Abstract
Renal medullary carcinoma, a highly aggressive tumor mainly occurring in patients with sickle cell hemoglobinopathy, is characterized by advanced stage at the time of presentation and poor response to treatment. Currently, the pathogenesis of this tumor is not well understood. In this study, the clinicopathologic features and molecular changes of 15 renal medullary carcinoma cases were evaluated. These cases demonstrated male predominance (M:F = 2:1) with a median age of 26 years. The tumors occurred predominantly in the right kidney with an average size of 5.9 cm. Immunohistochemistry analysis showed that the neoplastic cells were positive for CEA (7/8), AE1/3 (8/8), CAM5.2 (7/7), CK7 (5/5), CK20 (4/6), and vimentin (6/6). Absence of SMARCB1 protein expression in tumor cells was demonstrated in all of the 7 cases analyzed. By polymerase chain reaction–based microsatellite analysis, loss of heterozygosity of SMARCB1 was identified in 9 of 10 cases. These data suggest that inactivation of SMARCB1 may play a role in the pathogenesis of renal medullary carcinoma.
Keywords: renal medullary carcinoma, SMARCB1, loss of heterozygosity
Renal medullary carcinoma is a rare and aggressive, primary renal tumor occurring mainly in patients with sickle cell hemoglobinopathy.1 Typically, the tumor affects patients between ages 10 and 40 (mean age 26 y) with a male predominance (M:F = 2:1). Histologically, renal medullary carcinoma displays various morphologies including reticular, yolk sac, or adenoid cystic growth patterns and sometimes shows neoplastic cells admixed with neutrophils in a desmoplastic background. Margination of the lesions by lymphocytes is also commonly seen.1 On immunohistochemical analysis, the tumor cells were found to be positive for AE1/AE3, EMA, CEA, and CAM5.2 and negative for high molecular weight keratin. Although well recognized as an aggressive entity, little is known regarding the pathogenesis of renal medullary carcinoma.
SMARCB1 is a tumor suppressor gene located on the long arm of chromosome 22, which encodes a core subunit of the SWI/SNF complex known to regulate chromatin remodeling in an ATP-dependent manner. It has also been shown that SMARCB1 plays a critical role in cell cycle control and regulation of cytoskeleton dynamics.2–4 In mouse models, heterozygous inactivation of SMARCB1 induced tumors that were consistent with malignant rhabdoid tumors,5 and biallelic conditional inactivation of SMARCB1 led to rapid cancer development (CD8+ T-cell lymphomas and rhabdoid tumors) with 100% penetrance.6 Mutation/deletion of SMARCB1 gene and loss of its expression have been demonstrated in various tumors in humans, including malignant rhabdoid tumor of the kidney, atypical teratoid/rhabdoid tumor of the brain, extraskeletal myxoid chondrosarcomas, small cell undifferentiated hepatoblastomas, epithelioid sarcomas, and other neoplasms.7 Furthermore, it has been suggested that lack of SMARCB1 protein expression is associated with the aggressive biological behavior in certain tumors.8
Recently, absence of SMARCB1 protein expression has been demonstrated in renal medullary carcinoma by immunohistochemistry.9 However, its molecular mechanism and clinical significance are unclear.10–12 In the present study, we demonstrated that 9 of 10 renal medullary carcinoma cases showed loss of heterozygosity (LOH) of the SMARCB1 gene with corresponding negative SMARCB1 protein immunohistochemistry. In addition, the clinical, histopathologic, and immunohistochemical features of 15 renal medullary cell carcinoma cases were also reviewed. These data may provide molecular understanding of the pathogenesis of renal medullary carcinoma.
MATERIALS AND METHODS
Case Selection
Fifteen renal medullary carcinoma cases were collected from the pathology archive and consultation service (M.J.M.) of the Department of Pathology, the National Cancer Institute, National Institutes of Health, Bethesda, MD. Clinical information was obtained from submitted patients’ records or referring physicians. This study was approved by the Institute Review Board of the National Cancer Institute.
