Abstract
A 3.5-year-old intact male double-transgenic New Zealand white rabbit (Oryctolagus cuniculus), apoA-I and LCAT (apolipoprotein and lecithin:cholesterol acyltransferase), was presented with a discrete, raised facial mass (0.5 × 1.0 × 1.0 cm). The mass was surgically excised, with reoccurrence to the same site 88 days later. A second surgical excision was performed, and the rabbit died 3 weeks later from respiratory distress. At necropsy, multiple varying-sized masses were observed in the ventral mandibular region and throughout the lungs, pleura, and diaphragm. On histopathology, the masses were composed of moderately anisocytotic and anisokaryotic polygonal to spindloid cells with moderate finely granular, lightly eosinophilic cytoplasm, having round to oval nuclei with one to several nucleoli and finely stippled chromatin. Mitotic figures were frequent. Lymphatic and venous invasion were noted with neoplastic cells metastasized to the submandibular lymph nodes, lungs, liver, and adventitial surface of the aorta. Fontana-Masson stain was negative for melanin, thereby necessitating immunohistochemistry and transmission electron microscopy. Positive staining with MART-1 (a melanocyte protein marker) combined with transmission electron microscopy revealing type II melanosomes confirmed the diagnosis of an amelanotic melanoma.
Keywords: amelanotic melanoma, immunohistochemistry, rabbit, skin, transmission electron microscopy
A 3.5-year-old intact male double-transgenic New Zealand white rabbit (Oryctolagus cuniculus), apo-AI and LCAT (apolipoprotein and lecithin:cholesterol acyltransferase), initially presented with a discrete raised mass (0.5 × 1.0 × 1.0 cm) on the face approximately 1 cm rostral to the medial canthus of the left eye. The rabbit was part of a breeding colony of the Division of Veterinary Resources at the National Institutes of Health to study LCAT overexpression on atherosclerosis with the underlying health effects.3 ApoA-I activates LCAT for esterification of HDL—a critical step in reverse cholesterol transport.12 The National Institutes of Health is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International; all procedures were performed in accordance with the US Public Health Service guidelines and the Guide for the Care and Use of Laboratory Animals, and all were approved by the National Heart, Lung, and Blood Institute’s Animal Care and Use Committee.
The facial mass was surgically removed as an excisional biopsy. Within 3 months, the mass recurred and was raised and ulcerated, measuring 4.0 × 2.0 × 1.5 cm. A second surgery was performed to resect the tumor with wider margins. Over the next 3 weeks, the rabbit’s condition declined, and the rabbit developed respiratory distress and died acutely.
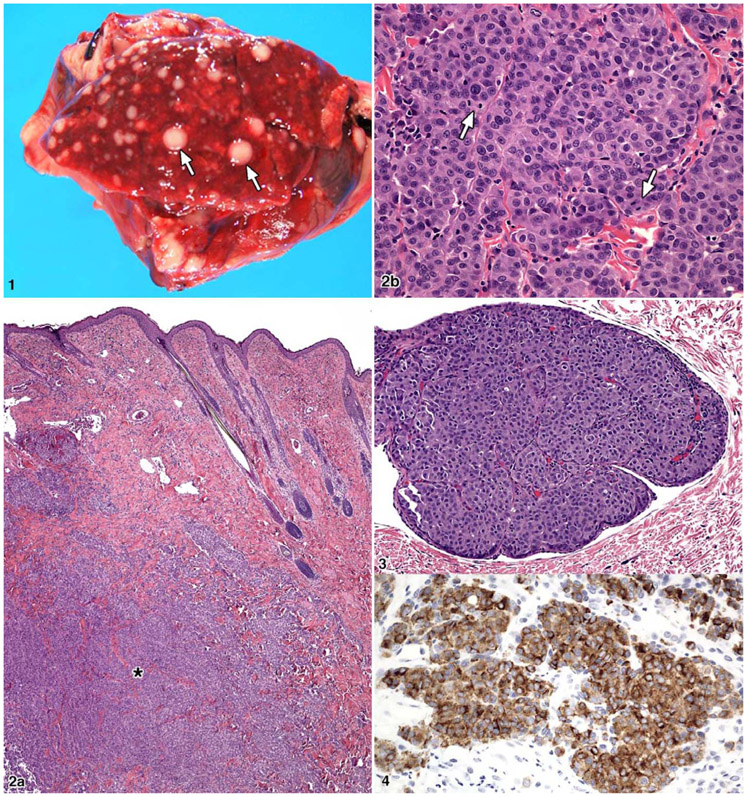
