality of life, employment and mortality.
Ethics and dissemination
The ADVANCE Study has approval from the Ministry of Defence Research Ethics Committee (protocol no:357/PPE/12) agreed 15 January 2013. Its results will be disseminated through manuscripts in clinical/academic journals and presentations at professional conferences, and through participant and stakeholder communications.
Trial registration number
The ADVANCE Study is registered at ISRCTN ID: ISRCTN57285353.
Keywords: cardiac epidemiology, rehabilitation medicine, mental health, musculoskeletal disorders
Strengths and limitations of this study.
ArmeD SerVices TrAuma and RehabilitatioN OutComE (ADVANCE) is, worldwide, the only longitudinal cohort study to evaluate the effect of combat trauma on a range of health indicators in military personnel who served in the Afghanistan war.
ADVANCE will provide a wide range of longitudinal data across sociodemographic, physical health and mental health outcomes, providing evidence for incidence and risk of disease and other non-disease related outcomes.
ADVANCE will provide high levels of evidence that will influence future healthcare of combat and major trauma patients.
Participants were injured between 5 and 16 years prior to baseline data collection, and the length of time since injury may have an effect on various physical and mental health indicators.
As with any cohort study, there is potential for response bias.
Introduction
During the Afghanistan war between 2003 and 2014, the UK military sustained over 2400 combat casualties.1 Many had such severe injuries that in previous conflicts they would have died, if it were not for the trauma care provided by the UK Defence Medical Services; they are frequently termed ‘unexpected survivors’.2 Rehabilitation took place at the Defence Medical Rehabilitation Centre (DMRC) at Headley Court, often over many months, and the short-term outcomes have been favourable.3–8 However, the longer term outcomes of this cohort of severely injured personnel are unclear. Understanding medical and psychosocial outcomes in this population will provide evidence for, and influence the future care of, patients in trauma and rehabilitation services worldwide.
Previous studies into war veterans from earlier conflicts, such as the Vietnam war and World War II, have investigated long-term health outcomes. However, studies investigating combat injury and consequent adverse cardiovascular disease (CVD) outcomes9–36 have low strength of evidence for cause and effect. Others have investigated only mental health outcomes37–39 or mortality.23 38–44 Studies of musculoskeletal (MSK) outcomes in combat amputees, such as osteoarthritis/osteopenia, pain and physical functioning, have been either retrospective, small in numbers, inconclusive,45–47 short-term,48 or focused on surrogates of outcome such as return to military duty.49 Many studies investigating veterans’ long-term outcomes are either not specifically related to combat trauma22–37 or are of cross sectional or retrospective design making it difficult to draw robust conclusions from them.10–13 15–25 27 29 30 32 34 38 39
Current knowledge
Cardiovascular disease
Combat injuries have been shown to be associated with CVD in Finnish war veterans,14 Israeli lower-limb amputee veterans18 and US Vietnam veterans.50 While this evidence suggests battlefield-injured ex-personnel or serving personnel are at higher risk of CVD, the strength of evidence is low,14 18 50 and the only UK data36 challenge these findings, suggesting that veterans may be at lower risk for acute myocardial infarction. Furthermore, the mechanisms that drive this potentially increased risk are poorly understood.
Systolic blood pressure and hypertension, diabetes mellitus, high sensitivity C-reactive protein (HsCRP), lipid profiles (eg, cholesterol, triglycerides, etc), heart rate, obesity and smoking are well-validated measures of CVD risk.51 Large artery stiffness, leading to increased pulse wave velocity (PWV) and accelerated arterial wave reflections causing an increase in myocardial demand, central systolic blood pressure and a decrease in coronary artery perfusion pressure, are promising additional risk markers. Increased PWV has been shown to be an independent predictor of cardiovascular morbidity and mortality in several population groups, including healthy controls,52–57 and has the potential to identify CVD risk earlier than traditional risk factors and to help better understand the aetiology of CVD.
Heart rate variability (HRV) is another risk factor for disease. Temporal changes in cardiac beat-to-beat intervals are subject to continuous autonomic nervous system influence and competing sympathetic versus parasympathetic control. As a marker for altered autonomic balance, HRV has been linked to adverse clinical conditions such as cardiac death, stroke and poor mental health.58–64
In a recent systematic review and meta-analysis,9 it was concluded that there is currently insufficient evidence to confidently link combat related traumatic injury to an increased risk of CVD and associated risk factors. The review identified the need for high-quality data from a more contemporary and prospective study.
Musculoskeletal health
The consequences of severe MSK trauma often result in functional limitations to the individual, and a significant socioeconomic cost to society.65 An improved understanding of the effect of trauma and amputation on the MSK system, and of disease processes and progress over time, is essential to provide effective long-term care of complex trauma casualties. There is some evidence to suggest an association between osteoarthritis and amputation,45 64 possibly reflecting alterations in the biomechanics of the amputee’s movement, by which degenerative changes such as osteoarthritis of the knee/hip can occur.66 67 However, few risk factors have been identified regarding hip/knee osteoarthritis.47 Similarly, femoral neck osteopenia45 46 68 and back pain69 70 appear to be prevalent in traumatic amputee populations. Long-term longitudinal prospective evidence of disease prevalence, risk and progression is required to understand the aetiology of these diseases.
Mental health
Among UK military personnel and veterans who deployed to the Iraq and Afghanistan wars, the prevalence of common mental disorders is estimated to be 22%.71 For veterans with physical impairments, reported rates of common mental disorders range from 10% to 46% for depression and 16% to 36% for anxiety disorders (excluding post-traumatic stress disorder (PTSD)).72 However, these figures come from a predominantly US population and have not been specifically investigated in combat injured populations.
The prevalence of PTSD in UK military veterans who served during the Iraq/Afghanistan conflicts has increased, from 4% in 200673 and 201037 to around 6% in 2018.71 This, along with reported rates of PTSD ranging from 2% to 59% in ex-/serving personnel with a physical impairment,72 highlights the need to be monitoring mental health in military personnel and especially in those who are combat casualties.
The incidence of other important long-term outcomes including all-cause mortality, hearing loss, drug and alcohol use, physical function, quality of life, social and employment outcomes is largely unknown, particularly in combat casualties.
Hypotheses and objectives
The objective of the ArmeD SerVices TrAuma RehabilitatioN OutComE (ADVANCE) study is to investigate the long-term medical and psychosocial outcomes of UK military personnel who sustained combat trauma. We hypothesise that combat trauma casualties will have an increased incidence of adverse medical, psychosocial and vocational long-term outcomes compared with equivalent but non-injured service personnel.
Methods and analysis
Study design
The ADVANCE Study is a prospective 20-year cohort study. The ADVANCE study aims to recruit ‘exposed’ adult males (n=600) who sustained physical combat trauma while on deployment in Afghanistan and required aeromedical evacuation to a UK hospital. A frequency matched unexposed comparison group (n=600) of males without combat injury will be also recruited.
Study population
Participants are recruited from ex-/serving UK military personnel who deployed on combat operations to Afghanistan between 2003 and 2014.
Recruitment
Recruitment started in March 2016 and will be finished by Autumn 2020; the inclusion and exclusion criteria are listed in box 1. Volunteers from both the ‘exposed’ and ‘comparison’ cohorts are recruited from two lists provided by Defence Statistics UK. The first is a list of serving and ex-serving military personnel who sustained a combat injury (n=1400). The second is a list of men who had not sustained an injury for the comparison group (n=2100), frequency matched to the injured group based on age, service, rank, role, regiment and deployment (figures 1 and 2). Deployment refers to a specific deployment period of interest. For the exposed (injured) group, this is the deployment period in which they sustained their injury. The unexposed (comparison) group was frequency matched based on deploying within the same period without sustaining a physical combat related injury. The following data sources were used to identify the potential participants: the initial Notification of Casualty System; the Defence Patient Tracking System; the Defence Medical Information Capability Programme; the DMRC Complex Trauma Database; the DRMC Prosthetic database; the Joint Theatre Trauma Registry; and the Joint Personnel Administration (JPA).
