Abstract
Purpose:
Standardized uptake values (SUV), total metabolic tumor volumes (TMTV), and total lesion glycolysis (TLG) based on positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG/positron emission tomography (PET) are established outcome predictors in FDG-avid lymphomas. We therefore investigated whether these biomarkers also have prognostic value in extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma), with a focus on patients treated with anti-CD20 antibody-based immunotherapy.
Procedures:
Pre-therapeutic [18F]FDG/PET scans of 61 treatment-naïve MALT lymphoma patients, including 35 scheduled for anti-CD20 antibody-based immunotherapy, were included in this retrospective study. SUVmean, SUVmax, TMTV, and TLG were measured and tested for 2 year progression-free survival (PFS) prognostication, using Cox regression analyses. Receiver-operating-characteristic curves were used to determine optimal cut-offs for prognostic [18F]FDG/PET parameters, and Kaplan-Meier estimates with log rank tests were performed.
Results:
After two years, progression had occurred in 12/61 patients (CD20-anitbody group: 6/35). TLG emerged as the only significant prognostic factor for 2 year PFS in the multivariate analyses with forward selection, both in entire cohort (hazard ratio HR, 1.001; 95% CI, 1.001–1.002; P < 0.0001) and in the CD20-antibody group (HR, 1.001; 95% CI, 1.001–1.002; P = 0.001). However, in the entire population, where 8/26 patients with a TLG>90 (30.8%) vs. 4/35 patients with a TLG≤90 (11.4%) showed progression within the 2 year observation period, TLG-based separation of risk groups failed (HR, 0.35; 95% CI, 0.10-1.15; P=0.069); whereas in the CD20-antibody group, where 6/16 patients with a TLG>90 (37.5%) vs. 0/19 patients with a TLG≤90 (0.0%) showed progression, risk group separation was successful (HR, 0.010; 95% CI, 0.0001-8.068; P = 0.003).
Conclusions:
TLG may improve early risk stratification of MALT lymphoma patients treated with CD20-antibody-based immunotherapy.
Keywords: Lymphoma, Deoxyglucose, Positron Emission Tomography, Prognosis
Introduction
Outcome prognostication and identification of risk groups play an important role in the management of patients with malignant lymphoma. An increasing number of recent studies suggest that, for patients with classical Hodgkin, diffuse large B-cell (DLBCL), follicular, as well as T-cell lymphomas, quantitative parameters derived from pre-therapeutic positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG)/positron emission tomography (PET) – such as maximum standardized uptake value (SUVmax), total metabolic tumor volume (TMTV), or total lesion glycolysis (TLG) – are prognostic for clinical outcomes, and may therefore improve risk stratification [1–5].
For extranodal marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma), only three studies have, so far, evaluated quantitative pre-therapeutic [18F]FDG/PET-derived parameters for outcome prognostication [6–8], probably because of the variable FDG avidity of this lymphoma subtype, due to which [18F]FDG/PET is not recommended for imaging of MALT lymphomas by the International Conference on Malignant Lymphoma (ICML) [9]. Notably, none of the these studies —including the single, very recently published study that included an evaluation of TMTV and TLG, and was unable to establish either parameter as a prognostic factor for overall survival (OS) or progression-free survival (PFS) [6]— focused on a specific treatment group, contrary to studies in other lymphoma subtypes. Instead, therapeutic approaches in the investigated MALT lymphoma populations ranged from antibiotics and radiation therapy to surgery and chemotherapy —a heterogeneity that may have influenced the results of the respective studies. Apart from antibiotics, CD20-antibody-based immunotherapy is the most commonly used systemic treatment for MALT lymphoma, and has been shown to carry significant anti-tumor activity in untreated as well as relapsed MALT lymphoma [10].
In the present study, it was therefore our aim to determine whether pre-therapeutic [l8F]FDG/PET-derived quantitative parameters, i.e., SUV, TMTV and TLG, carry prognostic information, with regard to 2 year PFS, in (1) MALT lymphoma patients treated with CD20-antibody-based immunotherapy, as compared to (2) a general population of MALT lymphoma patients receiving different types of treatment.
