Abstract
FAM3B mRNA has been predicted to have multiple splicing forms. Its secretory form PANDER is decreased in gastric cancers with high invasiveness and metastasis. Here we found that its non-secretory form FAM3B-258 was highly expressed in most colon cancer cell lines and colorectal adenocarcinoma tissues but not hepatocellular carcinoma, lung carcinoma and pancreatic adenocarcinoma cell lines. Elevation of FAM3B-258 was associated with poor cancer cell differentiation. Stable overexpression of FAM3B-258 in colon cancer cells downregulated adhesion proteins, upregulated Slug and Cdc42, promoted cell migration and invasion in vitro and metastasis in nude mice. Slug mediated FAM3B-258-induced downregulation of adhesion molecules, upregulation of Cdc42, and invasion of colon cancer cells. The expression of FAM3B-258 in human colorectal adenocarcinomas was positively correlated with Slug. These results suggest that FAM3B-258 promotes colon cancer cell invasion and metastasis through upregulation of Slug.
Keywords: FAM3B variant, Colon cancer cell, Slug, Cell migration, Metastasis
1. Introduction
Colorectal cancer is the third most common cancer in men and the second in women. The incidence of colorectal cancer in China is lower than that in western countries, but has increased in recent years, particularly in more developed areas [1]. Although there is good evidence demonstrating reduced morbidity and mortality resulted from early detection of invasive lesions and precursor adenomatous polyps for which surgical resection is highly effective [2], most colorectal cancers are diagnosed at an advanced stage. Metastasis is the cause of death in most colorectal cancer cases. Therefore, a better understanding of biological events contributing to colorectal cancer progression, especially those facilitating tumor invasion and metastasis, will yield novel targets for diagnosis and therapy.
Classical metastasis involves a series of steps which enable cancer cells spread from primary tumor to distant sites. In this process, cancer cells acquire the ability to migrate which will facilitate invasion to tissues and vessels. E-cadherin is a transmembrane protein which is mainly responsible for adherens junction between epithelial cells via complex formation with catenins [3]. Many studies demonstrate that loss of E-cadherin is associated with the development of invasive properties of colorectal cancer cells [4,5]. Studies on colon cancer cases show an overall decrease in the expression of E-cadherin compared to adjacent normal mucosa [6,7]. Several zinc-finger transcription factors, including Snail, Slug, ZEB1 and ZEB2, have been found binding to E-boxes of E-cadherin promoter and repressing E-cadherin expression [8–11]. However, the regulation of these transcription factors in colorectal cancer is poorly understood.
FAM3B belongs to FAM3 family which is a novel cytokine-like gene family identified in 2002 through searching for novel four-helix-bundle cytokines using structure-based methods. There are four members in this gene family (FAM3A, FAM3B, FAM3C and FAM3D) [12]. Most of the studies of FAM3B are focused on its secreting form PANDER (pancreatic-derived factor). PANDER is expressed at high levels in pancreatic α and β cells [12,13], and has been reported to be involved in regulation of β cell function under pathophysiological conditions [14–17] and in high fat diet induced fatty liver [18]. According to the NCBI Ace View database (http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/), human FAM3B mRNA has been predicted to have at least 7 alternative splicing variants. The mRNA encoding PANDER is one of them. The mRNA levels of PANDER are decreased in human gastric cancer tissues compared with normal gastric tissues [19,20]. Lower PANDER mRNA level in gastric cancer tissues was associated with deeper tumor invasion [20], suggesting that decreased expression of PANDER might be involved in gastric cancer initiation and progression. However, the functions of the proteins encoded by the other FAM3B mRNA isoforms are not clear.
In this study, we examined the mRNA levels of FAM3B mRNA variants in 6 colon adenocarcinoma cell lines, and found that one of the splicing forms of FAM3B mRNA, which consists of 940 bp nucleic acids and is predicted to encode a non-secretory protein with 258 amino acid residues, is highly expressed in most of these cell lines. We named this splicing isoform as FAM3B-258. We found that FAM3B-258 is upregulated in human colorectal adenocarcinoma tissues compared with paired normal tissues, and overexpression of FAM3B-258 in colon cancer cell line enhanced cell migration and invasion. We further explored the mechanisms involved in FAM3B-258-induced cell invasion and examined the effect of FAM3B-258 on colon cancer cell metastasis in nude mice.
2. Materials and methods
2.1. Cell lines
Human colon adenocarcinoma cell lines, hepatocellular carcinoma cell lines, lung carcinoma cell lines and pancreatic adenocarcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). HCT116, HT-29, SW480, HepG2 and CFPAC-1 cells were cultured in Dulbecco’s modified Eagle medium (DMEM). HCT-8, SW620, COLO205, HL-7702, SMMC-7721, Hep3B, NCI-H1299, A549, Capan-2 and SW1990 cells were cultured in RPMI 1640. All cell lines were cultured in medium containing 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2 at 37 °C.
2.2. Reverse transcription-PCR and quantitative real-time PCR
Surgically excised colorectal cancer tissues and their corresponding adjacent normal tissues were obtained from Zhongshan Hospital (Shanghai, China). The tissues were snap-frozen in liquid nitrogen and stored at −80 °C until mRNA extraction. Ethical approval was obtained from the Institutional Review Board of the Institute for Nutritional Sciences, and written informed consent was obtained from each patient. RNA was extracted from colorectal cancer samples and cell lines of colon adenocarcinoma, hapatocellular carcinoma, pancreatic adenocarcinoma using Trizol reagent (Invitrogen, Carlsbad, CA) and depleted of contaminating DNA with RNase-free DNase. cDNA was synthesized from 2 μg RNA with M-MuLV reverse transcriptase and oligo (dT) (Fermentas, Burlington, Ontario, Canada). cDNAs of lung carcinoma cell lines (NCI-H358, NCI-H1371, NCI-H520, NCI-H460, NCI-H522, NCI-H1437, NCI-H2126) were kindly provided by Professor Hongbin Ji (Institute of Biochemistry and Cell Biology, SIBS, Chinese Academy of Sciences, China). The PCR primers specifically targeting FAM3B-258 were: 5′-CCGCCACCAAGTCGTTCA-3′ (sense) and 5′-TTTGGGTATGCAGCCTTCTATCT-3′ (antisense). The primers for GAPDH were: 5′-CCACTCCTCCACCTTTGAC-3′ (sense) and 5′-ACCCTGTTGCTGTAGCCA-3′ (antisense). PCR products were visualized by ethedium bromide staining in 1% agarose gel.