Histology and Immunohistochemistry
The morphologic and immunophenotypic features (except SMARCB1) were studied on formalin-fixed and paraffin-embedded tissue sections. The immunohistochemical staining analyses were performed using an automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ) according to the company’s protocols with minor modifications.
Immunohistochemistry of SMARCB1
Immunohistochemical analyses were performed on formalin-fixed and paraffin-embedded tissue sections. The slides were deparaffinized in xylene, rehydrated in graded alcohol (100%, 95%, and 70%), and microwaved in a pressure cooker containing Target Retrieval Solution, pH 6.10 (Dako Corporation, Carpinteria, CA) for 8 minutes, followed by cooling down for 20 minutes. Slides were then washed in phosphate-buffered saline. Endogenous peroxidase activity was quenched by a peroxidase blocking reagent, and a blocking step for nonspecific antibody binding was performed using Protein Block Serum-Free (Dako Corporation) for 10 minutes. The slides were incubated with SMARCB1 (INI1) mouse monoclonal antibody (BD Transduction Labs, San Diego, CA) (dilution 1:200) for 2 hours at room temperature, followed by incubation with anti-mouse EnVision plus System-HRP-labeled polymer (Dako Corporation) for 30 minutes at room temperature. 3,3′-diaminobenzidine solution (Dako Corporation) was used as a chromogen, and hematoxylin was used for counterstaining.
Molecular Analysis of SMARCB1 (LOH)
Normal and tumor tissue were manually micro-dissected from hematoxylin and eosin-stained slides after a previously described method with minor modifications.13 To extract DNA, the cells were digested in 0.5 mg/mL proteinase K buffer at 56°C for 12 hours, followed by a 10-minute incubation at 95°C to inactivate proteinase K. DNA was amplified by polymerase chain reaction (PCR) using genetic markers D22S303, D22S257, D22S345, TOP1P2, and D22S310 (Applied Biosystems, Foster City, CA) for LOH of the SMARCB1 gene on chromosome 22. The PCR was performed in a Perkin-Elmer Model 480 thermal cycler and carried out in a 10 μL reaction mix containing 15mM Tris-HCl, pH 8.0, 50mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, 3 pmol each of forward and reverse primer, and 1.0 U Ampli-Taq gold DNA polymerase (Applied Biosystems). The PCR cycle was optimized for each primer set and carried out as follows: denaturing at 96°C for 2 minutes, followed by 35 cycles of amplification including denaturation at 94°C for 45 seconds, annealing at 54°C or 60°C, and extension at 72°C for 1 minute. The reaction was then kept in 72°C for 10 minutes. The amplified PCR products were analyzed using the ABI Prism model 310 Genetic Analyzer (Applied Biosystems) according to the manufacturer’s instructions. Cases were considered informative when DNA of the normal tissue showed heterozygous alleles. Homozygous alleles were considered noninformative. Allelic loss was calculated using a normalized allele ratio equation: LOH = (T1/T2)/(N1/N2), where T1, T2, N1, and N2 are peaks from the tumor (T) and normal (N) DNA. LOH was assumed when this ratio was < 0.6 or > 1.6.
RESULTS
Clinical and Histopathologic Findings of Renal Medullary Carcinoma
The 15 renal medullary carcinoma cases showed a median age of 26 years (range, 8 to 49 y) with a male to female ratio of 2:1. Five of the 15 cases had a known history of sickle cell trait, and the sickle cell status of the remaining patients was unavailable (Table 1). The tumors involved predominantly the right kidney (11/15 involving the right kidney, 4/15 involving the left kidney). The average size of the tumors was 5.9 cm (range, 1.1 to 15 cm). Among the 15 cases, 10/11 cases showed lymphovascular invasion, 2/12 showed positive vascular margin, 0/12 cases showed positive ureteral margin, and 8/8 cases had lymph node metastasis. All cases demonstrated the characteristic histologic features of renal medullary carcinoma, including reticular, yolk sac, or adenoid growth patterns. In some cases, poorly differentiated areas with highly desmoplastic stroma were seen. Various degrees of inflammatory infiltrate including neutrophils and lymphocytes were present (Fig. 1). All patients died of disease during the follow-up period of 3 to 6 months.