Box 1. Inclusion/exclusion criteria.
Inclusion criteria
UK Armed services personnel
Male
Exposed only: Sustaining physical battlefield trauma, while on deployment to Afghanistan, requiring aeromedical evacuation and direct UK hospital admission
Exposed only: injured between 2003 and the end of 2014.
Exclusion Criteria
Females
Patients who are unwilling or unable to give informed consent
Patients with established cardiovascular disease (previous stroke or transient ischaemic attack, ischaemic heart disease, peripheral vascular disease) prior to injury/deployment of interest
Medical history of diabetes prior to injury/deployment of interest
Past medical history of renal or liver disease prior to injury/deployment of injury
Aged <18 and >50 years
-
Active acute infection with at least 2two systemic features of sepsis*, at the time of recruitment, as defined below. Potential participant with active acute infection will be considered for recruitment once the acute illness is treated and resolved.
Temperature >38°C or <36°C
Heart rate >90 beats/min
Respiratory rate >20 breaths/min participants suffering from an acute infection will be excluded initially but will be reapproached to take part once the infection resolves.
Comparison group only: subsequent combat injury sustained while on deployment in Afghanistan after matching.
*American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. 1992
Figure 1.
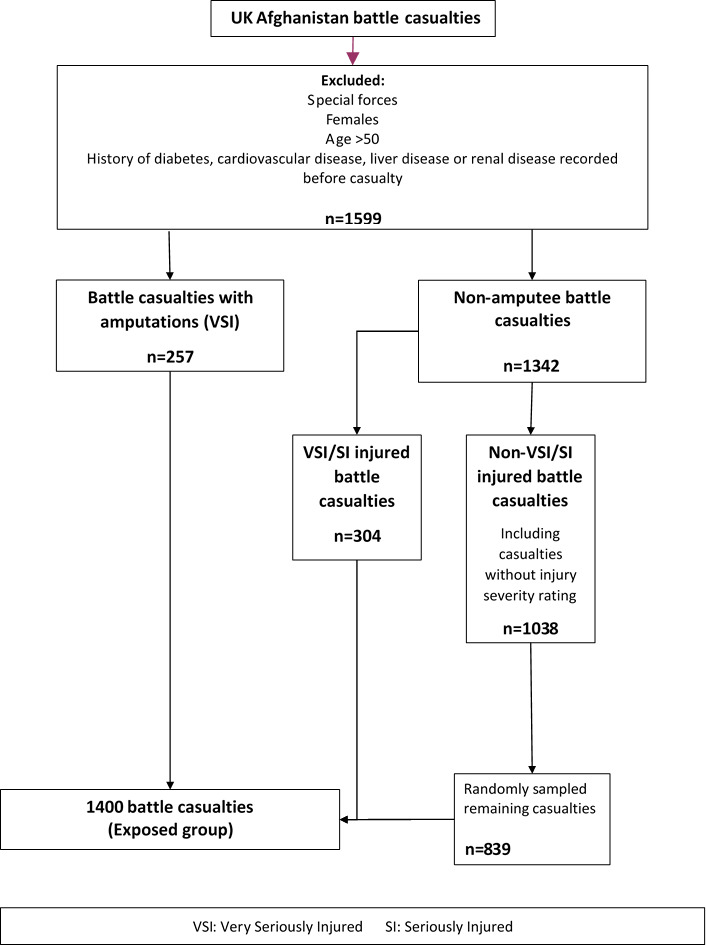
Sampling flow chart for injured personnel.
Figure 2.
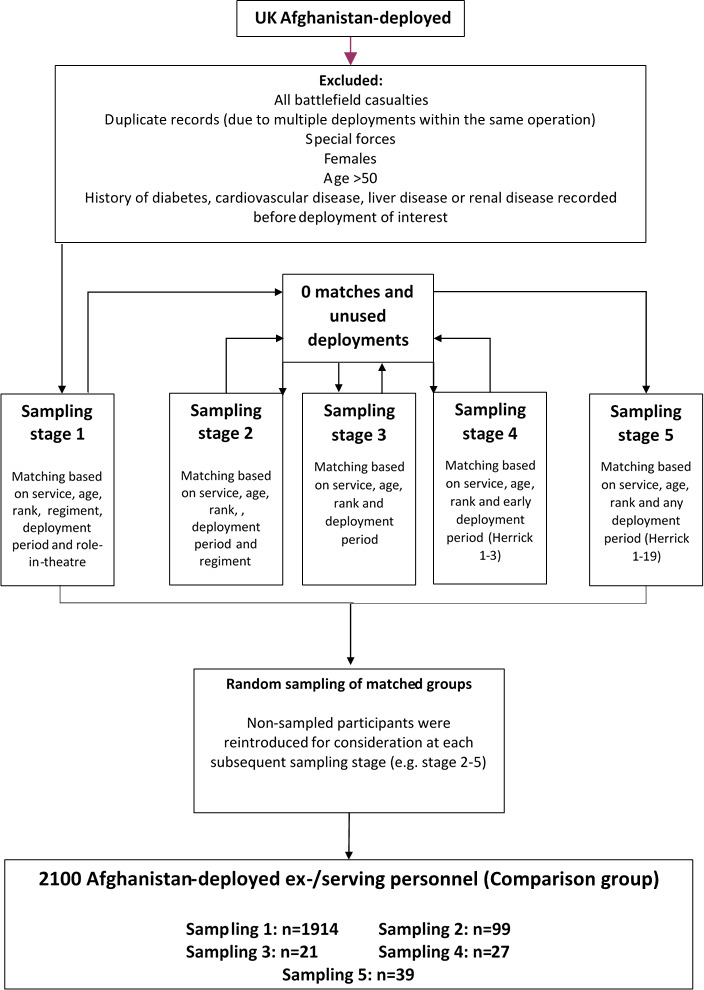
Sampling flow chart for comparison personnel.
Participants are recruited through postal mailouts, e-mail invitations and telephone calls, and where necessary traced via JPA contacts, if still serving, and through electoral roll data, social media or advertising via military charities.
Setting
Data is collected during a 1 day study visit to the DMRC at Headley Court (March 2016–August 2018) or Stanford Hall (after August 2018). The study day starts at 07:45, and participants arrive in a fasted state (8 hours); investigations are completed by 14:00 (figure 3). Participants will be invited to attend follow-up at 3, 6, 10, 15 and 20 years. Informed written consent is obtained from the participants by trained research personnel. This includes consent for access to central National Health Service and Ministry of Defence medical records and for required data linkages to be conducted.
Figure 3.
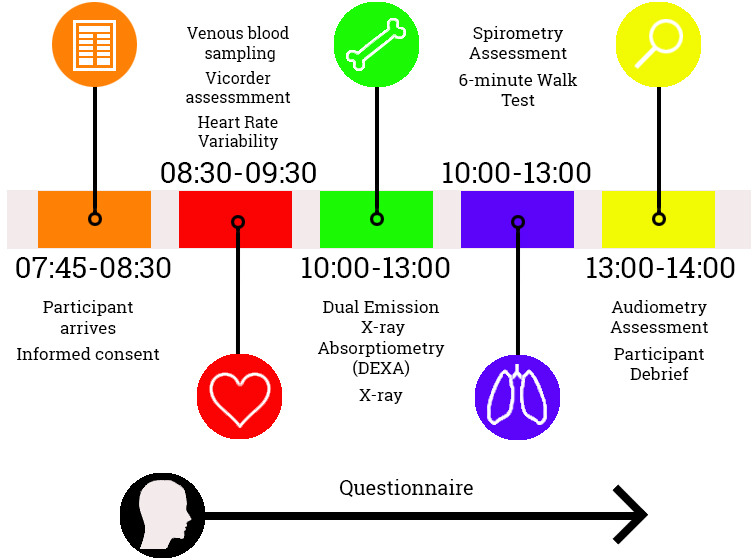
Timeline of ArmeD SerVices TrAuma RehabilitatioN OutComE study day.