Quantitative real-time PCR was performed by using 7900HT Fast Real-Time PCR System (Applied Biosystems Inc., Foster City, CA). Briefly, reverse-transcribed cDNA in duplicate samples were checked for target mRNA levels with SYBR Green PCR master kit (Applied Biosystems Inc., Foster City, CA). The PCR reaction consists of an initial denaturation at 95 °C for 10 min followed by 40 cycles of 30 s at 95 °C, 10 s at 60 °C and 30 s at 72 °C, and finally 10 min at 72 °C. Amplification of the target cDNA was normalized to β-actin expression. Relative levels of target mRNA expression were calculated using the 2−ΔΔCT method. The primers for real-time PCR were listed in Supplementary Table 1.
2.3. Stable transfection
The FAM3B-258 coding sequence was amplified from HT-29 cells by RT-PCR and cloned into pcDNA3.1 between the BamH I and EcoR I sites. The construct was confirmed by restriction enzyme digestion and sequencing. The PCR primers are: 5′-CGCGGATCCGGATGAAAAGCCACAGGG-3′ (sense), and 5′-GCGGAATTC CAGTGTCAGCTTCGTTC-3′ (antisense). HCT116 cells were transfected with pcDNA3.1-FAM3B-258 plasmid or pcDNA3.1 using Lipofectamine 2000 (Invitrogen). Stable transfectants were selected by treating the cells with 600 μg/ml G418 for about 3–4 weeks. Two stable clones highly expressing FAM3B-258, named FAM3B-258-1# and FAM3B-258-2#, were used in this study.
2.4. RNA interference
The small interfering RNA (siRNA) duplex oligos against Slug (siSlug) was synthesized by Genepharma (Shanghai, China). The sequence of siRNA against Slug was 5′-GCAUUUGCAGACAGGUCAATT-3′. The scrambled control siRNA sequence was: 5′-UUCUCCGAACGUGUCACGUTT-3′. HCT116 cells stably expressing FAM3B-258 were transfected with siSlug or scrambled siRNA with Lipofectamine 2000 (Invitrogen), the expression of adhesion molecules and cell invasion were examined after 48 h.
2.5. Western blot analysis
Western blot was performed as previously described [21]. The primary antibodies are as follows: E-cadherin (BD Biosciences), Slug (Santa Cruz, CA), Cdc42 (Cell Signaling, Beverly, MA) and β-actin (Sigma Aldrich, St. Louis, MO).
2.6. Cell migration and invasion assays
Cell migration was examined using the Boyden chamber. Cells (5 × 105/ml) in medium supplemented with 0.1% FBS were placed in the upper compartment of a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MA). The lower compartment was filled with medium containing 10% FBS. The two compartments were separated by a polycarbonate filter (GE Osmonics, Minnetonka, MN; 8 μm pore-size) pre-coated with 50 μg/ml collagen type I. After 7h of incubation at 37 °C, the filters were removed, stained, and the cells migrated across the filters were counted under light microscope.
For cell invasion assay, cells in serum-free medium were plated on 24-well Transwell inserts (Costar, Corning, NY) coated with Matrigel. The insert was incubated in a well containing medium supplemented with 10% FBS for 12 h. The cells that have moved through the pores to the lower side were stained and counted.
2.7. Experimental tumor metastasis assay
Tumor formation and metastatic ability of FAM3B-258/HCT116 cells or pcDNA3.1/HCT116 cells were determined by tail vein injection into 6-week-old male nude mice (BALB/cA-nu/nu, Shanghai Experimental Animal Center, China). After 4 weeks, the animals were ether-anesthetized and the lungs were removed and kept in liquid nitrogen. Frozen sections were stained with hematoxylin/eosin. Lung metastasis was quantified by counting metastatic lesions from 50 sections (~0.3 mm apart, representing about 50% of a lung volume) for each animal. Data were expressed as average numbers of metastases per 10 sections. All animals were maintained and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences.
2.8. Statistical analysis
The data represent mean ± SD from three independent experiments unless otherwise indicated. Statistical analyses were performed using Student’s t-tests. P < 0.05 was considered statistically significant.
3. Results
3.1. FAM3B-258 is highly expressed in human colon adenocarcinoma cell lines and colorectal adenocarcinoma
We first examined the mRNA levels of FAM3B-258 in various colon adenocarcinoma cell lines by RT-PCR. As shown in Fig. 1A, most of the cells express FAM3B-258, with higher levels in COLO205, HCT-8 and SW620 cells, a moderate level in SW480 cells and low levels in HCT116 and HT-29 cells. We also examined FAM3B-258 mRNA levels in cell lines derived from other types of cancers, including hepatocellular carcinoma, lung carcinoma and pancreatic adenocarcinoma cell lines. FAM3B-258 was undetected in all four hepatocellular carcinoma (Fig. 1B), nine lung carcinoma (Fig. 1C and data not shown) and three pancreatic adenocarcinoma (Fig. 1D) cell lines examined. These results suggest that FAM3B-258 is uniquely expressed in most of colorectal cancer cell lines. We then examined FAM3B-258 expression by real-time RT-PCR in 37 pairs of human colorectal adenocarcinoma samples, including primary colorectal adenocarcinoma tissues and paired normal colorectal tissues. We found FAM3B-258 mRNA significantly increased in 27 out of 37 (73%) colorectal tumors compared with the paired normal colorectal tissues (P < 0.001) (Fig. 1E). Therefore, the mRNA expression of FAM3B-258 was upregulated in most clinical colon cancer samples and cell lines.
Fig. 1.