Materials and Methods
Patients and design
Patients with histologically proven, treatment-naïve MALT lymphoma, who had undergone [18F]FDG/PET/CT (x-ray computed tomography) or [18F]FDG/PET/MRI (magnetic resonance imaging) for routine pre-therapeutic staging at the local tertiary care center between 2008 and 2015, had shown at least some degree of [18F]FDG avidity (equivalent to Deauville score of ≥2 [11]), and for whom follow-up over a period of ≥2 years after diagnosis/imaging, or until documented progression, was available, were included in our retrospective study. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Ethics committee approval was obtained; for this type of study (i.e., with a retrospective design), formal consent is not required. The histological diagnosis of MALT lymphoma was made by a reference pathologist who analyzed tissue samples (obtained by biopsy or surgery) according to the 2016 revision of the World Health Organization classification of lymphoid neoplasms. According to our institutional standard of care, the decision to perform [18F]FDG/PET was independent of age, performance status, laboratory findings, disease stage or localization, or treatment strategy.
Imaging protocols
For both [18F]FDG/PET/CT and [18F]FDG/PET/MRI, PET was performed 60 min after intravenous administration of a target dose of 3 MBq/kg (minimum injection dose, 200 MBq) of [18F]FDG. All patients had fasted for at least 5 h before imaging; the upper blood glucose level limit was 150 mg/dl.
Whole-body [18F]FDG/PET/CT was performed using a 64-row, multi-detector hybrid scanner (Biograph TruePoint 64; Siemens, Erlangen, Germany) with an axial field-of-view (FOV) of 216 mm, and a PET sensitivity of 7.6 cps/kBq. PET was performed with 3 min per bed position, four iterations and 21 subsets, a 5-mm slice thickness, and a 168x168 matrix, using the point spread function (PSF)-based reconstruction algorithm TrueX. Contrast-enhanced venous-phase CT was used for attenuation correction, and was performed after the intravenous injection of 90-120 ml of a tri-iodinated, non-ionic contrast medium at a rate of 4 ml/s, with a tube current of 230 mA, a tube voltage of 120 kVp, a collimation of 64x0.6 mm, a 5-mm slice thickness with a 3-mm increment, and a 512x512 matrix.
Whole-body [18F]FDG/PET/MRI was performed using an integrated, simultaneous, 3-Tesla PET/MRI hybrid scanner (Biograph mMR; Siemens, Erlangen, Germany), with an axial FOV of 258 mm, and a PET sensitivity of 13.2 cps/kBq. PET was performed with 5 min per bed position; three iterations and 21 subsets, a 4.2-mm slice thickness; and a 172x172 matrix, using the PSF-based reconstruction algorithm HD-PET. A coronal, two-point Dixon, three-dimensional, volume-interpolated, T1-weighted breath-hold MR sequence (VIBE) was obtained for attenuation correction, with a repetition time (TR)/echo times (TE), 3.6/TE1=1.23 ms, TE2=2.46 ms; one average, two echoes; a 10° flip angle; a 320x175 matrix with a 430x309 mm FOV; and a 3-mm slice thickness with 0.6-mm gap. In addition, an axial, echo-planar imaging SPAIR (spectral adiabatic inversion recovery)-based DWI sequence was obtained during free-breathing for the entire anatomy, using b-values of 50 and 800; a TR/TE of 6800/63 ms; six averages and one echo; a 180° flip angle; a 168x104 matrix with a 440x340 mm FOV; and a 6-mm slice thickness with a 1.2-mm gap.
Image analysis
A senior board-certified radiologist and a senior board-certified nuclear medicine physician rated all PET/MRI and PET/CT examinations in consensus, side-by-side. Blinded to the clinical data, pre-therapeutic Deauville scores —based on the lesion with the most intense uptake for patients with multiple lesions— were obtained, as previously recommended [11]. Using the Beth Israel PET/CT viewer plugin for FIJI (http://petctviewer.org/), total metabolic tumor volumes (TMTVs) were assessed based on isocontour volumes of interest, using the previously recommended 41% SUVmax threshold (Fig. 1) [12]. When there was low [18F]FDG uptake relative to the surrounding tissues (e.g., with Deauville scores 2 or 3), the CT or DWI components of PET/CT or PET/MRI could be used to assist with lesion delineation.