Outcome and confounder variables
Core outcome variables collected at baseline are detailed below. These may expand throughout the 20-year duration of the study.
Cardiovascular disease
Participants are assessed for onset of CVD at each visit, determined by the onset of individual components of the Major Adverse Cardiovascular Endpoint (MACE), a composite of cardiovascular death, non-fatal myocardial infarction, stroke, transient ischaemic attack, arterial revascularisation (coronary artery bypass grafting, percutaneous coronary intervention, carotid endarterectomy or stenting and peripheral arterial stenting or bypass) and the onset of peripheral vascular disease, angina or hypertension.
This combined cardiovascular endpoint is internationally recognised and validated in cardiovascular outcome research.74–81 Each participant will be flagged with the National Health Service Central Register to provide date and cause of death.
Cardiovascular risk
Biometric assessment
Height is measured using a stadiometer (SECA 704, UK); for men with bilateral leg amputations, reported preinjury height will be taken from medical records. Weight is measured using electronic scales (SECA 956, UK). Abdominal waist and hip circumference are measured manually using a tape measure. Body mass index is calculated using an adjusted formula for amputees (Tzamaloukas et al., 1994).82
Vicorder assessment
The Vicorder (Skidmore Medical Limited, Bristol, UK) is a validated device that measures arterial PWV and complex pulse wave form analysis (PWA).83–85 PWV is quantified by the simultaneous recording of arterial pulse waveforms at the carotid and femoral arteries using an integrated neck transducer and upper thigh cuff, respectively.84 Using the PWA, the Vicorder also provides a non-invasive measure of peripheral and central blood pressure and arterial central and peripheral augmentation index.83 Its measurement of PWV has been shown to have a within-subject coefficient of variation of 2.8%.85 The central and peripheral measurements of arterial stiffness have been shown to be independent predictors of CVD risk.86
The assessment takes place on a hospital bed at a 30° angle in the supine position, in a room that is temperature and noise regulated. Three measures of PWA are conducted using the upper left arm cuff. This cuff is then removed, and a neck transducer is used along with a cuff on the upper left thigh to conduct three measures of PWV. All tests are completed ipsilaterally unless this is impossible due to amputation, in which case assessments are completed contralaterally. Diastolic and systolic blood pressure are also taken in the supine position using the Vicorder device with a cuff attached to the upper left arm.
Heart rate variability
Cardiac interbeat intervals for the measurement of HRV is undertaken using an ECG device (Mega Motion Faros 180 recorder; Mega, Finland). Two consecutive 5 min recordings of ECG-derived cardiac interbeat intervals will be obtained using spontaneous breathing followed by a 5 min paced breathing exercise, both in a supine position.87 88 Tests are completed in a noise and temperature-controlled environment. Measures of HRV are undertaken offline using the recorded cardiac interbeat data.
Serum samples
Venous blood is drawn and analysed for full blood count, lipids, glucose, liver function, urea and electrolytes, Haemaglobin A1c (HbA1c) and HsCRP (online supplemental materials 1).
bmjopen-2020-037850supp001.pdf (79.2KB, pdf)
QRISK
10-year cardiovascular risk will be determined using the QRISK calculator89 with biometric, sociodemographic and clinical data.
Respiratory function
The participant’s respiratory function is measured using a PC Spirometer (Trueflow, NDD, Switzerland) to assess basic respiratory capability and its contribution to functional capacity. A forced expiratory test, without noseclips, is completed with the participant sitting upright. Three acceptable readings are sought, with a maximum of eight attempts made.
Traumatic/mild-traumatic brain injury (TBI/mTBI)
TBI/mTBI occurrence is assessed using a clinician administered interview90 and post-concussive symptoms are assessed using Participant Reported Outcome Measures (PROMS)91 (table 1).
Table 1.
Patient reported outcome measures: MSK, pain and physical function
| Measure | Questionnaire type | N items | |
| Musculoskeletal disease | |||
| Amputee physical functioning | Amputee Mobility Predictor Questionnaire/Assessment104–106 | Clinician assessment | 24 |
| Prosthetic functioning | Self-report | 6 (per missing limb) | |
| Prosthetic Socket Comfort Score109 | |||
| Special Interest Group in Amputee Medicine134 | Clinician assessment | 12 | |
| Pain | Brief Pain Inventory-Short Form115 | Self-report | 15 |
| Disability Arm, Shoulder and Hand Questionnaire110 111 | Self-report | 30 | |
| DN4*117 | Self-report | 7 | |
| Knee Osteoarthritis Outcomes Score135 136 | Self-report | 42 | |
| Manchester-Oxford Foot Questionnaire114 | Self-report | 16 | |
| Neuropathic Pain Symptom Inventory116 | Self-report | 12 | |
| Non-Arthritic Hip Score113 | Self-report | 20 | |
| Oswestry Disability Index112 | Self-report | 10 | |
| Pain Catastrophising Scale118 | Self-report | 13 | |
| Physical fitness | International Physical Activity Questionnaire137 | Self-report | 20 |
| Mental health | |||
| Alcohol and drug use | Alcohol Use Disorder Identification Toolkit122 | Self-report | 10 |
| Drug Use Disorder Identification Toolkit123 | Self-report | 11 | |
| Common mental disorders | Generalised Anxiety Disorder-7124 | Self-report | 7 |
| Patient Health Questionnaire-9125 | Self-report | 9 | |
| Post-traumatic growth | Deployment-related Post-Traumatic Growth Inventory*128 | Self-report | 21 |
| Post-traumatic stress disorder | Post Traumatic Check List-Civilian126 | Self-report | 17 |
| Post Traumatic Check List-DSM V127 | Self-report | 20 | |
| Sleep | Insomnia Severity Index*138 139 | Self-report | 4 |
| Pittsburgh Sleep Quality Index*140 | Self-report | 4 | |
| Other | |||
| Adverse childhood experiences | King’s Military Cohort: Health and Well-being Survey*121 | Self-report | 12 |
| Mild/traumatic brain injury | Ohio-State University Traumatic Brain Injury Identification Method Questionnaire90 | Clinician Interview | 13 |
| Rivermead Post-Concussion Questionnaire91 | Self-report | 18 | |
| Quality of life | Arizona Sexual Experiences Scale130 | Self-report | 5 |
| EQ-5D-5L129 | Self-report | ||
| Social support | Multidimensional Perceived Social Support Questionnaire131 | Self-report | 12 |
*Adapted versions.
MSK, musculoskeletal.