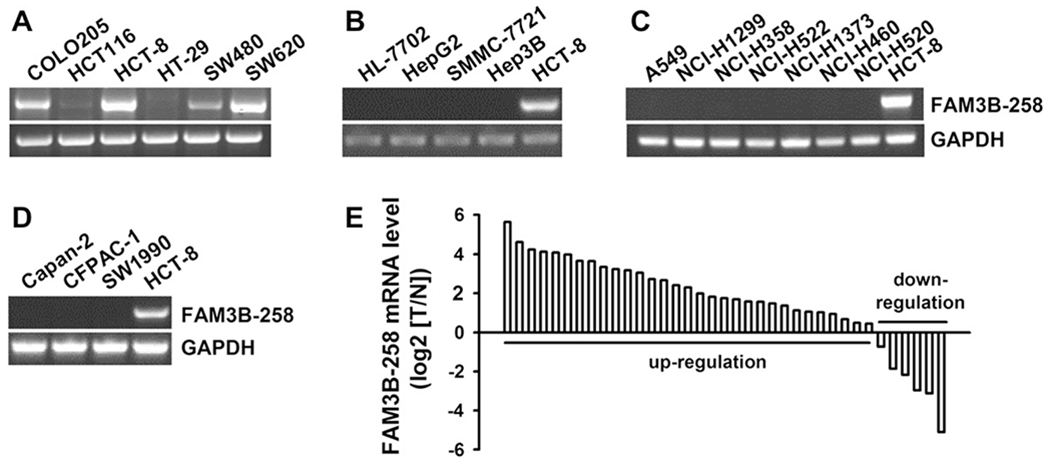
Expression profile of FAM3B-258 in human cancer cell lines and colorectal adenocarcinoma samples. RT-PCR analysis of FAM3B-258 expression in human colorectal adenocarcinoma cell lines (A), human hepatocellular carcinoma cell lines (B), human lung carcinoma cell lines (C) and human pancreatic adenocarcinoma cell lines (D). The expression of FAM3B-258 in HCT-8 cells was used as a positive control in B-D. GAPDH was used as an internal control. (E) Expression of FAM3B-258 mRNA in 37 paired colorectal adenocarcinomas and normal colorectal tissues was examined by real-time RT-PCR. T: tumor samples; N: matched normal tissues.
We further analyzed the correlation between FAM3B-258 mRNA levels and clinical and pathologic characteristics. The increase in FAM3B-258 expression in colorectal adenocarcinoma is associated with poor tumor differentiation (P = 0.042, Table 1). However, no significant correlation was identified between FAM3B-258 expression and patient gender, tumor site, tumor stage and metastasis.
Table 1.
Relationship between mRNA levels of FAM3B-258 in colorectal adenocarcinoma and clinical and pathologic features.
| Clinical characteristics | Increased FAM3B-258 (n = 27), n (%) | Non-increased FAM3B-258 (n = 10), n (%) | P value |
|---|---|---|---|
| Gender | 0.700 | ||
| Male | 17 (70.8) | 7 (29.2) | |
| Female | 10 (76.9) | 3 (23.1) | |
| Tumor site | 0.700 | ||
| Colon | 17 (70.8) | 7 (29.2) | |
| Rectum | 10 (76.9) | 3 (23.1) | |
| Tumor differentiation | 0.042* | ||
| II | 19 (67.9) | 9 (32.1) | |
| III | 8 (88.9) | 1 (11.1) | |
| T stage | 0.854 | ||
| T2 | 3 (75.0) | 1 (25.0) | |
| T3 | 10 (83.3) | 2 (16.7) | |
| T4 | 14 (66.7) | 7 (33.3) | |
| N stage | 0.443 | ||
| N0 | 17 (77.3) | 5 (22.7) | |
| N1 | 7 (70.0) | 3 (30.0) | |
| N2 | 3 (60.0) | 2 (40.0) | |
| Tumor grade | 0.392 | ||
| I | 2 (100.0) | 0 (0) | |
| II | 13 (72.2) | 5 (27.8) | |
| III | 9 (64.3) | 5 (35.7) | |
| IV | 3 (100.0) | 0 (0) |
Using SPSS 13.0 statistical software, Spearman’s rank correlation coefficient was performed to analyze the correlations of FAM3B-258 mRNA levels with clinical and pathologic parameters.
P < 0.05 was considered statistically significant.
3.2. Overexpression of FAM3B-258 promotes colon cancer cell migration and invasion
To investigate whether FAM3B-258 is involved in colorectal cancer progression, we developed stable cell clones overexpressing FAM3B-258 with HCT116 cells that had low level of endogenous FAM3B-258 (Fig. 2A). Overexpression of FAM3B-258 in HCT116 cells (FAM3B-258/HCT116) had no effect on cell proliferation and survival (data not shown). HCT116 cells transfected with control vector showed epithelial phenotype with cobblestone-like appearance and tight cell–cell attachment, while FAM3B-258 expressing HCT116 cells developed a spindle-like morphology with loose cell–cell connection (Fig. 2B). In order to explore whether the changes of morphology reflect enhanced cell motility, we examined the effect of FAM3B-258 on colon cancer cell migration and invasion by Boyden chamber assay and transwell assay, respectively. We found that FAM3B-258/HCT116 cells exhibited enhanced cell migration (Fig. 2C) and invasion (Fig. 2D) compared with HCT116 cells transfected with control vector, indicating that overexpression of FAM3B-258 promotes colon cancer cell migration and invasion.
Fig. 2.
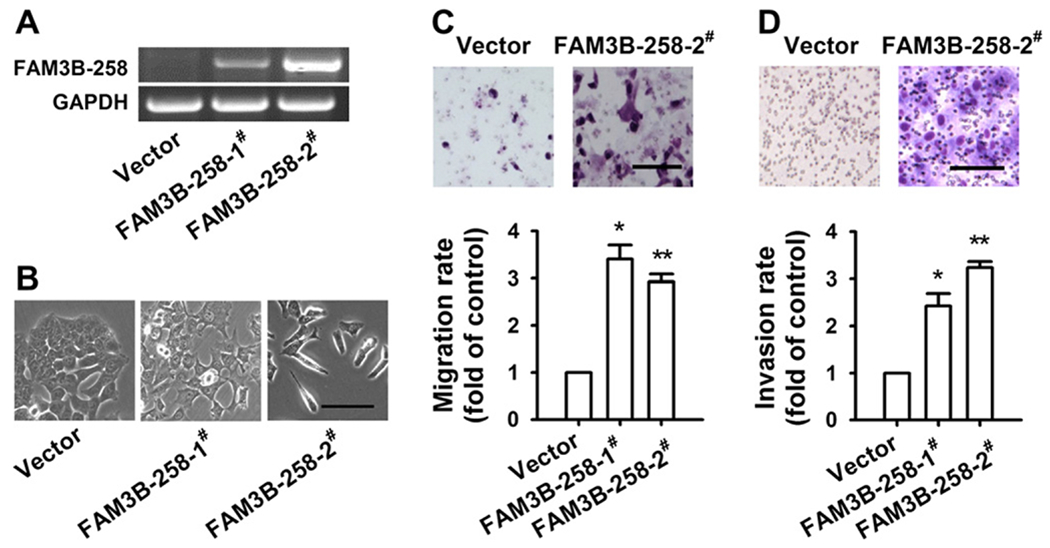
Overexpression of FAM3B-258 enhances cell migration and invasion. HCT116 cells stably transfected with pcDNA3.1 (vector) or FAM3B-258 expression plasmid (FAM3B-258-1# and FAM3B-258-2#) were examined for FAM3B-258 mRNA by RT-PCR (A). (B) The representative phase-contrast images of these cells. Scale bar = 60 μm. (C) Cell migration was measured with Boyden chamber assay. (D) Cell invasion was examined with transwell invasion assay. Data are mean ± SD, n = 3, *P < 0.05, **P < 0.01 vs. cell migration or invasion of pcDNA3.1/HCT116 cells. The representative pictures of cell migration and invasion were shown on the top panel of C and D, respectively. C and D: Scale bar = 100 μm.