Fig. 1.

A 53-year-old patient with MALT lymphoma. Total metabolic tumor volume (TMTV, blue areas) shown in a the [18F]FDG-PET maximum intensity projection includes the [18F]FDG-avid gastric MALT lymphoma (blue arrows) seen on both b contrast-enhanced CT and c PET, and the paraaortic nodal manifestations where the maximum standardized uptake value (SUVmax) was measured (red dot in a).
Post-reconstruction harmonization of SUVs, using the previously described ComBat method [13], was performed to correct for technical differences between the PET/CT and PET/MRI scanners that are known to affect SUVs [14]. Finally, TLG (product of TMTV and SUVmean) [15] was calculated.
Statistical analysis
Descriptive statistics for demographic, clinical and imaging parameters were calculated. Independent sample t-tests were used to test for significant differences of the imaging parameters between gastric and non-gastric MALT lymphomas. Univariate Cox regression analyses were performed to model the influence of demographic, clinical, and imaging parameters on the PFS. For all parameters that demonstrated statistical significance in the univariate analysis, a multivariate Cox regression analysis with forward selection (based on the likelihood ratio) was performed.
Receiver-operating-characteristic (ROC) curves were constructed, and used to determine optimal cut-offs for those [18F]FDG/PET parameters that differed significantly, at P ≤ 0.05, between patients who achieved, and those who did not achieve, 2 year PFS; areas under the ROC curve (AUC) were calculated. Survival functions based on parameters with statistical significance in the multivariate analysis were calculated, using Kaplan-Meier estimates, and log rank tests were used for group comparisons.
The above evaluation was performed for the entire patient cohort (regardless of the type of treatment received) on the one hand, and then also separately for the subgroup of patients that received CD20-antibody-based immunotherapy. All statistical tests were performed using IBM SPSS 23.0 (Armonk, NY, USA).
Results
Sixty-one consecutive patients (37 women and 24 men; mean age, 66.5 ± 12.6 years) met our criteria for participation in the study. Fifty-two patients had undergone pre-therapeutic [18F]FDG/PET using contrast-enhanced PET/CT, and nine patients using PET/MRI. Low [18F]FDG uptake (≤mediastinum; equivalent to Deauville 2) was observed in 12 patients (18.7%); uptake higher than that of the mediastinum, but not exceeding that of the liver (equivalent to Deauville 3) was observed in 11 patients (17.2%); and uptake greater than that of the liver (equivalent to Deauville 4 or 5) was observed in the remaining 41 patients (64.1%). Involved anatomic regions/organs are listed in Table 1.
Table 1.
Involvement of anatomic sites in the entire cohort of 61 MALT lymphoma patients, and in the 35 patients treated with CD20-antibody-based immunotherapy
| Entire cohort | CD20-antibody group | |
|---|---|---|
| Lymph nodes | 11 | 6 |
| Waldeyer ring | 2 | 1 |
| Lungs | 11 | 7 |
| Stomach | 15 | 7 |
| Small intestine | 1 | 1 |
| Large intestine | 1 | 1 |
| Soft-tissues | 6 | 4 |
| Breast | 5 | 3 |
| Liver | 2 | 2 |
| Kidney | 3 | 2 |
| Orbita | 15 | 10 |
| Urinary bladder / tract | 2 | 2 |
| Adrenal glands | 1 | 1 |
| Salivary glands | 3 | 2 |
| Total | 78 | 49 |
Thirty-five patients received CD20-antibody-based immunotherapy (rituximab, 27; ofatumumab, eight); seven received clarithromycin (one in combination with lenalidomide); five exclusively underwent helicobacter pylori eradication; four were treated with radiotherapy; two underwent surgery; and one patient each was treated with chlorambucil, zevalin, R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R-BENDA (rituximab, bendamustine), or R-2CDA (rituximab, cladribine). In three patients, a watch-and-wait strategy was used.