TABLE 1.
Clinical and Pathologic Features of Renal Medullary Carcinoma
| Margins |
Metastasis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case # | Age | Sex | Sickle Cell Status | Side | Size (cm) | L/V Invasion | V | Uret | ST | LN | Dist | Staging |
| 1 | 48 | M | NA | Right | 4.5 | + | — | — | — | N1 | Mx | pT3bN1Mx, III |
| 2 | 26 | M | Trait | Right | 15 | + | NA | NA | NA | NA | NA | Autopsy |
| 3 | 8 | M | NA | Left | 3.5 | + | — | — | — | N2 | Mx | NA |
| 4 | 29 | F | NA | Right | 6.1 | + | + | — | — | N1 | Mx | pT1bN1Mx, III |
| 5 | 45 | F | NA | Left | 2.5 | NA | — | — | — | N2 | Mx | pT1N2Mx, III-IV |
| 6 | 24 | M | NA | Right | 5.0 | + | — | — | — | Nx | Adrenal | pT3ANxM1,IV |
| 7 | 26 | F | Trait | Left | 10 | + | + | — | — | N1 | Adrenal | pT3N1M1, IV |
| 8 | 13 | M | Trait | Right | 1.1 | + | NA | NA | — | N1 | Mx | NA |
| 9 | 22 | F | Trait | Left | 7.1 | + | — | — | — | N1 | M1 | pT3bN1M1, IV |
| 10 | 29 | M | NA | Right | 4.7 | NA | — | — | NA | + | Mx | NA |
| 11 | 19 | F | NA | Right | 6.0 | + | — | — | — | NA | Liver | NA |
| 12 | 17 | M | Trait | Right | 7.2 | + | — | — | — | Nx | Mx | NA |
| 13 | 49 | M | NA | Right | 5.0 | NA | — | — | — | Nx | Mx | NA |
| 14 | 11 | M | NA | Right | N/A | NA | NA | NA | NA | NA | NA | Biopsy |
| 15 | 21 | M | NA | Right | 5.5 | — | — | — | — | Nx | Mx | NA |
| +/Total | 10/11 | 2/12 | 0/12 | 0/12 | 8/8 | |||||||
Dist indicates distal metastasis; L/V, lymphovascular invasion; LN, lymph node metastasis; NA, not available; ST, soft tissue margin; Uret, ureteral margin; V, vascular margin.
FIGURE 1.
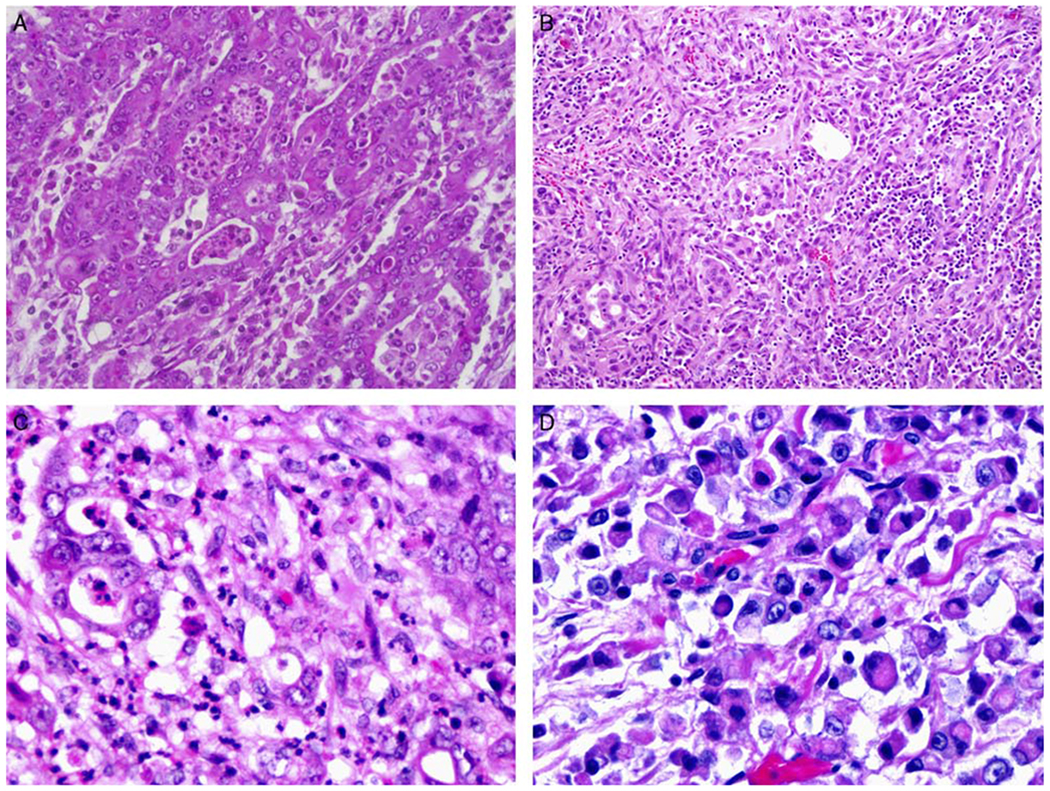