3.3. Effect of FAM3B-258 on the expression of adhesion proteins and related transcription factors
We next explored the mechanisms underlying FAM3B-258 induced colon cancer cell migration and invasion. We found that compared with vector transfected HCT116 cells, FAM3B-258 transfectants expressed lower levels of adhesion molecules E-cadherin and junctional adhesion molecule (JAM) (Fig. 3A). As transcription factors Snail and Slug function as E-cadherin transcriptional repressors [8,11], we examined their mRNA levels in FAM3B-258 and vector transfected HCT116 cells. We found that Slug and Snail significantly increased in FAM3B-258 expressing cells (Fig. 3A). The alteration of E-cadherin and Slug expression was also confirmed at protein level (Fig. 3B). However, the expression of Snail was not changed at the protein level (data not shown).
Fig. 3.
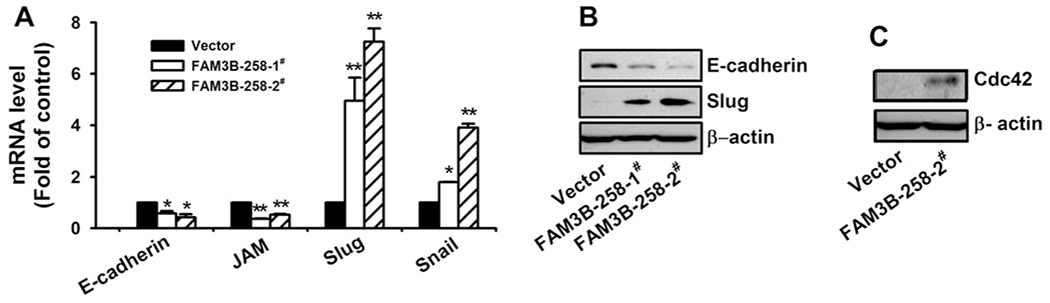
Overexpression of FAM3B-258 in HCT116 cells alters adhesion protein and related transcription factor expression. (A) HCT116 cells stably transfected with pcDNA3.1 (vector) or FAM3B-258 expression plasmid (FAM3B-258-1# and FAM3B-258-2#) were examined for mRNA levels of E-cadherin and junctional adhesion molecule (JAM), as well as transcriptional factors Slug and Snail by real-time RT-PCR. The expression of E-cadherin, Slug (B) and Cdc42 (C) at protein level was examined by Western blot. A: data are mean ± SD of three independent experiments. FAM3B-258 mRNA was normalized with that of β-actin. *P < 0.05, **P < 0.01 vs. pcDNA3.1/HCT116 cells. B and C: the experiments were performed at least 3 times and the representative results are shown.
Rho family small GTPases, such as RhoA, Rac1 and Cdc42, play pivotal roles in cancer cell migration and invasion through reorganization of actin cytoskeleton and modulation of cell–matrix interaction [22,23]. We examined the effect of FAM3B-258 on the expression of these molecules and found that overexpression of FAM3B-258 in HCT116 cells significantly induced Cdc42 expression (Fig. 3C). These results suggest that FAM3B-258 may induce colon cancer cell migration and invasion through upregulating Cdc42.
3.4. FAM3B-258 enhances cell invasion through Slug
We further asked whether Slug is involved in FAM3B-258 induced inhibition of adhesion molecule expression and promotion of cell migration and invasion. When we knocked down Slug in FAM3B-258 overexpressing HCT116 cells by RNAi (Fig. 4A), the expression of E-cadherin and JAM was significantly increased at mRNA level (Fig. 4B and C), and the expression of Cdc42 was decreased at protein level (Fig. 4D). Meanwhile, knockdown of Slug inhibited cell invasion (Fig. 4E). These results suggest that Slug mediates FAM3B-258-induced downregulation of adhesion molecules, upregulation of Cdc42, and invasion of colon cancer cells. We further examined the correlation between the expression of FAM3B-258 and Slug in 41 colorectal cancer samples. As shown in Fig. 4F, the expression of FAM3B-258 in tumor tissues is positively correlated with the level of Slug (P = 0.0001).
Fig. 4.
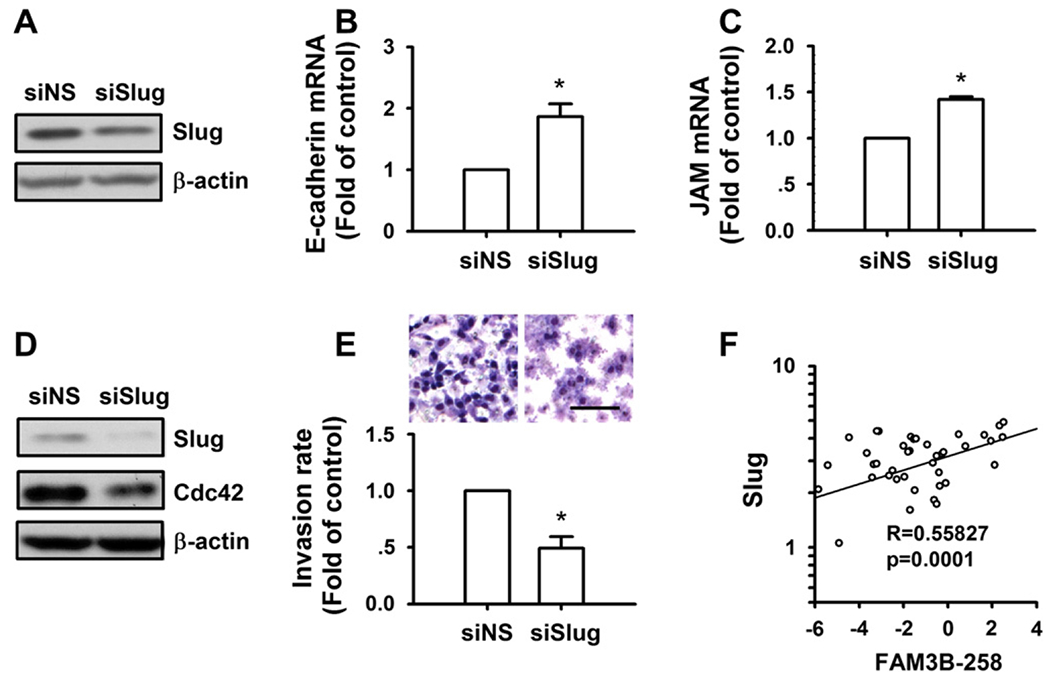
FAM3B-258 enhances cell invasion through Slug. FAM3B-258/HCT116 cells were transfected with nonspecific siRNA (siNS) or Slug specific siRNA (siSlug). After 48 h, the expression of Slug and Cdc42 was determined by Western blotting (A and D); E-cadherin and junctional adhesion molecule (JAM) mRNA levels were determined by real-time RT-PCR (B and C); cell invasion was examined with transwell assay (E, the upper panel showed the representative images of cell invasion, scale bar = 100 μm). Data are mean ± SD of three independent experiments. *P < 0.05 vs. FAM3B-258/HCT116 cells transfected with siNS. (F) mRNA levels of FAM3B-258 and Slug were examined in 41 human colorectal cancer tissues and analyzed for the correlation between FAM3B-258 mRNA level and that of Slug using SPSS13.0 by Pearson’s correlation coefficient.