At necropsy there was a healed incision at the site of the excisional biopsy. One mass (1.5 × 2.5 × 3.5 cm) was white, resilient, and somewhat lobulated, and a similar mass was present caudal to the first (1.0 × 1.0 × 1.5 cm). Two subcutaneous masses were present between the rami of the mandible. Within all lung lobes, there were multifocal to coalescing firm grayish-white variably sized masses, which measured up to 7 mm in diameter (Fig. 1). The masses extended into the parietal pleura and multifocally within the diaphragm. In the biopsy and necropsy, skin sample tumor cells were seen in the vessels and had invaded the dermis. Lesions were not observed in any other major organs.
Figure 1.
Rabbit; lung. Multiple expansile variably sized metastatic white nodules (arrows). Figure 2. Rabbit; skin. a, Cutaneous mass with invasion into the dermis (*). b, Cutaneous mass in a fibrous stroma with a packeting of cells that are round to polygonal with prominent mitotic figures (arrows). HE. Figure 3. Rabbit; skin. Tumor invasion within a dermal lymphatic vessel. HE. Figure 4. Rabbit; skin. Tumor cells with prominent cytoplasmic staining with MART-1.
Select tissues were collected, placed in 10% buffered formalin, processed, and embedded in paraffin. Five-micron-thick sections were cut and stained with hematoxylin and eosin. Three tumor sections were also stained with Fontana-Masson.
Formalin-fixed tissue was transferred to 1% osmium tetroxide for 2 hours for transmission electron microscopy. The tissue was washed again with 0.1M cacodylate buffer, serially dehydrated in ethanol and propylene oxide, and embedded in EMBed 812 resin (Electron Microscopy Sciences, Hatfield, PA). Thin sections, approximately 80 nm, were obtained with the Leica ultracut-UCT ultramicrotome (Leica, Deerfield, IL), placed onto 300 mesh copper grids, and stained with saturated uranyl acetate in 50% methanol and then lead citrate. The grids were viewed in the JEM-1200EXII electron microscope (JEOL Ltd, Tokyo, Japan) at 80 kV, and images were recorded on the XR611M midmounted 10.5-Mpixel CCD camera (Advanced Microscopy Techniques Corp, Danvers, MA).
For immunohistochemistry, paraffin-embedded tissue was cut to a thickness of 4 μm, placed on ProbeOn Plus slides (Fisher Scientific, Pittsburgh, PA), and stained with actin, AE1/AE3, HMB-45, MART-1, S-100, SMA, tyrosinase, and vimentin (Table 1). As a negative control, a monoclonal mouse immunoglobulin G was used in lieu of the antibody.
Table 1.
Immunohistochemistrya
| Antibody | Dilution | Company |
|---|---|---|
| Actin HHF35 | 1:100 | Enzo (Farmingdale, NY) |
| AE1/AE3b | 1:50 | Dako (Carpentaria, CA) |
| HMB-45 | 1:4 | Enzo |
| MART-1 | 1:100 | Cell Marque (Hot Springs, AL) |
| S-100 | 1:100 | Biocare (Concord, CA) |
| SMA | 1:160 | Dako |
| Tyrosinase | 1:20 | Novocastra (Leica Microsystems Inc, Bannockburn, IL) |
| Vimentin | 1:100 | Dako |
All species were mice. The pretreatment for all antibodies was citrate for 20 minutes (unless noted).