Entire cohort (all treatments)
After two years, progression had occurred in 12/61 patients (19.67%), including three deaths. Descriptive data for demographic, clinical, and imaging parameters, and results of the univariate Cox regression analyses are provided in Table 2. Gastric (n = 15) and non-gastric (n = 46) MALT lymphomas did not differ with regard to SUVmean (mean, 3.79 ± 1.56 vs. 3.68 ± 1.85; P = 0.84), SUVmax (mean, 7.62 ± 5.62 vs. 7.22 ± 4.17; P = 0.77), TMTV (mean, 88.17 ± 129.56 vs. 72.30 ± 200.59; P = 0.78), or TLG (mean, 472.22 ± 1009.59 vs. 224.79 ± 512.03; P = 0.22).
Table 2.
Demographic, clinical, and imaging data, and results of univariate Cox regression analyses for the 61 MALT lymphoma patients
| Univariate analysis of PFS | |||
|---|---|---|---|
| Characteristics | Frequency (%) or Mean (± SD) | HR (95% CI) | P-value |
| Age ≥70 years | 30 (49.2%) | 1.038 (0.34-3.22) | 0.94 |
| Ann Arbor stage | |||
| I | 36 (59.0%) | 1 | |
| II | 10 (16.4%) | 0.45 (0.10-2.01) | 0.30 |
| III | 2 (3.3%) | 1.44 (0.291-7.14) | 0.66 |
| IV | 13 (21.3%) | 5.10 (0.84-31.12) | 0.077 |
| Plasmacytic differentiation | 15 (24.6%) | 1.14 (0.31-4.20) | 0.85 |
| Elevated LDH | 5 (8.2%) | 0.38 (0.083-1.73) | 0.21 |
| Elevated β2 microglobulin Missing | 22 (36.1%) 6 (9.8%) | 0.81 (0.25-2.66) | 0.73 |
| ECOG ≥1 | 7 (11.5%) | 24.44 (0.12-49496.68) | 0.20 |
| SUVmax | 7.32 ± 4.52 | 1.13 (1.004-1.23) | 0.043 |
| SUVmean | 3.71 ± 1.77 | 1.16 (0.86-1.56) | 0.34 |
| TMTV (ml) | 76.20 ± 184.78 | 1.002 (1.001-1.004) | 0.002 |
| TLG | 258.63 ± 667.84 | 1.001 (1.001-1.002) | <0.0001 |
None of the demographic or clinical parameters, but all [18F]FDG/PET-derived parameters except SUVmean were significant at univariate analysis. In the multivariate analysis, only TLG (hazard ratio HR, 1.001; 95% CI, 1.001–1.002; P < 0.0001), but not SUVmax (P = 0.85) or TMTV (P = 0.27), retained the significant association with PFS.
The ROC analysis revealed an optimal TLG cut-off value of 90 for separation of patients who achieved, and those who did not achieve, 2 year PFS; the AUC was 0.68 (95% CI, 0.50-0.87; P = 0.05) (Fig. 2). Based on this threshold, 8/26 patients with a TLG > 90 (30.8%) vs. 4/35 patients with a TLG ≤ 90 (11.4%) showed progression within the 2 year observation period (HR, 0.35; 95% CI, 0.10-1.15; P = 0.069) (Fig. 3).
Fig. 2.

Receiver-operating-characteristic (ROC) curves for the four [18F]FDG-PET-derived quantitative parameters SUVmean, SUVmax, TMTV, and TLG, with regard to separation of MALT lymphoma patients achieving, and those not achieving, 2 year PFS.
Fig. 3.