3 5. Overexpression of FAM3B-258 promotes metastasis of colon cancer cells in vivo
Based on our in vitro results that FAM3B-258 overexpression promoted colon cancer cell migration and invasion, we evaluated the effect of FAM3B-258 on colon cancer cell experimental metastasis in nude mice. After 4 weeks, mice intravenously injected with FAM3B-258/HCT116 cells showed higher incidence of lung metastasis with more metastatic lesions compared with mice injected with pcDNA3.1/HCT116 cells (Fig. 5). These results suggest FAM3B-258 promotes metastasis of colon cancer cells in vivo.
Fig. 5.
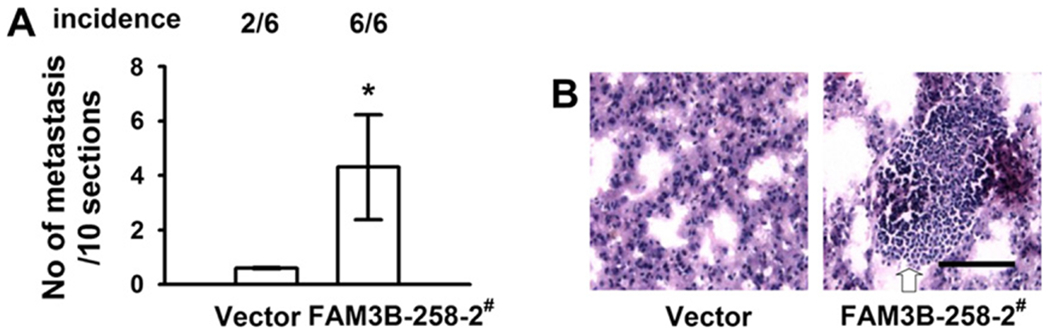
Overexpression of FAM3B-258 promotes establishment of metastases of colon cancer cells in vivo. FAM3B-258/HCT116 cells (FAM3B-258-2#) or pcDNA3.1/HCT116 cells (vector) were inoculated intravenously into athymic nude mice and assessed for lung metastasis after 4 weeks. (A) Incidence (top) and mean numbers of lung metastases (bottom, mean ± SD, *P < 0.05 vs. vector). (B) Representative images from histological assessment of lungs with H&E staining. Metastatic lesions were indicated by arrow. Scale bar = 100 μm.
4. Discussion
In this study, we found that FAM3B-258, a splicing isoform of FAM3B, is highly expressed in colorectal adenocarcinoma. Although we did not detect the expression of FAM3B-258 at protein level due to the lack of specific antibody against FAM3B-258, we established a functional role for this protein as a contributing factor to invasiveness of colon cancer cells. We further demonstrated that the effect of FAM3B-258 is mediated by Slug.
Human FAM3B mRNA has been predicted to have multiple alternative splicing forms. We found that one splicing isoform of FAM3B mRNA, which we named FAM3B-258, was expressed by most colon cancer cell lines but not hepatocellular carcinoma, pancreatic adenocarcinoma and lung carcinoma cell lines examined. These results suggest that FAM3B-258 is specifically expressed in colorectal cancer cells. Consistent with the results of colorectal cancer cell lines, we further found that the expression of FAM3B-258 mRNA was significantly upregulated in human colorectal adenocarcinoma tissues (27/37, 73%) than in paired normal colorectal tissues. Among the colorectal cancer cell lines, HCT-8, COLO205 and SW620 express higher levels of FAM3B-258, SW480 expresses medium level of FAM3B-258, HCT116 and HT-29 cells express relative low levels of FAM3B-258. COLO205 cell line was derived from metastatic ascites of a colon adenocarcinoma patient. SW480 was established from a primary colon adenocarcinoma, while SW620 was derived from a lymph node metastasis of the same patient from which the SW480 cell line was established. These results suggest that higher expression of FAM3B-258 may associate with colon adenocarcinoma invasion and metastasis.
By establishing FAM3B-258 stable expressing cells, we found that FAM3B-258 induced cell morphological changes from epithelial-like to spindle-like, and promoted cell migration and invasion. Furthermore, we found that FAM3B-258/HCT116 cells had higher incidence of lung metastasis and more metastatic lesions in nude mice than that of pcDNA3.1/HCT116 cells. All together, our in vitro and in vivo results demonstrated that elevation of FAM3B-258 promotes colorectal cancer cell migration and metastasis. Although the clinical data from colorectal adenocarcinoma patients showed that there was no correlation between FAM3B-258 mRNA level and metastasis, we found that the high level of FAM3B-258 mRNA was associated with poor differentiation of the tumor (Table 1). Cancer metastasis comprises multiple steps, including cancer cell escape from the primary tumor site, penetration to local stroma, entry into local vascular or lymphatic vessels, interaction with and adhesion to vascular endothelia, extravasation, recolonization, and expansion [24]. In primary colorectal cancers, FAM3B-258 may only contribute to some steps of cancer metastasis, and may work together with other factors to promote cancer metastasis. These possibilities remain further investigation.