Pretreatment: proteinase K.
Histological examination of the original excised skin biopsy revealed a raised mass that invaded the dermis, which was partially ulcerated, nonencapsulated, and well delineated and composed of tightly packed polygonal to spindloid cells with a fine fibrovascular stroma (Fig. 2A). The cells were moderately anisocytotic and anisokaryotic with moderate lightly eosinophilic, finely granular cytoplasm and indistinct cell borders. Nuclei were round to oval with finely stippled chromatin, 1 to 4 nucleoli, and frequent mitoses (5 to 7 per high-power field; Fig. 2B). Vascular invasion was evident, with tumor cells present within multiple vascular and lymphatic channels in multiple tissues (Fig. 3). Within the skin, neoplastic cells were seen fingering into the adjacent dermis and extending to the cut border of the facial mass. Similar neoplastic foci with a packeting of cells were present in the submandibular lymph nodes, lungs, parietal pleura, and liver. Tumor cell morphology in these metastatic sites resembled the morphology of the tumor from the excisional biopsies.
The masses from the biopsies and necropsy were stained with the Fontana-Masson method (melanin stain). Results were negative. Immunohistochemistry demonstrated positive cytoplasmic staining for vimentin in polygonal and spindloid cells. There was positive cytoplasmic staining for MART-1 and S-100 in the polygonal cells but negative staining in the spindloid cells (Fig. 4). Staining with actin, HHF35, AE1/AE3, HMB-45, SMA, and tyrosinase yielded negative findings.
Transmission electron microscopy of the neoplastic cells revealed small numbers of clustered stage II melanosomes with a size range of 100 to 900 nm (Fig. 5) with myelin-like membranes in sheets. Most nuclei were small and pleomorphic, but a few had nuclear infoldings (Fig. 5). Based on these findings, a diagnosis of amelanotic melanoma was made.
Figure 5.
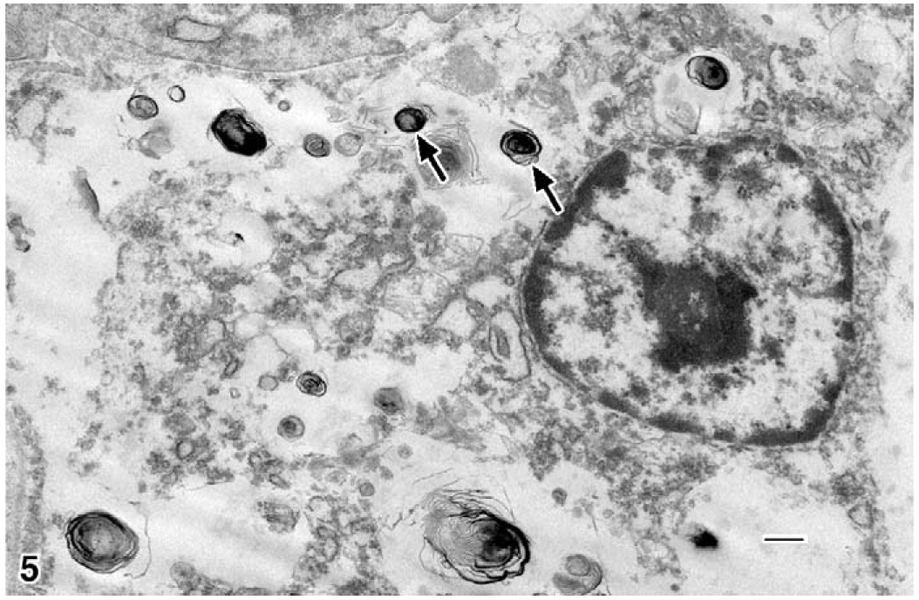
Rabbit; skin neoplasm. Electron microscopy of cutaneous polygonal mass: type II melanosomes (arrows) arranged in myelin-like sheets; a nucleus with randomly dispersed clumped chromatin and a prominent nucleolus; few cellular organelles. Uranyl acetate and lead citrate. Bar=500 nm.