Histopathologic features of renal medullary carcinoma. Renal medullary carcinoma displays various morphologic features (A). The tumor cells often form a reticular pattern or sheets with large, prominent nucleoli, some displaying rhabdoid features (D). Desmoplastic stroma (B) and mucin in the background are not uncommon (D). Neutrophilic and lymphocytic infiltrates are also often admixed with tumor cells (C).
Immunohistochemical Profiles
Immunohistochemical analyses were performed using various stains, including AE1/3, CK7, CK20, CAM5.2, CK903, EMA, polyclonal CEA, Ulex, and vimentin. Seven of 8 cases were positive for CEA (polyclonal), and 7/7 cases were positive for CAM5.2. Positive staining was also seen with CK20, CK7, AE1/3, and vimentin (Table 2, Fig. 2).
TABLE 2.
Immunohistochemistry of Renal Medullary Carcinoma
| Case # | AE1/3 | CK7 | CK20 | CAM5.2 | CK903 | EMA | pCEA | Ulex | Vimentin |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NA | + | + | + | NA | + | NA | NA | + |
| 2 | + | NA | NA | NA | NA | + | + | NA | NA |
| 3 | + | NA | NA | NA | NA | NA | NA | NA | + |
| 4 | + | NA | NA | + | NA | NA | + | NA | NA |
| 5 | NA | + | + | NA | NA | NA | − | − | NA |
| 6 | NA | NA | + | + | − | NA | NA | NA | NA |
| 7 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 8 | + | NA | NA | NA | − | + | + | + | + |
| 9 | NA | + | − | + | − | NA | + | + | NA |
| 10 | NA | NA | NA | NA | NA | NA | + | NA | NA |
| 11 | + | NA | NA | NA | NA | NA | NA | NA | + |
| 12 | + | NA | NA | + | + | NA | + | + | NA |
| 13 | + | + | − | NA | NA | + | NA | NA | + |
| 14 | + | + | + | + | − | NA | NA | NA | NA |
| 15 | NA | NA | NA | + | NA | NA | NA | + | + |
| +/Total | 8/8 | 5/5 | 4/6 | 7/7 | 1/5 | 4/4 | 7/8 | 4/5 | 6/6 |
NA indicates not available.
FIGURE 2.