Musculoskeletal disease
Radiographic assessment for osteoarthritis and sacroiliitis
Participants have radiographic assessment for osteoarthritis of the knees and hips and for chronic sacroiliitis. Posterior–anterior views with the knees in semiflexed position (7–10°) using the Synaflexer frame are performed as per recommendations for the assessment of osteoarthritis.92–95 Anterior–lateral and skyline views (inferior–superior) of the patellofemoral joint with the knees in 30° of flexion are taken.95 96 Hips are also assessed radiographically with an AP pelvis film (focal length 100 cm, hips internally rotated 15°).97 Radiographs are scored according to the Kellgren and Lawrence radiographic osteoarthritis scoring method98 for both the hip and the tibiofemoral joints of the knee. The patella femoral joint will be scored using the Osteoarthritis Research Society International scoring method.99 AP pelvis X-rays will also be scored for sacroiliitis via the modified New York score.100
Dual emission X-ray absorptiometry (DEXA) assessment for osteoporosis and body composition
Total body composition, visceral fat, lean muscle mass and bone mineral density are recorded using body composition DEXA (Headley court: Vertec Horizon, UK; Stanford Hall: Vertec Discovery, UK)101 which has previously been used in a military population.102 103 Scans of the whole body, right and left proximal femur and lumbar spine are performed. For the whole-body scan, participants are laid in the supine position, with their head and spine aligned with the centre of the DEXA table, with legs apart and feet turned in; participants remain in this position for approximately 10 min. For the right and left proximal femur, the relevant leg is abducted to allow the shaft of the femur to be parallel to the table, and the relevant foot strapped in. Participants remain in this position for 7 min for each leg. For the lumbar spine, legs are elevated onto a square block and hips flexed at a 70° angle; participants remain in this position for 7 min.
Physical function and pain
Physical function is assessed using a mixture of clinician administered tests, including the Amputee Mobility Predictor Questionnaire (AMP-Q), Special Interest Group in Amputee Medicine (SIGAM) mobility grades, the 6 min walk test, and PROMS (table 1).
The AMP-Q assesses an amputee’s ability to complete physical tasks ranging from balance, reach, weight distribution/gait and walking,104–106 from which a SIGAM mobility grading is assigned. The 6 min walk test evaluates functional capacity by measuring the distance an individual is able to walk over a total of 6 min on a hard, flat surface; it is valid in both the able-bodied and amputees.107 108 The goal is for the individual to walk as far as possible in 6 min at a self-directed pace with rest as needed as they traverse back and forth along a marked walkway.
PROMS used (table 1) assess prosthetic functioning, including socket comfort109 and usage of prosthetics. Pain is assessed in specific areas of the body (shoulders, arm, hand,110 111 back,112 hip,113 foot,114 phantom pain and overall pain115) as well as type of pain (eg, neuropathic)116 117 and effects of pain (eg, pain catastrophising).118
Axial spondyloarthritis
The presence of the gene HLA-B27, inflammatory back pain and the spondyloarthritis criteria119 120 is used to assess the prevalence of spondyloarthritis (axSpA). Inflammatory back pain is assessed using the Assessment of Spondyloarthritis International Society (ASAS) experts’ Inflammatory back pain criteria120 and classification of axSpA through the ASAS classification criteria.119
Mental health
Mental health is assessed using PROMS (table 1) investigating adverse childhood events,121 alcohol and drug use,122 123 common mental disorders,124 125 PTSD,126 127 post-traumatic growth,128 quality of life129 130 and social support.131
Sociodemographic and educational/employment history and outcomes
Sociodemographic
Sociodemographic information from time of injury/deployment including age, rank and regiment are provided by Defence Statistics. Other sociodemographic data (eg, postcode, ethnicity, etc) will be collected at baseline and, where there may be changes, at all subsequent visits via questionnaire.
Employment outcomes
Current and historic employment/education are recorded using an employment history questionnaire. Reasons for leaving the Armed Forces, highest level of educational attainment and veteran specific outcomes will be measured as per the King’s Military Cohort: Health and Well-being Survey.37 71 73
Bio-sample storage and other serum samples
Approximately 20 mL of blood (whole blood/plasma/serum/DNA [dried whole blood spots stored on Whatman FTA cards]) and 50 mL of urine are stored at −80°C for assay of any future biomarkers of cardiovascular, MSK or other disease. Venous blood will also be assayed for a standard profile of male hormones including testosterone, follicular stimulating hormone, luteinising hormone and sex hormone binding globulin at each follow-up visit.
Audiology
Following simple otoscopic examination, an Amplivox CA850 4A audiometer with headphones is used to test hearing in a soundproofed booth. Both the audiometry and otoscopic examination follow recommended procedures from the British Society of Audiology.132
Sample size
Sample size calculations (GraphPad StatMate V.2.00 for Windows (GraphPad Software)) were based on the primary composite CVD endpoint. Published data have shown a greater risk of a CVD event (HR of >1.70) among those with traumatic injury compared with healthy controls.14 18 Given the age and demographic of the target population, event rates are likely to be low. However, the study is using a well-defined, published and measurable, broad composite CVD primary endpoint and has a prolonged follow-up period which both significantly reduce the sample size needed to maintain statistical power.
The rate of the primary MACE endpoint has been estimated using data from similarly aged populations.76 79 A primary composite CVD event rate of >10% at 20 years is expected in the comparison group with a HR of >1.7 in the combat trauma group. Based on this assumption we have calculated that a sample size of at least 400 in both the battlefield trauma exposed group and the non-exposed group would provide >80% power to detect a HR of >1.7 at an alpha of 0.05 (two sided) over a 20-year follow-up period. It is estimated that the initial recruitment of 600 participants will have a natural dropout rate of approximately 10% every 5 years. This would result in a sample size of approximately 400 at 20 years, and therefore still be sufficient to identify differences in composite CVD endpoints between the groups.
Sample size calculations were also performed for the other primary study outcomes; cardiovascular risk as determined by PWV, and osteoarthritis as determined by radiograph, each of which required smaller sample sizes than the sample size required for the primary composite CVD end-point analysis.
Statistical methods
The characteristics of non-responders—at recruitment and at follow-up—will be examined and compared with those who (continue to) participate. Differences between responders and non-responders will be examined with logistic regression analysis. Response weights will be generated to compensate for unequal probabilities of response based on any significant differences between responders and non-responders on age, rank, service and deployment.
The association between CVD and exposure will be assessed using the χ2 or Fisher’s exact test where appropriate. T-tests and one-way analysis of variance will be used to evaluate the association between CVD risk and exposure. Multiple comparisons will be assessed using the Bonferroni correction or similar when appropriate. At baseline, multivariable linear regression will be used to assess the association between primary outcomes and exposure. Generalised linear models with a binomial distribution will be used to assess the relative risk of CVD. If the model does not converge, then a modified Poisson regression approach would be considered. Multicollinearity will be assessed using the Variance Inflation Factor diagnostic test.
For repeated measures (baseline, 3, 6 and 10 years), we will use mixed effects models. Cox proportional hazard models will be used to evaluate the association between exposure and disease development over time while adjusting for confounders.
Multiple imputation133 will be considered for missing data. A priori confounders will be adjusted for in the analysis and any other potential confounders will be considered using univariable analyses. A p-value of <0.05 will be considered statistically significant. Data will be analysed using STATA V.16 (Stata Corp) with the svy command to take account of the sample and response weights.
Data storage and retention
All data will be handled in accordance with current legislation, at present the GDPR 2018 and the Data Protection Act 2018. Physical data will be pseudoanonymised and stored accessible only by the research team. Digital data will be secured using dedicated data management software. After the last participant’s final follow-up, all data will be stored for a minimum of 15 years.
Ethics and dissemination
The ADVANCE Study has Ministry of Defence Research Ethics Committee approval (protocol no: 357/PPE/12), granted on 15 January 2013. The trial will be performed in accordance with the recommendations guiding ethical research involving human subjects adopted by the 18th World Medical Association General Assembly, Helsinki, Finland, June 1964, amended at the 64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013. Results will be disseminated through manuscripts in clinical/academic journals, presentations at clinical/academic conferences and communications with participants and other stakeholders and via the ADVANCE study website www.advancestudydmrc.org.uk.