This case of an amelanotic melanoma in an albino rabbit is rare. Albinism is an autosomal recessive disorder in which, despite an adequate number of normally distributed melanocytes, the melanin is not synthesized in a great enough quantity owing to a point mutation in the gene for tyrosinase. Amelanotic melanomas have been reported in human albinos and have been experimentally induced in albino guinea pigs.19,25 Human amelanotic melanomas are rare, accounting for only 5% of all melanoma cases.25 The risk factors cited are family history, familial predisposition genes, precursor nevi, childhood sunburn, and lack of skin pigmentation.11,25 Although melanomas are most commonly found in skin, any tissue that has melanin can be a site of tumor development.11
Melanomas are uncommon in rabbits.1,2,4,9,10,24 Amelanotic melanomas are extremely rare, with only one report in the literature.10 The first case of melanoma in a rabbit was reported more than 80 years ago.24 Melanomas have been reported in pet and laboratory rabbits, with New Zealand white rabbit strain being overrepresented.10 In a retrospective study at the School of Veterinary Medicine at the University of Pennsylvania with 179 pet rabbits and a total of 190 tumors, 8 were diagnosed as malignant melanoma by hematoxylin and eosin and immunohistochemistry.2 These melanomas were on the skin of the pinna, eyelid, head, limb, and scrotum. They also had the typical appearance of aggressively growing tumors with high cellular pleomorphism and large numbers of mitotic figures. All tumors contained abundant melanin.2
Melanomas have been reported in other species, including cat,18 opossum,16 monkey,20 rat,15 dog,14 ferret,27 and horse.17 Melanomas account for 7% of all malignant tumors in dogs and are most often found in the oral cavity, with metastases commonly occurring in the lung and lymph nodes. Metastases can be found in the brain, heart, and spleen and in rare cases, the bone marrow.14 Melanomas are rare in cats, with only 4 diagnoses out of 3,145 cases (0.1%) necropsied at the Animal Medical Center in New York. The most common site was intraocular, with metastasis in 63%. The prognostic factors for these cats were similar to those of humans, with location, size, cell type, and melanin content being determining factors and with depth of invasion being the most important factor.18 Tumors thicker than 2 mm in human cases often metastasize to lymph nodes, skin, subcutaneous tissue, lung, liver, small intestine, pancreas, heart, brain, and spleen.11
The ability for melanomas to express a range of antigens complicates diagnoses. Antibodies that are key to differentiating melanomas include S-100, HMB-45, MART-1, and MiTF.26 Immunohistochemistry is often necessary for a definitive diagnosis of melanomas because of their ability to be the “great pretender.” In a retrospective study of 48 feline melanoma cases, Melan-A and S-100 positive staining was found in 67.0% and 88.5% of these masses, respectively. The cells of the masses had different morphological appearances, including spindle, epithelioid, and signet ring.21 In the present case, the neoplasm was undifferentiated and contained spindloid and polygonal cells. The initial biopsy closely mimicked a basal cell carcinoma; however, results from pan-cytokeratin stain were negative. To rule out melanoma, a Fontana-Masson stain was performed, and findings were negative for melanin. Next, a panel of antibodies revealed the mass to be diffusely vimentin positive yet only MART-1 and S-100 positive for polygonal cells. The inconclusiveness of negative spindloid staining warranted transmission electron microscopy.