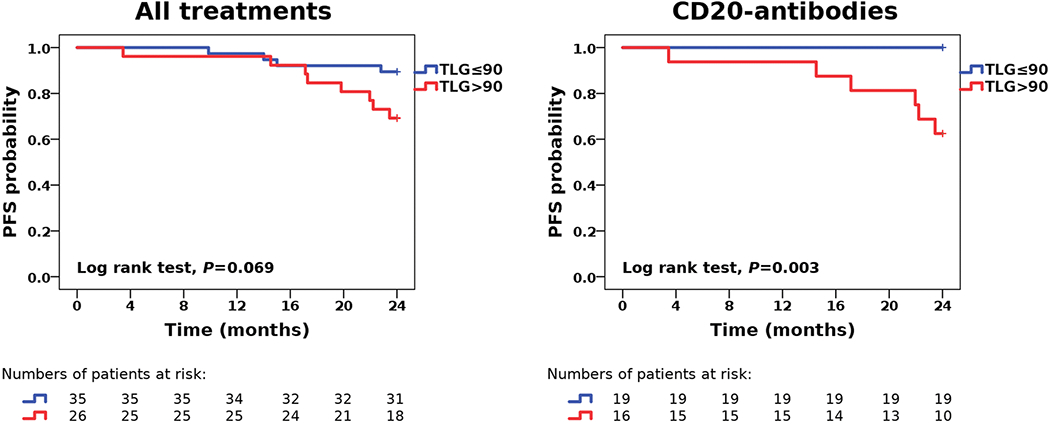
Kaplan-Meier estimates for 2 year PFS suggest that risk stratification by total lesion glycolysis (TLG) is feasible in MALT lymphoma patients treated with CD20-antibody-based immunotherapy, but not in a more heterogeneous population of MALT lymphoma patients treated with different therapeutic regimens.
CD20-antibody group
After two years, progression in this treatment group had occurred in 6/35 patients (17.1%), including one death. Descriptive data for demographic, clinical, and imaging parameters, and results of the univariate Cox regression analyses are provided in Table 3. Similar to the entire cohort, gastric (n=7) and non-gastric (n = 28) MALT lymphomas did not differ with regard to SUVmean (mean, 4.02 ± 1.98 vs. 3.89 ± 1.57; P = 0.86), SUVmax (mean, 9.21 ± 7.92 vs. 7.42 ± 3.99; P = 0.40), TMTV (mean, 124.32 ± 176.11 vs. 61.68 ± 111.59; P = 0.25), or TLG (mean, 762.30 ± 1447.90 vs. 228.93 ± 411.30; P = 0.37).
Table 3.
Demographic, clinical, and imaging data, and results of univariate Cox regression analyses for the subgroup of 35 patients receiving CD20-antibody-based immunotherapy
| Univariate analysis of PFS | |||
|---|---|---|---|
| Characteristics | Frequency (%) or Mean (± SD) | HR (95% CI) | P-value |
| Age ≥70 years | 16 (45.7%) | 1.91 (0.35-10.44) | 0.46 |
| Ann Arbor stage | |||
| I | 21 (60.0%) | 1 | |
| II | 6 (17.1%) | 1.23 (0.13-11.78) | 0.86 |
| III | 0 (0.0%) | - | - |
| IV | 8 (22.9%) | 3.34 (0.30-36.97) | 0.33 |
| Plasmacytic differentiation | 8 (22.9%) | 1.54 (0.18-13.21) | 0.69 |
| Elevated LDH | 3 (8.6%) | 0.13 (0.023-0.70) | 0.018 |
| Elevated β2 microglobulin Missing | 17 (48.6%) 1 (2.9%) | 1.54 (0.26-9.24) | 0.64 |
| ECOG ≥1 | 6 (17.1%) | 27.60 (0.004-200104.21) | 0.46 |
| SUVmax | 7.78 ± 4.92 | 1.13 (0.98-1.32) | 0.091 |
| SUVmean | 3.92 ± 1.63 | 1.17 (0.72-1.88) | 0.53 |
| TMTV (ml) | 74.21 ± 126.52 | 1.006 (1.002-1.01) | <0.0001 |
| TLG | 335.61 ± 742.39 | 1.002 (1.001-1.003) | <0.0001 |
TMTV (HR, 1.006; 95% CI, 1.002-1.01; P<0.0001) and TLG (HR, 1.002; 95% CI, 1.001-1.003; P < 0.0001), but not SUVmean (P = 0.53) or SUVmax (P = 0.091) were prognostic for 2 year PFS at univariate analysis; whereas in the multivariate analysis, only TLG (HR, 1.001; 95% CI, 1.001–1.002; P = 0.001), but not TMTV (P = 0.71) retained its significant association with PFS.