Participant involvement
Ex-patients of DMRC Headley Court were involved in the development and design of the study from the outset as were a number of experienced clinicians regarding appropriate outcomes, feasibility, tolerability, priorities and recruitment. Ongoing participant consultations continue to influence recruitment, outcome measure priorities and acceptability.
Supplementary Material
Acknowledgments
The authors wish to thank all the nursing and ancillary staff who have helped with the ongoing ADVANCE Study. They would like to acknowledge in particular our senior research nurses Helen Blackman, Louise Young and Seamus Wilson, and our project managers Maria-Benedicta Edwards, Melanie Chesnokov, Maija Maskuniitty and Emma Coady.
Footnotes
Collaborators: Prof Alexander N Bennett, Prof Paul Cullinan, Prof Nicola T Fear, Prof Anthony M J Bull, Prof Christopher J Boos, Dr Susie Schofield, Mr Daniel M Dyball.
Contributors: The study concept and design were conceived by AB, CJB, AMJB, NTF and PC. AB and DMD were the main authors of the paper. SS provided significant portions of the data analysis section and provided critical revisions to the whole paper. NTF, CJB, AMJB and PC provided critical revisions to the whole paper. Final approval of the whole paper was given by all authors. All authors agree to be accountable for all aspects of the accuracy and integrity of this protocol paper.
Funding: The ADVANCE Study is supported by research grants from Help for Heroes, Her Majesty’s Treasury, Headley Court Trustees, Nuffield Trust for the Forces of the Crown, Blesma the limbless Veterans charity and also generously supported by the MoD.
Competing interests: AB works for the Ministry of Defence. NTF receives funding from the MoD and is a trustee of a veteran charity. AMJB directs a research centre that receives funding from veteran’s charities and is supported by the Ministry of Defence.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
On behalf of the ADVANCE Study:
Alexander N Bennett, Paul Cullinan, Nicola T Fear, Anthony M J Bull, Christopher J Boos, Susie Schofield, and Daniel M Dyball
References
- 1. Keene DD, Penn-Barwell JG, Wood PR, et al. Died of wounds: a mortality review. J R Army Med Corps 2016;162:355–60. 10.1136/jramc-2015-000490 [DOI] [PubMed] [Google Scholar]
- 2. Russell RJ, Hodgetts TJ, McLeod J, et al. The role of trauma scoring in developing trauma clinical governance in the defence medical services. Philos Trans R Soc Lond B Biol Sci 2011;366:171–91. 10.1098/rstb.2010.0232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jarvis HL, Bennett AN, Twiste M, et al. Temporal spatial and metabolic measures of walking in highly functional individuals with lower limb amputations. Arch Phys Med Rehabil 2017;98:1389–99. 10.1016/j.apmr.2016.09.134 [DOI] [PubMed] [Google Scholar]
- 4. Etherington J, Bennett AN, Phillip R, et al. Outcomes for UK service personnel indicate high quality trauma care and rehabilitation. BMJ 2016;354:i4741. 10.1136/bmj.i4741 [DOI] [PubMed] [Google Scholar]
- 5. Ladlow P, Phillip R, Etherington J, et al. Functional and mental health status of United Kingdom military amputees Postrehabilitation. Arch Phys Med Rehabil 2015;96:2048–54. 10.1016/j.apmr.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 6. Dharm-Datta S, Gough MRC, Porter PJ, et al. Successful outcomes following neurorehabilitation in military traumatic brain injury patients in the United Kingdom. J Trauma Acute Care Surg 2015;79:S197–203. 10.1097/TA.0000000000000721 [DOI] [PubMed] [Google Scholar]
- 7. Dharm-Datta S, Etherington J, Mistlin A, et al. The outcome of British combat amputees in relation to military service. Injury 2011;42:1362–7. 10.1016/j.injury.2011.06.028 [DOI] [PubMed] [Google Scholar]
- 8. Bahadur S, McRann J, McGilloway E. Long-Term employment outcomes following rehabilitation for significant neurological impairment in UK military personnel: a 3-year study. J R Army Med Corps 2017;163:355–60. 10.1136/jramc-2016-000703 [DOI] [PubMed] [Google Scholar]
- 9. Boos CJ, De Villiers N, Dyball D, et al. The relationship between military combat and cardiovascular risk: a systematic review and meta-analysis. Int J Vasc Med 2019;2019:1–14. 10.1155/2019/9849465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hrubec Z, Ryder RA. Traumatic limb amputations and subsequent mortality from cardiovascular disease and other causes. J Chronic Dis 1980;33:239–50. 10.1016/0021-9681(80)90068-5 [DOI] [PubMed] [Google Scholar]
- 11. Vollmar JF, Paes E, Pauschinger P, et al. Aortic aneurysms as late sequelae of above-knee amputation. Lancet 1989;2:834–5. 10.1016/S0140-6736(89)92999-1 [DOI] [PubMed] [Google Scholar]
- 12. Labouret G, et al. Systolic arterial hypertension in patients amputated for injury. Presse medicale 1983;12:1349–50. [PubMed] [Google Scholar]
- 13. Rose HG, Schweitzer P, Charoenkul V, et al. Cardiovascular disease risk factors in combat veterans after traumatic leg amputations. Arch Phys Med Rehabil 1987;68:20–3. [PubMed] [Google Scholar]
- 14. Kunnas T, Solakivi T, Renko J, et al. Late-life coronary heart disease mortality of Finnish war veterans in the TAMRISK study, a 28-year follow-up. BMC Public Health 2011;11:71. 10.1186/1471-2458-11-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lorenz M, Panitz K, Grosse-Furtner C, et al. Lower‐limb amputation, prevalence of abdominal aortic aneurysm and atherosclerotic risk factors. Br J Surg 1994;81:839–40. 10.1002/bjs.1800810615 [DOI] [PubMed] [Google Scholar]
- 16. Yekutiel M, Brooks ME, Ohry A, et al. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia 1989;27:58–62. 10.1038/sc.1989.9 [DOI] [PubMed] [Google Scholar]
- 17. Stewart IJ, Sosnov JA, Howard JT, et al. Retrospective analysis of long-term outcomes after combat injury: a hidden cost of war. Circulation 2015;132:2126–33. 10.1161/CIRCULATIONAHA.115.016950 [DOI] [PubMed] [Google Scholar]
- 18. Modan M, Peles E, Halkin H, et al. Increased cardiovascular disease mortality rates in traumatic lower limb amputees. Am J Cardiol 1998;82:1242–7. 10.1016/S0002-9149(98)00601-8 [DOI] [PubMed] [Google Scholar]
- 19. Shahriar SH, Masumi M, Edjtehadi F, et al. Cardiovascular risk factors among males with war-related bilateral lower limb amputation. Mil Med 2009;174:1108–12. 10.7205/milmed-d-00-0109 [DOI] [PubMed] [Google Scholar]
- 20. Peles E, Akselrod S, Goldstein DS, et al. Insulin resistance and autonomic function in traumatic lower limb amputees. Clin Auton Res 1995;5:279–88. 10.1007/BF01818893 [DOI] [PubMed] [Google Scholar]
- 21. Ejtahed H-S, Soroush M-R, Hasani-Ranjbar S, et al. Prevalence of metabolic syndrome and health-related quality of life in war-related bilateral lower limb amputees. J Diabetes Metab Disord 2017;16:17. 10.1186/s40200-017-0298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bullman TIMA, Kang HANK, Watanabe KK. Proportionate mortality among US army Vietnam veterans who served in military region I. Am J Epidemiol 1990;132:670–4. 10.1093/oxfordjournals.aje.a115708 [DOI] [PubMed] [Google Scholar]
- 23. Macfarlane GJ, Thomas E, Cherry N. Mortality among UK Gulf War veterans. Lancet 2000;356:17–21. 10.1016/S0140-6736(00)02428-4 [DOI] [PubMed] [Google Scholar]
- 24. Schlenger WE, Corry NH, Williams CS, et al. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. Am J Epidemiol 2015;182:kwv217–90. 10.1093/aje/kwv217 [DOI] [PubMed] [Google Scholar]