We explored the mechanisms underlying FAM3B-258-induced cell migration and invasion, and found that elevation of FAM3B-258 inhibited the expression of E-cadherin and JAM. E-cadherin is a well-studied tumor suppressor which belongs to a family of calcium-dependent cell adhesion molecules. Abnormal expression of this protein in a number of colon cancer cases has been described [25,26]. Recent findings suggest that downregulation of E-cadherin contributes to tumor cell invasion [27]. JAM (also named F11R) belongs to the junctional adhesion molecule superfamily. It is also an important regulator of tight junction assembly in epithelia [28]. Breakdown of cell-cell interactions and downregulation of junctional and adhesion proteins are believed to be key steps in cancer invasion and metastasis. Therefore, downregulation of E-cadherin and JAM in colon adenocarcinoma cells by FAM3B-258 may contribute to enhanced colon cancer cell migration and invasion.
Slug has been reported to be overexpressed in various types of cancers [29]. Elevated expression of Slug is associated with reduced E-cadherin expression, advanced tumor grade, lymph node metastasis, and poor prognosis [11,29–32]. Slug expression was shown to be positive in 37% of cases of primary colorectal cancer, which correlated significantly with metastasis [33]. Recent studies showed that Slug overexpression in colorectal cancer cells led to epithelial to mesenchymal transition and enhanced cell migration, invasion, and in vivo tumor development [31]. In the present study, we found that overexpression of FAM3B-258 in HCT116 leads to upregulation of Slug and enhancement of cell migration and invasion. Furthermore, we found that knockdown of Slug in FAM3B-258/HCT116 cells reduced E-cadherin and JAM expression and inhibited cell invasion. These results demonstrate that FAM3B-258 promotes cell invasion through Slug dependent inhibition of E-cadherin and JAM expression. Supporting the in vitro results, we found that FAM3B-258 expression in colorectal cancer tissues was positively correlated with the expression of Slug.
Overexpression of Rho GTPases is associated with reorganization of actin cytoskeleton, and increase in cell migration, invasion and metastasis which are important aspects of cancer progression [34]. RhoA, Rac1, and Cdc42 are the best studied members of small GTPase family [35,36]. We found that overexpression of FAM3B-258 in HCT116 cells enhanced Cdc42 expression but had no effect on Rho and Rac1 expression (data not shown). Thus, upregulation of Cdc42 may contribute to FAM3B-258-induced colon cancer cell migration and invasion. A positive association between higher Cdc42 and lower E-cadherin expression has been reported in lung cancer tissues, and this abnormal expression profile is associated with tumor progression [37]. As we observed that upregulation of Cdc42 by FAM3B-258 was in company with Slug-dependent E-cadherin downregulation, we then examined if Cdc42 is also a downstream effector of Slug in FAM3B-258/HCT116 cells. We found for the first time that FAM3B-258 upregulated Cdc42 expression through Slug. Overall, our data suggest that FAM3B-258 promotes colorectal cancer cell migration and invasion through Slug mediated downregulation of E-cadherin and JAM and upregulation of Cdc42.
Multiple signaling molecules have been reported to be involved in regulation of Slug expression. The promoter region of Slug has E-boxes and binding sites for activator protein 1 (AP1), Smad, and LEF1/T cell factor [32]. Twist1 directly binds to E-box on the Slug promoter to induce Slug transcription [38]. ERK-Fra-1/c-Jun signaling pathway regulates breast cancer cell migration through AP1 dependent upregulation of Slug expression [39]. Transforming growth factor-β (TGFβ) induces Slug expression through binding of Smad complex to the promoter of Slug [40]. Wnt signaling activates the T cell factor-β-catenin complex which binds directly to the promoter of Slug and actives Slug expression [41]. We found that overexpression of FAM3B-258 in HCT116 cells had no effect on the mRNA levels of Twist, MEK, Jun and TGFβ, on the phosphorylation of ERK and Smad 2, and on the translocation of β-catenin into nucleus. Treatment of FAM3B-258/HCT116 cells with MEK inhibitor had no effect on Slug expression (data not shown). These results suggest that Twist, ERK, Wnt signaling and TGFβ-Smad signaling may not be involved in FAM3B-258 induced Slug upregulation. Whether Slug is a direct target of transcriptional activation by FAM3B-258 or whether other signaling molecules are situated between them as part of a regulatory cascade remains further investigation.
In addition to transcriptional regulation, Slug is also regulated at posttranslational level. Wild-type p53 has been reported to facilitate Slug degradation through upregulation of MDM2 [42]. We found that overexpression of FAM3B-258 in HCT116 cells had no effect on p53 expression at both mRNA and protein levels (data not shown), indicating that FAM3B-258 upregulates Slug through p53 signaling independent pathway.
In summary, our results show that FAM3B-258 is highly expressed in most colorectal adenocarcinomas. Elevation of FAM3B-258 promoted colon cancer cell migration and invasion through Slug dependent downregulation of E-cadherin and JAM. Slug-dependent enhancement of Cdc42 expression by FAM3B-258 may also contribute to colon cancer cell migration and invasion (Fig. 6). Although due to the higher sequence similarity among FAM3B variants we could not design specific siRNA against FAM3B-258 and utilize RNAi to get reverse results of FAM3B-258 overexpressing cells, our in vitro and in vivo studies with FAM3B-258 overexpressing cells, and clinical data strongly support that FAM3B-258 plays an important role in invasion and metastasis of colon cancer cells. Our findings provide new insights into the progression of colorectal cancer and potential therapeutic targets against this disease.
Fig. 6.