Similar to findings in entire cohort, the ROC analysis revealed an optimal TLG cut-off value of 90 for separation of patients who achieved, and those who did not achieve, 2 year PFS; the AUC was 0.86 (95% CI, 0.72-1.00; P = 0.006) (Fig. 2). Based on this threshold, 6/16 patients with a TLG>90 (37.5%) vs. 0/19 patients with a TLG≤90 (0.0%) showed progression within the 2 year observation period (HR, 0.010; 95% CI, 0.0001-8.068; P = 0.003) (Fig. 3).
Non-CD20-antibody group (all other treatments)
After two years, progression in this treatment group had occurred in 6/26 patients (23.1%), including two deaths. Gastric (n = 8) and non-gastric (n = 18) MALT lymphomas did not differ with regard to SUVmean (mean, 3.59 ± 1.19 vs. 3.35 ± 2.23; P = 0.73), SUVmax (mean, 6.22 ± 2.14 vs. 6.91 ± 4.55; P=0.60), TMTV (mean, 56.54 ± 67.36 vs. 88.81 ± 293.70; P = 0.66), or TLG (mean, 218.41 ± 289.55 vs. 218.34 ± 652.12; P = 1.0).
Only Ann Arbor stage (P = 0.039), but none of the PET-based parameters, and also none of the other clinical and laboratory parameters showed a significant association with PFS at univariate analysis, and therefore, no multivariate analyses were performed.
Discussion
There are two main conclusions that can be drawn from the results of our study. First, TLG may be a useful prognostic factor for 2 year PFS in patients treated with CD20-antibody-based immunotherapy, but not necessarily in an unselected population of MALT lymphoma patients receiving different types of treatment - here, contrary to the CD20-antibody cohort, TLG did not reach statistical significance for separation of groups at risk for progression (Fig. 3). Second, in accordance with previous findings in MALT lymphoma and DLBCL, TLG seems to be slightly superior to TMTV as a prognostic biomarker, and clearly superior to the clinical standard PET parameter, the SUVmax, in MALT lymphoma patients [3, 4, 6]. The latter may be explained by the variable degree of [18F]FDG avidity in this lymphoma subtype [9] - both metabolic tumor volume and the degree of glucose metabolism, which the TLG combines in a single measure, appear to carry prognostic information here.
Contrary to other lymphoma subtypes, there is currently no universally accepted standard treatment for MALT lymphoma [16]. For instance, limited disease may be treated with radiation therapy or surgery, and in gastric MALT lymphoma, eradication of H. pylori may even be sufficient. The randomized, multicenter IELSG-19 trial suggested that PFS and EFS (event-free survival) were comparable between MALT lymphoma patients that received CD20-antibody-based immunotherapy with rituximab (monotherapy) and those that received standard cytostatic chemotherapy with chlorambucil [17]. Unlike rituximab, the fully humanized, second-generation CD20-antibody ofatumumab can bind both the small and large extracellular loop of CD20 [18]; it has already been approved by the FDA (Food and Drug Administration) for the treatment of chronic lymphocytic leukemia, and has shown promise in different lymphoma subtypes, although head-to-head comparisons with rituximab in randomized controlled trials are presently still lacking [19]. In our study, TLG demonstrated a potential utility as a prognostic biomarker in the CD20-antibody group: the dichotomized TLG enabled the identification of a “low-risk population” in which not a single patient showed progression within the two-year observation period, suggesting a very favorable response to this type of treatment (Fig. 2). We hypothesize that, in such a low-risk population, the use of longer time intervals between follow-up examinations may be an option.