- 25. Barth SK, Kang HK, Bullman T. All-cause mortality among US veterans of the Persian Gulf war: 13-year follow-up. Public Health Rep 2016;131:822–30. 10.1177/0033354916676278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang HK, Li B, Mahan CM, et al. Health of US veterans of 1991 Gulf war: a follow-up survey in 10 years. J Occup Environ Med 2009;51:401–10. 10.1097/JOM.0b013e3181a2feeb [DOI] [PubMed] [Google Scholar]
- 27. Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National health interview survey. Chronic Illn 2018. [DOI] [PubMed] [Google Scholar]
- 28. Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation 2014;129:1813–20. 10.1161/CIRCULATIONAHA.113.005407 [DOI] [PubMed] [Google Scholar]
- 29. Thomas MM, Harpaz-Rotem I, Tsai J, et al. Mental and physical health conditions in US combat veterans: results from the National health and resilience in veterans study. Prim Care Companion CNS Disord 2017;19. 10.4088/PCC.17m02118. [Epub ahead of print: 22 Jun 2017]. [DOI] [PubMed] [Google Scholar]
- 30. Sheffler JL, Rushing NC, Stanley IH, et al. The long-term impact of combat exposure on health, interpersonal, and economic domains of functioning. Aging Ment Health 2016;20:1202–12. 10.1080/13607863.2015.1072797 [DOI] [PubMed] [Google Scholar]
- 31. Johnson AM, Rose KM, Elder GH, et al. Military combat and burden of subclinical atherosclerosis in middle aged men: the ARIC study. Prev Med 2010;50:277–81. 10.1016/j.ypmed.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. O'Toole BI, Marshall RP, Grayson DA, et al. The Australian Vietnam veterans health study: II. self-reported health of Veterans compared with the Australian population. Int J Epidemiol 1996;25:319–30. 10.1093/ije/25.2.319 [DOI] [PubMed] [Google Scholar]
- 33. Granado NS, Smith TC, Swanson GM, et al. Newly reported hypertension after military combat deployment in a large population-based study. Hypertension 2009;54:966–73. 10.1161/HYPERTENSIONAHA.109.132555 [DOI] [PubMed] [Google Scholar]
- 34. Eisen SA, Kang HK, Murphy FM, et al. Gulf War veterans' health: medical evaluation of a U.S. cohort. Ann Intern Med 2005;142:881–90. 10.7326/0003-4819-142-11-200506070-00005 [DOI] [PubMed] [Google Scholar]
- 35. Johnson AM, Rose KM, Elder GH, et al. Military combat and risk of coronary heart disease and ischemic stroke in aging men: the Atherosclerosis risk in communities (ARIC) study. Ann Epidemiol 2010;20:143–50. 10.1016/j.annepidem.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergman BP, Mackay DF, Pell JP. Acute myocardial infarction in Scottish military veterans: a retrospective cohort study of 57,000 veterans and 173,000 matched nonveterans. Am J Epidemiol 2014;179:1434–41. 10.1093/aje/kwu082 [DOI] [PubMed] [Google Scholar]
- 37. Fear NT, Jones M, Murphy D, et al. What are the consequences of deployment to Iraq and Afghanistan on the mental health of the UK armed forces? a cohort study. Lancet 2010;375:1783–97. 10.1016/S0140-6736(10)60672-1 [DOI] [PubMed] [Google Scholar]
- 38. Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol 2006;16:248–56. 10.1016/j.annepidem.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 39. Chin DL, Zeber JE. Mental health outcomes among military service members after severe injury in combat and TBI. Mil Med 2020;185:e711–8. 10.1093/milmed/usz440 [DOI] [PubMed] [Google Scholar]
- 40. Bramsen I, Deeg DJH, van der Ploeg E, et al. Wartime stressors and mental health symptoms as predictors of late-life mortality in World War II survivors. J Affect Disord 2007;103:121–9. 10.1016/j.jad.2007.01.014 [DOI] [PubMed] [Google Scholar]
- 41. Kang HK, Bullman TA. Mortality among US veterans of the Persian Gulf war: 7-year follow-up. Am J Epidemiol 2001;154:399–405. 10.1093/aje/154.5.399 [DOI] [PubMed] [Google Scholar]
- 42. Watanabe KK, Kang HK. Mortality patterns among Vietnam veterans: a 24-year retrospective analysis. J Occup Environ Med 1996;38:272–8. 10.1097/00043764-199603000-00012 [DOI] [PubMed] [Google Scholar]
- 43. Fett MJ, NAIRN JR, COBBIN DM, et al. Mortality among Australian conscripts of the Vietnam conflict era. II. causes of DEATH1. Am J Epidemiol 1987;125:878–84. 10.1093/oxfordjournals.aje.a114604 [DOI] [PubMed] [Google Scholar]
- 44. Fett MJ, Adena MA, Cobbin DM, et al. Mortality among Australian conscripts of the Vietnam conflict era. I. death from all causes. Am J Epidemiol 1987;125:869–77. 10.1093/oxfordjournals.aje.a114603 [DOI] [PubMed] [Google Scholar]
- 45. Kulkarni J, Adams J, Thomas E, et al. Association between amputation, arthritis and osteopenia in British male war veterans with major lower limb amputations. Clin Rehabil 1998;12:348–53. 10.1191/026921598672393611 [DOI] [PubMed] [Google Scholar]
- 46. Sherk VD, Bemben MG, Bemben DA. Bmd and bone geometry in transtibial and transfemoral amputees. J Bone Miner Res 2008;23:1449–57. 10.1359/jbmr.080402 [DOI] [PubMed] [Google Scholar]
- 47. Farrokhi S, Mazzone B, Yoder A, et al. A narrative review of the prevalence and risk factors associated with development of knee osteoarthritis after traumatic unilateral lower limb amputation. Mil Med 2016;181:38–44. 10.7205/MILMED-D-15-00510 [DOI] [PubMed] [Google Scholar]
- 48. Ramasamy A, Hill AM, Masouros S, et al. Outcomes of IED foot and ankle blast injuries. J Bone Joint Surg Am 2013;95:e25–1. 10.2106/JBJS.K.01666 [DOI] [PubMed] [Google Scholar]
- 49. Ramasamy A, Hill AM, Phillip R, et al. The modern “deck-slap” injury—calcaneal blast fractures from vehicle explosions. J Trauma 2011;71:1694–8. 10.1097/TA.0b013e318227a999 [DOI] [PubMed] [Google Scholar]
- 50. Boehmer TKC, Flanders WD, McGeehin MA, et al. Postservice mortality in Vietnam veterans: 30-year follow-up. Arch Intern Med 2004;164:1908–16. 10.1001/archinte.164.17.1908 [DOI] [PubMed] [Google Scholar]
- 51. National Heart L, Institute B. Assessing cardiovascular risk: systematic evidence review from the risk assessment work group. Bethesda, MD: National Institutes of Health, 2013. [Google Scholar]
- 52. Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005;111:3384–90. 10.1161/CIRCULATIONAHA.104.483628 [DOI] [PubMed] [Google Scholar]
- 53. Mourad JJ, Pannier B, Blacher J, et al. Creatinine clearance, pulse wave velocity, carotid compliance and essential hypertension. Kidney Int 2001;59:1834–41. 10.1046/j.1523-1755.2001.0590051834.x [DOI] [PubMed] [Google Scholar]
- 54. Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–90. 10.1161/01.cir.0000033824.02722.f7 [DOI] [PubMed] [Google Scholar]
- 55. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001;37:1236–41. 10.1161/01.HYP.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 56. Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184–9. 10.1161/01.CIR.0000105767.94169.E3 [DOI] [PubMed] [Google Scholar]