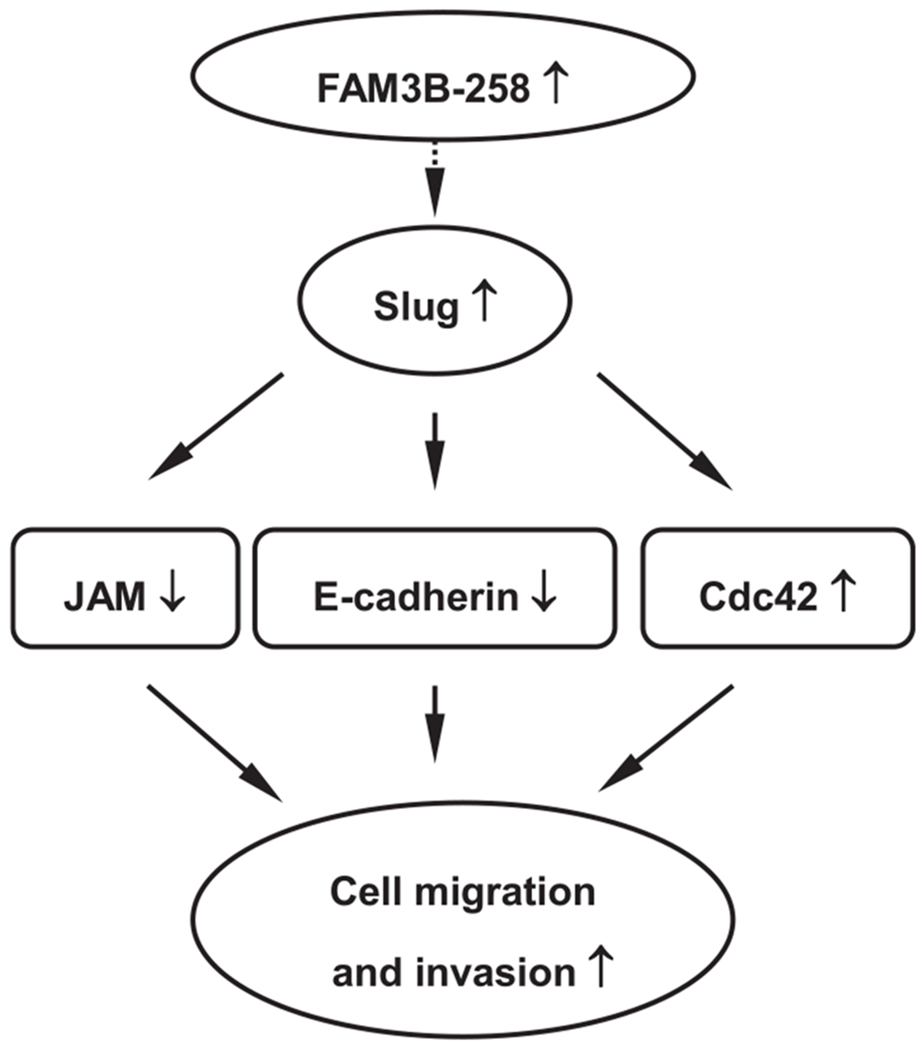
Schematic summary of FAM3B-258-induced colorectal cancer cell migration and invasion. Elevation of FAM3B-258 in colorectal cancer cells upregulates Slug expression which in turn represses the expression of E-cadherin and junctional adhesion molecule (JAM), and enhances the expression of Cdc42. Alterations in these molecules ultimately lead to enhanced migration and invasion of colon cancer cells.
Supplementary Material
Acknowledgements
We thank Dr. Jiaqiang Huang (SABiosciences, Qiagen, USA) for technical assistance and Professor Hongbin Ji (Institute of Biochemistry and Cell Biology, SIBS, Chinese Academy of Sciences, China) for providing cDNAs of lung carcinoma cell lines. This work was supported by research Grants from the National Basic Research Program of China (2011CB504002), National Natural Science Foundation of China (81172316, 30970586), and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-R-10).
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canlet.2012.09.026.
References
- [1].Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, Liu JH, Lou QY, Gan AH, Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey, World J. Gastroenterol 16 (2010) 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Umar A, Greenwald P, Alarming colorectal cancer incidence trend: a case for early detection and prevention, Cancer Epidemiol. Biomarkers Prev 18 (2009) 1672–1673. [DOI] [PubMed] [Google Scholar]
- [3].Gumbiner BM, McCrea PD, Catenins as mediators of the cytoplasmic functions of cadherins, J. Cell Sci 17 (1993) 155–158. [DOI] [PubMed] [Google Scholar]
- [4].Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA, Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways, Cancer Res. 15 (2008) 3645–3654. [DOI] [PubMed] [Google Scholar]
- [5].Tsanou E, Peschos D, Batistatou A, Charalabopoulos A, Charalabopoulos K, The E-cadherin adhesion molecule and colorectal cancer. A global literature approach, Anticancer Res. 28 (2008) 3815–3826. [PubMed] [Google Scholar]
- [6].Schuhmacher C, Becker I, Oswald S, Atkinson MJ, Nekarda H, Becker KF, Mueller J, Siewert JR, Hofler H, Loss of immunohistochemical E-cadherin expression in colon cancer is not due to structural gene alterations, Virchows Arch. 434 (1999) 489–495. [DOI] [PubMed] [Google Scholar]
- [7].Rosivatz E, Becker I, Bamba M, Schott C, Diebold J, Mayr D, Hofler H, Becker KF, Neoexpression of N-cadherin in E-cadherin-positive colon cancers, Int. J. Cancer 111 (2004) 711–719. [DOI] [PubMed] [Google Scholar]
- [8].Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA, The transcription factor snail controls epithelialmesenchymal transitions by repressing E-cadherin expression, Nat. Cell Biol 2 (2000) 76–83. [DOI] [PubMed] [Google Scholar]
- [9].Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, Helfrich BA, Garrett-Mayer E, Bunn PA, Drabkin HA, ZEB1-responsive genes in non-small cell lung cancer, Cancer Lett. 300 (2011) 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F, The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion, Mol. Cell 7 (2001)1267–1278. [DOI] [PubMed] [Google Scholar]
- [11].Hajra KM, Chen DY, Fearon ER, The SLUG zinc-finger protein represses Ecadherin in breast cancer, Cancer Res. 62 (2002) 1613–1618. [PubMed] [Google Scholar]
- [12].Zhu Y, Xu G, Patel A, McLaughlin MM, Silverman C, Knecht K, Sweitzer S, Li X, McDonnell P, Mirabile R, Zimmerman D, Boyce R, Tierney LA, Hu E, Livi GP, Wolf B, Abdel-Meguid SS, Rose GD, Aurora R, Hensley P, Briggs M, Young PR, Cloning, expression, and initial characterization of a novel cytokine-like gene family, Genomics 80 (2002) 144–150. [DOI] [PubMed] [Google Scholar]
- [13].Carnegie JR, Robert-Cooperman CE, Wu J, Young RA, Wolf BA, Burkhardt BR, Characterization of the expression, localization, and secretion of PANDER in alpha-cells, Mol. Cell. Endocrinol 325 (2010) 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cao X, Gao Z, Robert CE, Greene S, Xu G, Xu W, Bell E, Campbell D, Zhu Y, Young R, Trucco M, Markmann JF, Naji A, Wolf BA, Pancreatic-derived factor (FAM3B), a novel islet cytokine, induces apoptosis of insulin-secreting beta-cells, Diabetes 52 (2003) 2296–2303. [DOI] [PubMed] [Google Scholar]
- [15].Cao X, Yang J, Burkhardt BR, Gao Z, Wong RK, Greene SR, Wu J, Wolf BA, Effects of overexpression of pancreatic derived factor (FAM3B) in isolated mouse islets and insulin-secreting betaTC3 cells, Am. J. Physiol. Endocrinol. Metab 289 (2005) E543–550. [DOI] [PubMed] [Google Scholar]
- [16].Yang J, Wang C,Li J, Burkhardt BR, Robert-Cooperman CE, Wilson C, Gao Z, Wolf BA, PANDER binds to the liver cell membrane and inhibits insulin signaling in HepG2 cells, FEBS Lett. 583 (2009) 3009–3015. [DOI] [PubMed] [Google Scholar]