The literature on the use of quantitative [18F]FDG/PET parameters in MALT lymphoma is scarce, and in part, contradictory. Qi, et al recently reported, in a series of 123 patients with FDG-avid disease, that the SUV was an independent prognostic factor for 5-year OS, but not PFS [7] - the latter finding being partly in accordance with our results. The patients included in this study had received different types of treatment: in Ann Arbors stages I and II, they were predominantly treated with radiotherapy, with only 8% receiving immunotherapy not further specified; in stages III and IV, 47% received systemic treatment not further specified, and 36% underwent active surveillance. Contrary to these results, Albano et al very recently reported, based on a cohort of 94 patients with [18F]FDG-avid MALT lymphoma, a significant association of TMTV and TLG, but not SUV, with PFS, albeit only in the univariate, but not the multivariate analysis; no imaging parameter was associated with OS [6]. Their cohort also consisted of a mixed population of MALT lymphoma patients treated with antibiotic therapy, surgery, radiotherapy, chemotherapy or a combination thereof. The fact that none (Albano et al), or only a small percentage (Qi, et al), of the patients were treated with immunotherapy, limits the comparability between these study and our own. Nevertheless, the TLG cut-off of 84 in the study by Albano et al was similar to that in our study (TLG>90), and then again, in our own mixed population of 61 patients that received different types of treatment, TLG was also unable to separate groups at risk for progression, despite a trend in that direction.
We used PFS instead of overall survival (OS) as the clinical endpoint of our study, mainly because MALT lymphoma is a less frequently seen, although not uncommon, lymphoma subtype, with quite a good prognosis, compared to other Non-Hodgkin lymphoma subtypes, such as mantle cell lymphoma. As a consequence, with PFS, smaller patient populations, as well as shorter observation periods, may be used to obtain a sufficiently large patient sample with the outcome of interest (i.e., progression). Furthermore, PFS is recognized by the FDA as a valid surrogate endpoint for Non-Hodgkin lymphomas [20]. For DLBCL, a recent analysis of multiple randomized trials with a total sample size of 7,507 patients also clearly supported the use of PFS, and showed that 2 year PFS significantly correlates with OS [21].
While our study is limited by its modest cohort size, this was a hypothesis-generating study, as [18F]FDG/PET is not considered a routine procedure for MALT lymphoma according to the ICML guidelines [9]. The fact that —contrary to our own institutional standard of care - CT is still the standard test for MALT lymphomas in the vast majority of institutions, despite its known limitations for treatment response assessment in lymphomas [22], prevented us from using a multi-centric approach that would have increased the number of study participants. For the survival analysis, we did not further subdivide our patient population (e.g., into gastric and non-gastric lymphomas), because only 12/61 patients overall, and 6/35 patients in the CD20-antibody group, showed progression. Finally, [18F]FDG/PET data were obtained from a PET/CT scanner on the one hand, and a PET/MRI scanner on the other hand, which probably affected SUV as well as SUV-based measurements. To correct for the latter, we applied a post-reconstruction harmonization technique that was specifically designed for this task [13]; still, remaining minor differences between the datasets of the two scanners cannot be completely ruled out.
Conclusion
In conclusion, our study results suggest that TLG may have prognostic meaning in MALT lymphoma patients treated with CD20-antibody-based immunotherapy. More specifically, TLG appears to have a high negative predictive value for 2 year PFS, and may, therefore, improve early risk stratification, and possibly, risk-adapted management of MALT lymphoma patients treated with CD20-antibody-based immunotherapy. For the general population of MALT lymphomas that receive different types of treatment, the prognostic value of [18F]FDG-PET-based quantitative parameters remains questionable. Larger patient cohorts are required to validate our results, and longer observation periods that will enable assessment of OS in addition to PFS.
Footnotes
Conflict of Interest and Ethical Approval
Marius E. Mayerhoefer received honoraria for lectures as well as research support (not related to the present study) from Siemens Healthineers.