- 57. Shechter M, Issachar A, Marai I, et al. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 2009;134:52–8. 10.1016/j.ijcard.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 58. Cardoso CR, Moraes RA, Leite NC, et al. Relationships between reduced heart rate variability and pre-clinical cardiovascular disease in patients with type 2 diabetes. Diabetes Res Clin Pract 2014;106:110–7. 10.1016/j.diabres.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 59. Fyfe-Johnson AL, Muller CJ, Alonso A, et al. Heart rate variability and incident stroke: the Atherosclerosis risk in Communities study. Stroke 2016;47:1452–8. 10.1161/STROKEAHA.116.012662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kemp AH, Quintana DS, Felmingham KL, et al. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One 2012;7:e30777. 10.1371/journal.pone.0030777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol 2013;89:288–96. 10.1016/j.ijpsycho.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 62. Sessa F, Anna V, Messina G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 2018;10:166–77. 10.18632/aging.101386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thayer JF, Ahs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 2012;36:747–56. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 64. Tucker P, Pfefferbaum B, Jeon-Slaughter H, et al. Emotional stress and heart rate variability measures associated with cardiovascular risk in relocated Katrina survivors. Psychosom Med 2012;74:160–8. 10.1097/PSY.0b013e318240a801 [DOI] [PubMed] [Google Scholar]
- 65. Edwards DS, Phillip RD, Bosanquet N, et al. What is the magnitude and long-term economic cost of care of the British military Afghanistan amputee cohort? Clin Orthop Relat Res 2015;473:2848–55. 10.1007/s11999-015-4250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gailey R, Allen K, Castles J, et al. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J Rehabil Res Dev 2008;45:15–30. 10.1682/JRRD.2006.11.0147 [DOI] [PubMed] [Google Scholar]
- 67. Ding Z. Biomechanical evidence of increased rates of knee osteoarthritis in high functioning amputees: a pilot study. [DOI] [PubMed]
- 68. Leclercq MM, Bonidan O, Haaby E, et al. [Study of bone mass with dual energy x-ray absorptiometry in a population of 99 lower limb amputees]. Ann Readapt Med Phys 2003;46:24–30. 10.1016/s0168-6054(02)00350-1 [DOI] [PubMed] [Google Scholar]
- 69. Hammarlund CS, Carlström M, Melchior R, et al. Prevalence of back pain, its effect on functional ability and health-related quality of life in lower limb amputees secondary to trauma or tumour: a comparison across three levels of amputation. Prosthet Orthot Int 2011;35:97–105. 10.1177/0309364610389357 [DOI] [PubMed] [Google Scholar]
- 70. Sivapuratharasu B, Bull AMJ, McGregor AH. Understanding low back pain in traumatic lower limb amputees: a systematic review. Arch Rehabil Res Clin Transl 2019;1:100007 10.1016/j.arrct.2019.100007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stevelink SAM, Jones M, Hull L, et al. Mental health outcomes at the end of the British involvement in the Iraq and Afghanistan conflicts: a cohort study. Br J Psychiatry 2018;213:690–7. 10.1192/bjp.2018.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stevelink SAM, Malcolm EM, Mason C, et al. The prevalence of mental health disorders in (ex-)military personnel with a physical impairment: a systematic review. Occup Environ Med 2015;72:243–51. 10.1136/oemed-2014-102207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hotopf M, Hull L, Fear NT, et al. The health of UK military personnel who deployed to the 2003 Iraq war: a cohort study. Lancet 2006;367:1731–41. 10.1016/S0140-6736(06)68662-5 [DOI] [PubMed] [Google Scholar]
- 74. Heart Protection Study Collaborative Group MRC/BHF heart protection study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–33. 10.1016/S0140-6736(02)09328-5 [DOI] [PubMed] [Google Scholar]
- 75. Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000;101:1899–906. 10.1161/01.CIR.101.16.1899 [DOI] [PubMed] [Google Scholar]
- 76. AIM-HIGH Investigators, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. 10.1056/NEJMoa1107579 [DOI] [PubMed] [Google Scholar]
- 77. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557–65. 10.1056/NEJMoa021993 [DOI] [PubMed] [Google Scholar]
- 78. Abola MTB, Bhatt DL, Duval S, et al. Fate of individuals with ischemic amputations in the reach Registry: three-year cardiovascular and limb-related outcomes. Atherosclerosis 2012;221:527–35. 10.1016/j.atherosclerosis.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 79. Raiko JRH, Magnussen CG, Kivimäki M, et al. Cardiovascular risk scores in the prediction of subclinical atherosclerosis in young adults: evidence from the cardiovascular risk in a young finns study. Eur J Cardiovasc Prev Rehabil 2010;17:549–55. 10.1097/HJR.0b013e3283386419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anderson TJ, Charbonneau F, Title LM, et al. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the firefighters and their endothelium (fate) study. Circulation 2011;123:163–9. 10.1161/CIRCULATIONAHA.110.953653 [DOI] [PubMed] [Google Scholar]
- 81. Sehestedt T, Jeppesen J, Hansen TW, et al. Can ambulatory blood pressure measurements substitute assessment of subclinical cardiovascular damage? J Hypertens 2012;30:513–21. 10.1097/HJH.0b013e32834f6f60 [DOI] [PubMed] [Google Scholar]
- 82. Tzamaloukas AH, Patron A, Malhotra D. Body mass index in amputees. JPEN J Parenter Enteral Nutr 1994;18:355–8. 10.1177/014860719401800414 [DOI] [PubMed] [Google Scholar]
- 83. Butlin M, Qasem A, Battista F, et al. Carotid-Femoral pulse wave velocity assessment using novel cuff-based techniques: comparison with tonometric measurement. J Hypertens 2013;31:2237–43. 10.1097/HJH.0b013e328363c789 [DOI] [PubMed] [Google Scholar]
- 84. van Leeuwen-Segarceanu EM, Tromp WF, Bos W-JW, et al. Comparison of two instruments measuring carotid-femoral pulse wave velocity: Vicorder versus SphygmoCor. J Hypertens 2010;28:1687–91. 10.1097/HJH.0b013e32833a8b83 [DOI] [PubMed] [Google Scholar]
- 85. Hickson SS, Butlin M, Broad J, et al. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res 2009;32:1079–85. 10.1038/hr.2009.154 [DOI] [PubMed] [Google Scholar]
- 86. Woodman RJ, Kingwell BA, Beilin LJ, et al. Assessment of central and peripheral arterial stiffness: studies indicating the need to use a combination of techniques. Am J Hypertens 2005;18:249–60. 10.1016/j.amjhyper.2004.08.038 [DOI] [PubMed] [Google Scholar]
- 87. Camm AJ. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of cardiology and the North American Society of pacing and electrophysiology. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- 88. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ 2017;357:j2099. 10.1136/bmj.j2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Corrigan JD, Bogner J. Initial reliability and validity of the Ohio state university TBI identification method. J Head Trauma Rehabil 2007;22:318–29. 10.1097/01.HTR.0000300227.67748.77 [DOI] [PubMed] [Google Scholar]
- 91. King NS, Crawford S, Wenden FJ, et al. The Rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995;242:587–92. 10.1007/BF00868811 [DOI] [PubMed] [Google Scholar]
- 92. Buckland-Wright JC, Ward RJ, Peterfy C, et al. Reproducibility of the semiflexed (metatarsophalangeal) radiographic knee position and automated measurements of medial tibiofemoral joint space width in a multicenter clinical trial of knee osteoarthritis. J Rheumatol 2004;31:1588–97. [PubMed] [Google Scholar]
- 93. Buckland-Wright JC, Wolfe F, Ward RJ, et al. Substantial superiority of semiflexed (MTP) views in knee osteoarthritis: a comparative radiographic study, without fluoroscopy, of standing extended, semiflexed (MTP), and schuss views. J Rheumatol 1999;26:2664–74. [PubMed] [Google Scholar]
- 94. Nevitt M, Felson D, Lester G. The Osteoarthritis Initiative: Protocol for the Cohort Study. In: Sequence parameters: the osteoarthritis initiative, 2006. [Google Scholar]