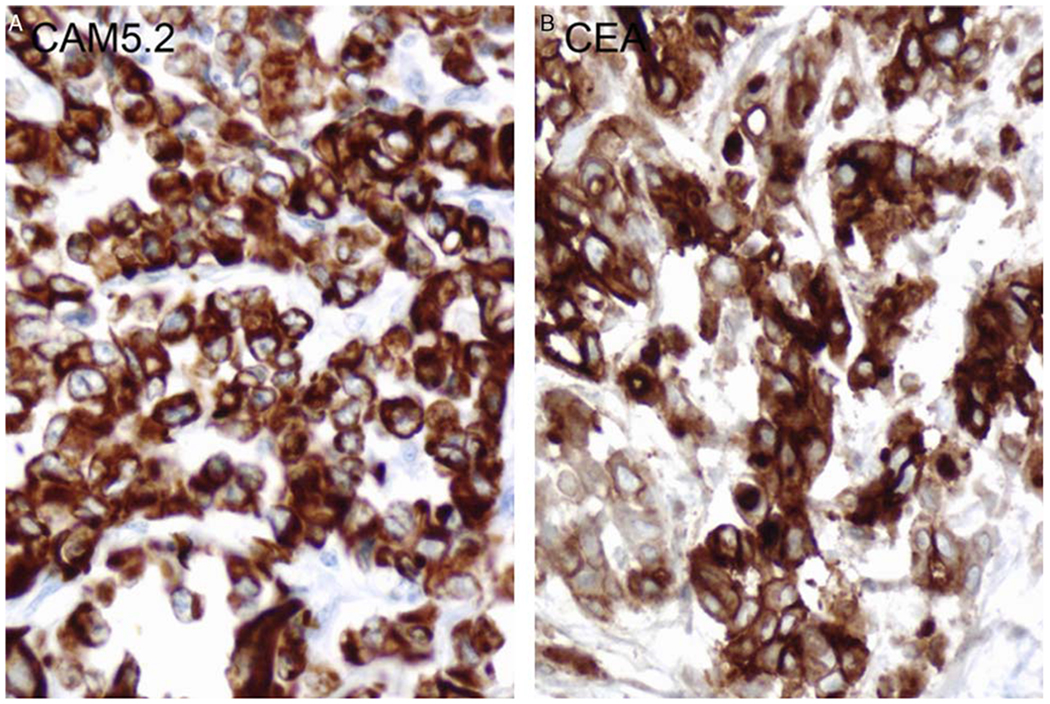
Immunohistochemistry of CAM5.2 and CEA of renal medullary carcinoma (case #4). CAM5.2 shows diffuse and strong positivity in tumor cells (A);CEA polyclonal antibody staining shows cytoplasmic positivity in tumor cells (B).
LOH of SMARCB1 Gene by PCR-based Microsatellite Analysis
LOH analysis of the SMARCB1 gene was performed on 10 renal medullary carcinoma cases. LOH was identified in the regions flanking the SMARCB1 gene on chromosome 22q in 9 of 10 cases analyzed (90%, Table 3, Fig. 3). One of the 10 cases (case #10) failed to demonstrate LOH of the SMARCB1 gene but did show the absence of SMARCB1/INI1 protein expression by immunohistochemical stain.
TABLE 3.
LOH and Immunohistochemistry of hSNF5/INI1 in Renal Medullary Carcinoma
| Microsatellite Markers |
INI1 IHC |
|||||
|---|---|---|---|---|---|---|
| Case# | D22S303 | D22S257 | D22S345 | TOP1P2 | D22S310 | |
| 1 | LOH | LOH | LOH | LOH | LOH | Absent |
| 2 | NI | LOH | LOH | LOH | LOH | Absent |
| 3 | RH | RH | LOH | LOH | LOH | NA |
| 4 | RH | LOH | LOH | No result | LOH | Absent |
| 5 | LOH | LOH | NI | No result | RH | Absent |
| 6 | LOH | NI | RH | RH | LOH | NA |
| 7 | LOH | RH | NI | No result | NI | Absent |
| 8 | NI | RH | LOH | RH | No result | NA |
| 9 | RH | NI | NI | LOH | NI | Absent |
| 10 | RH | RH | NI | RH | RH | Absent |
IHC indicates immunohistochemistry; NA, not available; NI, not informative; RH, retention of heterozygosity.
FIGURE 3.

LOH analysis of renal medullary carcinoma. A representative example of PCR-based microsatellite LOH analysis from case #2 using primer set D22S257 is shown here. The upper panel shows the 2 alleles from normal renal tissue, and the lower panel shows the 2 alleles from tumor cells. LOH is calculated using the formulation as indicated in the Materials and methods section. The ratio of allelic peaks between tumor and normal tissue indicates LOH in this region.