Transmission electron microscopy can show melanin granules that may be few in number and small in size.6 The melanosomes are generally granular and myelin-like, with shapes including oval, round, spindle-shaped, rodlike, and irregular, and they are found singular, not in groups. Type I melanosomes are difficult to recognize by morphology because they lack pigment but will stain with tyrosinase. Type II melanosomes are ovoid, have a myelin-like membranous structure, and lack pigmentation. Type III melanosomes are similar to type I but have no melanin deposits on the fibrils. Type IV melanosomes are dark because they have complete melanin and may be confused with other structures. Transmission electron microscopy can be used to identify type II and III melanosomes, which are considered diagnostic for melanoma because this is a tumor of melanocytes.6 The present case demonstrated features of type II melanosomes (Fig. 5). Nuclei in melanomas could be pleomorphic and large or exhibit numerous foldings of the nuclear membrane—features that were observed in melanomas of this case.5,7,22 One possible explanation for the usual and aggressive nature of the tumor in this rabbit could be the location of the transgene in an oncogene promoter region; or, as increasingly reported in transgene mice lines, the transgene alters the background pathology of the animal. This animal was maintained in longitudinal studies and was a member of a breeding colony. The New Zealand white rabbit is also the most frequently used strain in biomedical research. As based in the National Institutes of Health, 9 cases of basal cell carcinoma include genetically manipulated animals containing the LCAT gene (average age, 4.2 years).
The list of differentials is extensive because melanoma can mimic almost any tumor owing to the diversity of cell size and shape for this neoplasm. The principal differential diagnoses in this report include a basal cell carcinoma and sebaceous gland carcinoma. At the NIH basal cell carcinoma was diagnosed in 17/1103 (1.5%) rabbits (unpublished data).
Morphologically, this mass closely resembled a basal cell carcinoma by its ovoid and deeply basophilic nucleus, scant cytoplasm, and small and polygonal cells that are not pleomorphic. Basal cell tumors are positive for HMWCK, CK14, BerEP4, and CK7 and are negative for melanocytic markers, including S-100, HMB45, and MART-1.13
A sebaceous gland carcinoma is lobular with basaloid cells, a varying degree of differentiation, and a high mitotic rate.8 By immunohistochemistry, the tumor is negative for melanocytic markers (HMB45, MART-1, and S-100) and positive for CAM5.2, Ber-ep4, CK7, EMA, and androgen receptor marker.23 Diagnosis of this mass was inconclusive by morphology and negative Fontana-Masson. Positive immunohistochemistry staining with MART-1, a melanocyte protein marker along with the demonstration of Type II melanosomes on TEM were used to confirm the diagnosis of metastatic amelanotic melanoma.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health. Excellent technical support was provided by Jorge Chavez and Erik Lasker. Thanks to Rick Dreyfuss for photography.
Financial Disclosure/Funding
The authors received no financial support for the research and/or authorship of this article.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
References
- 1.Beniashvili DS: Spontaneous rabbit melanoblastoma. Vopr Onkol 18:84–85, 1972. [PubMed] [Google Scholar]
- 2.Bomhard W, Goldschmidt MH, Shofer FS, Perl L, Rosenthal KL, Mauldin EA: Cutaneous neoplasms in pet rabbits: a retrospective study. Vet Pathol 44:579–588, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Brousseau ME, Kauffman RD, Herderick EE, Demosky SJ Jr, Evans W, Marcovina S, Santamarina-Fojo S, Brewer HB Jr, Hoeg JM: LCAT modulates atherogenic plasma lipoproteins and the extent of atherosclerosis only in the presence of normal LDL receptors in transgenic rabbits. Arterioscler Thromb Vasc Biol 20:450–458, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Brown WH, Pearce L: Melanoma (sarcoma) of the eye in a syphilitic rabbit. J Exp Med 43:807–813, 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinoza CG, Lavallee-Grey MC: Malignant melanoma In: Pathology of Human Neoplasms, ed. Azar HA, 9th ed., pp. 405–434. Raven Press, New York, NY, 1988. [Google Scholar]