- [17].Robert-Cooperman CE, Carnegie JR, Wilson CG, Yang J, Cook JR,Wu J, Young RA, Wolf BA, Burkhardt BR, Targeted disruption of pancreatic-derived factor (PANDER, FAM3B) impairs pancreatic beta-cell function, Diabetes 59 (2010) 2209–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li J, Chi Y, Wang C, Wu J, Yang H, Zhang D, Zhu Y, Wang N,Yang J, Guan Y, Pancreatic-derived factor promotes lipogenesis in the mouse liver: role of the Forkhead box 1 signaling pathway, Hepatology 53 (2011) 1906–1916. [DOI] [PubMed] [Google Scholar]
- [19].Shao Y, Yang SB, Wang MW, Wu BY, You WD, Li H, Gene expression profile of human adenocarcinoma by cDNA microarray and clustering, Zhonghua Yi Xue Yi Chuan Xue Za Zhi 21 (2004) 110–115. [PubMed] [Google Scholar]
- [20].Huang H, Wu B, Yang S, Shao Y, You W, Wang W, Wang M, Downregulation of PANDER expression in gastric cancer, World Chin. J. Digestol 16 (2008) 1513–1518. [Google Scholar]
- [21].Wang O, Cai K, Pang S, Wang T, Qi D, Zhu Q, Ni Z, Le Y, Mechanisms of glucose-induced expression of pancreatic-derived factor in pancreatic beta-cells, Endocrinology 149 (2008) 672–680. [DOI] [PubMed] [Google Scholar]
- [22].Price LS, Collard JG, Regulation of the cytoskeleton by Rho-family GTPases: implications for tumour cell invasion, Semin. Cancer Biol 11 (2001) 167–173. [DOI] [PubMed] [Google Scholar]
- [23].Yamazaki D, Kurisu S, Takenawa T, Regulation of cancer cell motility through actin reorganization, Cancer Sci. 96 (2005) 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liotta LA, Stetler-Stevenson WG, Steeg PS, Cancer invasion and metastasis: positive and negative regulatory elements, Cancer Invest. 9 (1991) 543–551. [DOI] [PubMed] [Google Scholar]
- [25].Van der Wurff AA, Arends JW, Van der Linden EP, Ten Kate J, Bosman FT, L-CAM expression in lymph node and liver metastases of colorectal carcinomas, J. Pathol 172 (1994) 177–181. [DOI] [PubMed] [Google Scholar]
- [26].Ngan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, Hemmi H, Takemasa I, Ikeda M, Sekimoto M, Monden M, A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer, J. Surg. Oncol 15 (2007) 652–662. [DOI] [PubMed] [Google Scholar]
- [27].Thiery JP, Acloque H, Huang RY, Nieto MA, Epithelial-mesenchymal transitions in development and disease, Cell 139 (2009) 871–890. [DOI] [PubMed] [Google Scholar]
- [28].Naik UP, Eckfeld K, Junctional adhesion molecule 1 (JAM), J. Biol. Regul. Homeost. Agents 17 (2003) 341–347. [PubMed] [Google Scholar]
- [29].Alves CC, Carneiro F, Hoefler H, Becker KF, Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers, Front. Biosci 14 (2009) 3035–3050. [DOI] [PubMed] [Google Scholar]
- [30].Uchikado Y, Natsugoe S, Okumura H, Setoyama T, Matsumoto M, Ishigami S, Aikou T, Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma, Clin. Cancer Res. 11 (2005) 1174–1180. [PubMed] [Google Scholar]
- [31].Camp ER, Findlay VJ, Vaena SG, Walsh J, Lewin DN, Turner DP, Watson DK, Slug expression enhances tumor formation in a noninvasive rectal cancer model, J. Surg. Res 170 (2011) 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shih JY, Yang PC, The EMT regulator slug and lung carcinogenesis, Carcinogenesis 32 (2011) 1299–1304. [DOI] [PubMed] [Google Scholar]
- [33].Shioiri M, Shida T, Koda K, Oda K, Seike K, Nishimura M, Takano S, Miyazaki M, Slug expression is an independent prognostic parameter for poor survival in colorectal carcinoma patients, Br. J. Cancer 94 (2006) 1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rathinam R, Berrier A, Alahari SK, Role of Rho GTPases and their regulators in cancer progression, Front. Biosci 17 (2011) 2561–2571. [DOI] [PubMed] [Google Scholar]
- [35].Barber MA, Welch HC, PI3K and RAC signalling in leukocyte and cancer cell migration, Bull. Cancer 93 (2006) E44–E52. [PubMed] [Google Scholar]
- [36].Wennerberg K, Der CJ, Rho-family GTPases: it is not only Rac and Rho (and I like it), J. Cell Sci 117 (2004) 1301–1312. [DOI] [PubMed] [Google Scholar]
- [37].Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, Yang LH, Li QC, Zhao C, Wang EH, Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer, Lung Cancer 63 (2009) 375–382. [DOI] [PubMed] [Google Scholar]
- [38].Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J, Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis, Cancer Res. 71 (2011) 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen H, Zhu G, Li Y, Padia RN, Dong Z, Pan ZK, Liu K, Huang S, Extracellular signal-regulated kinase signaling pathway regulates breast cancer cell migration by maintaining slug expression, Cancer Res. 69 (2009) 9228–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brandl M, Seidler B, Haller F, Adamski J, Schmid RM, Saur D, Schneider G, IKK(α) controls canonical TGF(ß)-SMAD signaling to regulate genes expressing SNAIL and SLUG during EMT in panc1 cells, J. Cell Sci 123 (2010) 4231–4239. [DOI] [PubMed] [Google Scholar]
- [41].Nelson WJ, Nusse R, Convergence of Wnt, beta-catenin, and cadherin pathways, Science 303 (2004) 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH, Yuan A, Lin CW, Yang SC, Chan WK, Li KC, Hong TM, Yang PC, P53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug, Nat. Cell Biol 11 (2009) 694–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