SMARCB1 Expression by Immunohistochemistry
Immunohistochemical analysis of SMARCB1 was performed on 7 renal medullary carcinoma cases. Absence of nuclear staining of SMARCB1 in the tumor cells was observed in all 7 renal medullary carcinoma cases, whereas nuclear staining of SMARCB1 was positive in the lymphocytes in the background (Fig. 4). The results of SMARCB1 immunohistochemistry and its correlation with LOH assay are summarized in Table 3.
FIGURE 4.

Immunohistochemistry of SMARCB1 (INI1) in renal medullary carcinoma. Immunohistochemical staining of SMARCB1 (INI1) in case #4 (A, B) and case #7 (C, D) are shown here. Tumor cells are negative for SMARCB1 (INI1) nuclear staining. In contrast, lymphocytes in the background show positive nuclear staining of SMARCB1 (INI1).
DISCUSSION
Renal medullary carcinoma was first recognized as the seventh sickle cell nephropathy in 1995.1 Presently, the genetic changes of these tumors are largely unknown. In an immunohistochemical study of 40 renal medullary carcinoma cases, it was suggested that the tumorogenesis appeared to be associated with the expression of TP53, VEGF, or HIF.12 Increased amplification of the ABL gene was also observed in renal medullary carcinoma.14 Recently, Cheng et al9 found that SMARCB1 protein was not detectable in renal medullary carcinoma by immunohistochemistry, suggesting that the absence of SMARCB1 may be involved in the pathogenesis of renal medullary carcinoma. In our study, 9 of 10 renal medullary carcinoma cases showed LOH of the SMARCB1 gene, which correlated well with the absence of protein expression. These data support the hypothesis that deletions of chromosome 22q may play an important role in renal medullary carcinoma.
Interestingly, in 1 of the cases studied, neither SMARCB1/INI1 protein nor LOH of SMARCB1 could be detected. A similar phenomenon has been reported in the studies of other tumors in which the tumor cells showed loss of SMARCB1 protein expression while lacking detectable mutations or deletions of the gene.8,15–18 These data imply that there may be an alternative post-transcriptional modification mechanism to explain the lack of protein expression.19–22
Renal medullary carcinoma is known for its close relation with the sickle cell hemoglobinopathy, particularly the sickle cell trait. Swartz et al12 proposed a hypothesis that hypoxia in the kidney induces the expression of HIF and VEGF in the absence of normal p53, which leads to the development of tumor through the neovascular network formation. It remains to be elucidated whether inactivation of the SMARCB1 gene is connected with the hypoxia hypothesis.
In our study, we observed a male predominance (M:F = 2:1) and right kidney predilection. These data are consistent with a series of cases described previously.1 In addition, a significant number of tumors are positive for polyclonal CEA and CAM5.2. If further studies confirm these findings, the detection of polyclonal CEA and CAM5.2, in conjunction with the loss of SMARCB1 expression, may be helpful to assist the diagnosis of renal medullary carcinoma.
In conclusion, renal medullary carcinoma is a highly aggressive tumor with characteristic clinicopathologic features. Recognition of the entity is important to establish appropriate forms of treatment. LOH at chromosome 22q11 and q12 and the lack of SMARCB1 protein expression are commonly seen in these tumors. Absence of SMARCB1 protein expression suggests that inactivation of the SMARCB1 gene may play an important role in the pathogenesis of renal medullary carcinoma.