- 6.Eyden B, Moss J, Shore I, Banerjee SS: Metastic small cell malignant melanoma: a case requiring immunoelectronmicroscopy for the demonstration of lattice-deficient melanosomes. Ultrastruct Pathol 29:71–78, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Ghadially FN: The role of electron microscopy in the determination of tumour histogenesis. Diagn Histopathol 4:245–262, 1981. [PubMed] [Google Scholar]
- 8.Goldschmidt MH: Epithelial tumors In: Tumors in Domestic Animals, ed. Meuten DJ, 4th ed., pp. 46–63. Iowa State Press, Ames, IA, 2002. [Google Scholar]
- 9.Holz K, Heutgens W: Multiple Melanombildungen bein einem Kaninchen. Dtsch Tierarztl Wochenschr 62:146–148, 1955. [Google Scholar]
- 10.Hotchkiss CE, Norden H, Collins BR, Ginn PE: Malignant melanoma in two rabbits. Lab Anim Sci 44:377–379, 1994. [PubMed] [Google Scholar]
- 11.Hussein MR: Extracutaneous malignant melanomas. Cancer Invest 26:516–534, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Jones MK, Catte A, Li L, Segrest JP: Dynamics of activation of lecithin: cholesterol acyltransferase by apolipoprotein A-I. Biochem 48:11196–11210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamil ZS, Tong LCB, Habeeb AA, Ghazarian D: Non-melanocytic mimics of melanoma, part I: intradermal and intraepidermal mimics. J Clin Pathol 62:120–127, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kim DY, Royal AB, Villamil JA: Disseminated melanoma in a dog with involvement of leptomeninges and bone marrow. Vet Pathol 46:80–83, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Kurotaki T, Tomonari Y, Kanno T, Wako Y, Tsuchitani M: Malignant amelanotic melanoma behind the left eye in a female Crj:CD(SD)IGS rat: a case report. Vet Pathol 45:681–684, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kusewitt DF, Applegate LA, Bucana CD Ley RD: Naturally occurring malignant melanoma in the South American opossum (Monodelphis domestica). Vet Pathol 27:66–68, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Murphy J, Young S: Intraocular melanoma in a horse. Vet Pathol 16:539–542, 1979. [DOI] [PubMed] [Google Scholar]
- 18.Patnaik AK, Mooney S: Feline melanoma: a comparative study of ocular, oral, and dermal neoplasms. Vet Pathol 25:105–112, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Pawlowski A, Heaberman HF, Menon A: Skin melanoma induced by 7, 12-dimethylbenzanthracene in albino guinea pigs and it similarities to skin melanoma of humans. Cancer Res 40:3652–3660, 1980. [PubMed] [Google Scholar]
- 20.Pellegrini G, Bienvenu JG, Meehan JT, Mischler SA, Perry RW, Scott DW, Anderson WI: Cutaneous melanoma with metastasis in a cynomolgus monkey (Macaca fascicularis). J Med Primatol 38:444–447, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Ramos-Vara JA, Miller MA, Johnson GC, Turnquist SE, Kreeger JM, Watson GL: Melan A and S-100 protein immunohistochemistry in feline melanomas: 48 cases. Vet Pathol 39:127–132, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Marks MS: Melanosomes-dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol 8:786–797, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sramek B, Lisle A, Loy T: Immunohistochemistry in ocular carcinomas. J Cutan Pathol 35:641–646, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Sustmann: Multiple Melanombildungen bein Kaminchen. Dtsch Tierarztl Wochenschr 30:402, 1922. [Google Scholar]
- 25.Terenziani M, Spreafico F, Serra A, Podda M, Cereda S: Amelanotic melanoma in a child with oculocutaneous albinism. Med Pediatr Oncol 41:179–180, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Tong LCB, Kamil ZS, Habeeb A Al, Ghazarian D: Non-melanocytic mimics of melanoma, part II: intradermal and intraepidermal mimics. J Clin Pathol 62:290–307, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Tunev SS, Wells MG: Cutaneous melanoma in a ferret (Mustela putorius furo). Vet Pathol 39:141–143, 2002. [DOI] [PubMed] [Google Scholar]