Barbara Kiesewetter and Markus Raderer received honoraria for lectures from Celgene, Ipsen, and Novartis.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Mettler J, Müller H, Voltin CA, et al. (2018) Metabolic Tumour Volume for Response Prediction in Advanced-Stage Hodgkin Lymphoma. J Nucl Med DOI: 10.2967/jnumed.118.210047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meignan M, Cottereau AS, Versari A, et al. (2016) Baseline Metabolic Tumor Volume Predicts Outcome in High-Tumor-Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies. J Clin Oncol 34:3618–3626 [DOI] [PubMed] [Google Scholar]
- 3.Mikhaeel NG, Smith D, Dunn JT, et al. (2016) Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging 43:1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceriani L, Martelli M, Zinzani PL, et al. (2015) Utility of baseline 18FDG-PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B-cell lymphoma. Blood 126:950–956 [DOI] [PubMed] [Google Scholar]
- 5.Becker S, Vermeulin T, Cottereau AS, et al. (2017) Predictive value of 18F-FDG PET/CT in adults with T-cell lymphoblastic lymphoma: post hoc analysis of results from the GRAALL-LYSA LLO3 trial. Eur J Nucl Med Mol Imaging 44:2034–2041 [DOI] [PubMed] [Google Scholar]
- 6.Albano D, Bosio G, Camoni L, et al. (2018) Prognostic role of baseline 18F-FDG PET/CT parameters in MALT lymphoma. Hematol Oncol DOI: 10.1002/hon.2563 [DOI] [PubMed] [Google Scholar]
- 7.Qi S, Huang MY, Yang Y, et al. (2018) Uptake of [18F]fluorodeoxyglucose in initial positron-emission tomography predicts survival in MALT lymphoma. Blood Adv 2:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JP, Lim I, Byun BH, Kim BI, Choi CW, Lim SM (2016) Prognostic value of SUVmax measured by pretreatment 18F-FDG PET/CT in patients with primary gastric lymphoma. Nucl Med Commun 37:1267–1272 [DOI] [PubMed] [Google Scholar]
- 9.Barrington SF, Mikhaeel NG, Kostakoglu L, et al. (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32:3048–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conconi A, Martinelli G, Thiéblemont C, et al. (2003) Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood 102:2741–2745 [DOI] [PubMed] [Google Scholar]
- 11.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C (2009) Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma 50:1257–1260 [DOI] [PubMed] [Google Scholar]
- 12.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. (2015) FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 42:328–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlhac F, Boughdad S, Philippe C, et al. (2018) A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J Nucl Med 59:1321–1328 [DOI] [PubMed] [Google Scholar]
- 14.Eiber M, Takei T, Souvatzoglou M, et al. (2014) Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med 55:191–197 [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Bhattarai A, Korkola P, et al. (2017) The Association Between Liver and Tumor [18F]FDG Uptake in Patients with Diffuse Large B Cell Lymphoma During Chemotherapy. Mol Imaging Biol 19:787–794 [DOI] [PubMed] [Google Scholar]
- 16.Raderer M, Kiesewetter B, Ferreri AJ (2016) Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin 66:153–171 [DOI] [PubMed] [Google Scholar]
- 17.Zucca E, Conconi A, Martinelli G, et al. (2017) Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival With Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J Clin Oncol 35:1905–1912 [DOI] [PubMed] [Google Scholar]
- 18.Pierpont TM, Limper CB, Richards KL (2018) Past, Present, and Future of Rituximab-The World’s First Oncology Monoclonal Antibody Therapy. Front Oncol 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falchi L, Ferrajoli A, Jacobs I, et al. (2018) An Evidence-based Review of Anti-CD20 Antibody-containing Regimens for the Treatment of Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Diffuse Large B-cell Lymphoma, or Follicular Lymphoma. Clin Lymphoma Myeloma Leuk 18:508–518 [DOI] [PubMed] [Google Scholar]
- 20. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM614369.xlsx.
- 21.Shi Q, Schmitz N, Ou FS, et al. (2018) Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Diffuse Large B-Cell Lymphoma: An Individual Patient-Level Analysis of Multiple Randomized Trials (SEAL). J Clin Oncol 36:2593–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez León N, Delgado-Bolton RC, Del Campo Del Val L, et al. (2017) Multicenter Comparison of Contrast-Enhanced FDG PET/CT and 64-Slice Multi-Detector-Row CT for Initial Staging and Response Evaluation at the End of Treatment in Patients With Lymphoma. Clin Nucl Med 42:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]