Acknowledgments
Conflicts of Interest and Source of Funding: Supported by funding from the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Davis CJ Jr, Mostofi FK, Sesterhenn IA. Renal medullary carcinoma. The seventh sickle cell nephropathy. Am J Surg Pathol. 1995;19:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Klochendler-Yeivin A, Picarsky E, Yaniv M. Increased DNA damage sensitivity and apoptosis in cells lacking the Snf5/Ini1 subunit of the SWI/SNF chromatin remodeling complex. Mol Cell Biol. 2006:26:2661–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medjkane S, Novikov E, Versteege I. et al. The tumor suppressor hSNF5/INI1 modulates cell growth and actin cytoskeleton organization. Cancer Res. 2004;64:3406–3413. [DOI] [PubMed] [Google Scholar]
- 4.Versteege I, Medjkane S, Rouillard D. et al. A key role of the hSNF5/INI1 tumour suppressor in the control of the G1-S transition of the cell cycle. Oncogene. 2002;21:6403–6412. [DOI] [PubMed] [Google Scholar]
- 5.Roberts CW, Galusha SA, McMenamin ME. et al. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci USA. 2000:97:13796–13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts CW, Leroux MM, Fleming MD, et al. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer Cell. 2002;2:415–425. [DOI] [PubMed] [Google Scholar]
- 7.Roberts CW, Orkin SH. The SWI/SNF complex—chromatin and cancer. Nat Rev Cancer. 2004;4:133–142. [DOI] [PubMed] [Google Scholar]
- 8.Haberler C, Laggner U, Slavc I, et al. Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: lack of INI1 in atypical teratoid/rhabdoid tumors and in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol. 2006;30:1462–1468. [DOI] [PubMed] [Google Scholar]
- 9.Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008;21:647–652. [DOI] [PubMed] [Google Scholar]
- 10.Avery RA, Harris JE, Davis CJ Jr, et al. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer. 1996;78:128–132. [DOI] [PubMed] [Google Scholar]
- 11.Stahlschmidt J, Cullinane C, Roberts P, et al. Renal medullary carcinoma: prolonged remission with chemotherapy, immunohistochemical characterisation and evidence of bcr/abl rearrangement. Med Pediatr Oncol. 1999;33:551–557. [DOI] [PubMed] [Google Scholar]
- 12.Swartz MA, Karth J, Schneider DT, et al. Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology. 2002;60:1083–1089. [DOI] [PubMed] [Google Scholar]
- 13.Vortmeyer AO, Devouassoux-Shisheboran M, Li G, et al. Microdissection-based analysis of mature ovarian teratoma. Am J Pathol. 1999;154:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson L, He X, Pins M, et al. Renal medullary carcinoma and ABL gene amplification. J Urol. 2005;173:1883–1888. [DOI] [PubMed] [Google Scholar]
- 15.Bourdeaut F, Freneaux P, Thuille B, et al. Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer. 2008;51:363–368. [DOI] [PubMed] [Google Scholar]
- 16.Bourdeaut F, Freneaux P, Thuille B, et al. hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol. 2007;211:323–330. [DOI] [PubMed] [Google Scholar]
- 17.Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–1491. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CY, Wong TT, Lee YH, et al. Intact INI1 gene region with paradoxical loss of protein expression in AT/RT: implications for a possible novel mechanism associated with absence of INI1 protein immunoreactivity. Am J Surg Pathol. 2012;36:128–133. [DOI] [PubMed] [Google Scholar]
- 19.Albanese P, Belin MF, Delattre O. The tumour suppressor hSNF5/INI1 controls the differentiation potential of malignant rhabdoid cells. Eur J Cancer. 2006;42:2326–2334. [DOI] [PubMed] [Google Scholar]
- 20.Betz BL, Strobeck MW, Reisman DN, et al. Re-expression of hSNF5/INI1/BAF47 in pediatric tumor cells leads to G1 arrest associated with induction of p16ink4a and activation of RB. Oncogene. 2002;21:5193–5203. [DOI] [PubMed] [Google Scholar]
- 21.Caramel J, Medjkane S, Quignon F, et al. The requirement for SNF5/INI1 in adipocyte differentiation highlights new features of malignant rhabdoid tumors. Oncogene. 2008;27:2035–2044. [DOI] [PubMed] [Google Scholar]
- 22.Gresh L, Bourachot B, Reimann A, et al. The SWI/SNF chromatin-remodeling complex subunit SNF5 is essential for hepatocyte differentiation. EMBO J. 2005;24:3313–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]