- 95. Wesseling J, Dekker J, van den Berg WB, et al. Check (cohort hip and cohort knee): similarities and differences with the osteoarthritis initiative. Ann Rheum Dis 2009;68:1413–9. 10.1136/ard.2008.096164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum 1996;39:988–95. 10.1002/art.1780390616 [DOI] [PubMed] [Google Scholar]
- 97. Leyland KM, Hart DJ, Javaid MK, et al. The natural history of radiographic knee osteoarthritis: a fourteen-year population-based cohort study. Arthritis Rheum 2012;64:2243–51. 10.1002/art.34415 [DOI] [PubMed] [Google Scholar]
- 98. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 2007;15:A1–56. 10.1016/j.joca.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 100. Linden SVD, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 101. Lohman TG, Harris M, Teixeira PJ, et al. Assessing body composition and changes in body composition. another look at dual-energy X-ray absorptiometry. Ann N Y Acad Sci 2000;904:45–54. 10.1111/j.1749-6632.2000.tb06420.x [DOI] [PubMed] [Google Scholar]
- 102. Nindl BC, Friedl KE, Frykman PN, et al. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med 1997;18:317–24. 10.1055/s-2007-972640 [DOI] [PubMed] [Google Scholar]
- 103. Nindl BC, Friedl KE, Marchitelli LJ, et al. Regional fat placement in physically fit males and changes with weight loss. Med Sci Sports Exerc 1996;28:786–93. 10.1097/00005768-199607000-00003 [DOI] [PubMed] [Google Scholar]
- 104. Gailey RS, Roach KE, Applegate EB, et al. The amputee mobility predictor: an instrument to assess determinants of the lower-limb amputee's ability to ambulate. Arch Phys Med Rehabil 2002;83:613–27. 10.1053/apmr.2002.32309 [DOI] [PubMed] [Google Scholar]
- 105. Hafner BJ, Willingham LL, Buell NC, et al. Evaluation of function, performance, and preference as transfemoral amputees transition from mechanical to microprocessor control of the prosthetic knee. Arch Phys Med Rehabil 2007;88:207–17. 10.1016/j.apmr.2006.10.030 [DOI] [PubMed] [Google Scholar]
- 106. Miller WC, Deathe AB. A prospective study examining balance confidence among individuals with lower limb amputation. Disabil Rehabil 2004;26:875–81. 10.1080/09638280410001708887 [DOI] [PubMed] [Google Scholar]
- 107. Balke B. A simple field test for the assessment of physical fitness. Rep 63-6. Rep Civ Aeromed Res Inst US 1963;63:1–8. [PubMed] [Google Scholar]
- 108. Lin S-J, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil 2008;89:2354–9. 10.1016/j.apmr.2008.05.021 [DOI] [PubMed] [Google Scholar]
- 109. Hanspal RS, Fisher K, Nieveen R. Prosthetic socket fit comfort score. Disabil Rehabil 2003;25:1278–80. 10.1080/09638280310001603983 [DOI] [PubMed] [Google Scholar]
- 110. Germann G, Wind G, Harth A. [The DASH(disability of arm-shoulder-hand) questionnaire--a new instrument for evaluating upper extremity treatment outcome]. Handchir Mikrochir Plast Chir 1999;31:149–52. 10.1055/s-1999-13902 [DOI] [PubMed] [Google Scholar]
- 111. Davidson J. A comparison of upper limb amputees and patients with upper limb injuries using the disability of the arm, shoulder and hand (DASH). Disabil Rehabil 2004;26:917–23. 10.1080/09638280410001708940 [DOI] [PubMed] [Google Scholar]
- 112. Fairbank JC, Couper J, Davies JB, et al. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3. [PubMed] [Google Scholar]
- 113. Christensen CP, Althausen PL, Mittleman MA, et al. The nonarthritic hip score: reliable and validated. Clin Orthop Relat Res 2003;406:75–83. 10.1097/00003086-200301000-00013 [DOI] [PubMed] [Google Scholar]
- 114. Morley D, Jenkinson C, Doll H, et al. The Manchester-Oxford foot questionnaire (MOXFQ): development and validation of a summary index score. Bone Joint Res 2013;2:66–9. 10.1302/2046-3758.24.2000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tan G, Jensen MP, Thornby JI, et al. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain 2004;5:133–7. 10.1016/j.jpain.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 116. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the neuropathic pain symptom inventory. Pain 2004;108:248–57. 10.1016/j.pain.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 117. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 118. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 119. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of spondyloarthritis International Society classification criteria for axial spondyloarthritis (Part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 120. Sieper J, Rudwaleit M, Baraliakos X, et al. The assessment of spondyloarthritis International Society (ASAS) Handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68:ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 121. Iversen AC, Fear NT, Simonoff E, et al. Influence of childhood adversity on health among male UK military personnel. Br J Psychiatry 2007;191:506–11. 10.1192/bjp.bp.107.039818 [DOI] [PubMed] [Google Scholar]
- 122. Bohn MJ, Babor TF, Kranzler HR. The alcohol use disorders identification test (audit): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995;56:423–32. 10.15288/jsa.1995.56.423 [DOI] [PubMed] [Google Scholar]
- 123. Berman AH, Bergman H, Palmstierna T, et al. Evaluation of the drug use disorders identification test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 2005;11:22–31. 10.1159/000081413 [DOI] [PubMed] [Google Scholar]
- 124. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 125. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Weathers FW, et al. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. in annual convention of the International Society for traumatic stress studies. San Antonio, TX, 1993. [Google Scholar]
- 127. Blevins CA, Weathers FW, Davis MT, et al. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress 2015;28:489–98. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- 128. Tedeschi RG, Calhoun LG. The posttraumatic growth inventory: measuring the positive legacy of trauma. J Trauma Stress 1996;9:455–71. 10.1002/jts.2490090305 [DOI] [PubMed] [Google Scholar]
- 129. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona sexual experience scale (ASEX): reliability and validity. J Sex Marital Ther 2000;26:25–40. 10.1080/009262300278623 [DOI] [PubMed] [Google Scholar]
- 131. Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Pers Assess 1988;52:30–41. 10.1207/s15327752jpa5201_2 [DOI] [Google Scholar]
- 132. BSA British Society of audiology, 2019. Available: www.thebsa.org
- 133. Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- 134. Ryall NH, Eyres SB, Neumann VC, et al. The SIGAM mobility grades: a new population-specific measure for lower limb amputees. Disabil Rehabil 2003;25:833–44. 10.1080/0963828021000056460 [DOI] [PubMed] [Google Scholar]
- 135. Roos EM, Lohmander LS. The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64. 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1:17. 10.1186/1477-7525-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport 2000;71:114–20. 10.1080/02701367.2000.11082794 [DOI] [PubMed] [Google Scholar]
- 138. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 139. Greenberg N, Jones N. Operational mental health needs evaluation, 2019. Available: https://www.kcl.ac.uk/kcmhr/research/admmh/OMHNE
- 140. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037850supp001.pdf (79.2KB, pdf)


