Abstract
The entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) has been widely studied against a wide range of arthropod pests, including many of medical and veterinary importance. New investigators must sort through a wide array of published methods for the production, harvest, storage, and bioassay methods for this pathogen. Simplified methods for production of conidia using Sabouraud dextrose agar with yeast (SDYA) plates and two conidial harvesting methods are described. Dry harvesting yields conidia that are ready to incorporate into dusts and food baits, but the fungal product includes mycelial debris that can hamper quantification and introduces variable amounts of unwanted bulk. Wet harvesting with filtration produces a cleaner product that is immediately ready for testing in liquid formulations. Examples of bioassays with house flies are presented that include conidia applied topically to the dorsal thorax for dose–mortality assays and conidial suspensions applied to filter paper disks for concentration mortality assays.
Keywords: house fly, Musca domestica, microbial control, biological control
Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae) has been isolated and subsequently used to manage various pest insects for decades (Fargues and Remaudiere 1977, Roberts et al. 1991, Watson et al. 1995, Kaufman et al. 2005, Uma Devi et al. 2008, Barbarin et al. 2012, Hasaballah et al. 2017). In addition to causing direct insect mortality, infection with B. bassiana can result in a 90% reduction of life-time fecundity (Acharya et al. 2015).
Information on culturing, storing, and harvesting of B. bassiana for bioassays with pests of medical and veterinary pests is available, but a new investigator who wishes to test new strains or formulations must consult multiple sources to get a sense of what the entire process entails. Inglis et al. (2012) is an excellent resource for propagation, enumeration, and many other aspects of researching hypocrealean fungi, but it covers a diverse group of fungi and has little specific information on harvesting conidia from cultures. For the novice, deciding on collecting/harvesting methods for B. bassiana conidia from agar plates can be challenging. That is, conidia can be scraped off the plates in a dry state or washed off by flooding and scraping plates, but details for these methods and their relative advantages differ across a wide swath of published articles (Arcas et al. 1999, Kirkland et al. 2004, Moslim et al. 2005, Leemon and Jonsson 2008, Jackson et al. 2010, Mishra et al. 2013, Sun et al. 2013, Lopes and Faria 2019, Moloinyane and Nchu 2019).
The intent of this paper is to present examples of methods to provide start-to-finish procedures for working with this important entomopathogen. The intended audience is entomologists with an interest in working with this pathogen who do not have expertise in insect pathology or mycology. Different harvesting methods will be provided, and individual methods are selected based on experimental design and bioassay differences. The two bioassay types discussed are used for dose–response experiments (topical application), and concentration–response experiments (treated filter paper application). These assays can be used to compare virulence of various strains and products and to determine if B. bassiana is effective against target insects. The goal is to provide a protocol that will benefit scientists who are new to working with B. bassiana and similar entomopathogenic fungi.
Experimental Design
Propagation
The following methods are currently used at the USDA-ARS-CMAVE laboratory in Gainesville, FL, to produce B. bassiana. Cultures are maintained on Sabouraud dextrose agar with yeast (SDYA). This can be made by following the recipe provided in Inglis et al. (2012), or from commercially available powders (see below). Stock solutions of newly acquired and currently used strains should be prepared and stored at −80°C to preserve genetic integrity, as repeated subculturing can affect growth and virulence properties (Butt et al. 2006). These freezer stocks will be referred to as ‘parental’ stocks. To make a parental stock, first add an inoculum (conidia or vegetative growth from a plate) to 50 ml SDY broth (without agar) in an Erlenmeyer flask. Place on a shaker at approximately 120 rpm for 72 h at 22–26°C. The broth will become increasingly turbid during this time as the culture proliferates. Pipette 750 μl of this liquid culture and 750 μl of autoclaved 50% glycerol into a sterile 2-ml screw cap cryogenic storage tube. Label and store at −80°C for future use. The glycerol is included as a cryoprotectant for the conidia. Several vials of parental stocks can be prepared so that there are backup cultures in one becomes contaminated. Other long-term preservation methods are described in Smith (1993), Chandler (1994), Smith and Onions (1994), and Humber (2012), including the use of porous Microbank beads.
To make a batch of 20–25 SDYA plates, weigh 15 g of Sabouraud dextrose (SD) broth powder (Table 1), 5 g of yeast extract, and 7.5 g of agar and place in autoclavable glassware with a magnetic stir rod (commercial sources of SDA powders are also available that have the agar included in the premix). Add 500 ml of deionized (DI) water and stir on a magnetic stirrer for 10 min. This can be done under heat to fully dissolve the agar prior to autoclaving. Autoclave at 121°C for at least 15 min per label instructions. We have found that using the 50-min ‘liquid’ cycle at 121°C and 17 PSI (pounds per square inch) provides excellent assurance of sterility for this volume of media in our countertop autoclave. When the autoclave cycle is complete remove the glassware containing the SDYA media once it has reached a safe handling temperature and place back on the stirrer until the temperature drops to approximately 60°C. A cool water bath can be used in conjunction with stirring to speed up this process but the agar will start to set if the temperature drops substantially below 60°C. Dispense approximately 20 ml each into sterile plastic Petri dishes. This can be done using a sterile serological pipette but with a little practice the plates can be poured directly from the flask by eye using sterile technique. Allow agar to cool and set in a biosafety cabinet before storing at 4°C.
Table 1.
List of materials used in production and harvesting Beauveria bassiana for bioassays
| Item | Source | Catalog no. | Costa |
|---|---|---|---|
| BD Difco Sabouraud Dextrose Broth | www.fischersci.com | 238230 | $92.10 for 500 mg |
| Fisher BioReagents Microbiology Media Additives: Yeast Extract | www.fischersci.com | BP14422-100 | $98.00 for 100 g |
| Corning Falcon Bacteriological Petri Dishes with Lid, 100 × 15 mm | www.fischersci.com | 351029 | $234.50 for 500 |
| GE Healthcare Whatman Qualitative Filter Paper: Grade 2 Circles, 90 mm | www.fischersci.com | 1002090 | $22.00/pack of 100 |
| CELLTREAT L-Shaped Cell Spreader, Individually Wrapped, Sterile | www.fischersci.com | 229616 | $48.60/case of 100 |
| DWK Life Sciences Wheaton Glass 20-ml Scintillation Vials and Urea Caps | www.fischersci.com | 986542 | $384.05/case of 500 |
| Fisherbrand Disposable Sterile Spatula | www.fischersci.com | 14375253 | $114.10/case of 100 |
| Aquatrols CapSil Adjuvant Nonionic Surfactant | www.seedranch.com | N/A | $134.75/gallon |
| Fisherbrand Reusable Glass Narrow-Mouth Erlenmeyer Flasks | www.fischersci.com | FB500250 | $82.15/case of 12 |
| Fisherbrand Reusable Glass Short-Stem Funnels, 60 ml | www.fischersci.com | FB6015865 | $144.46/case of 12 |
| Hausser Scientific Bright-Line and Hy-Lite Counting Chambers 3100, Hemacytometer | www.fischersci.com | 0267110 | $325.50 |
| Fisherbrand Easy-Squeeze Wash Bottles, 500 ml | www.fischersci.com | 0289713 | $51.70/case of 6 |
| PYREX Glass Wool | www.fischersci.com | 11388 | $167.04/454 g |
| Corning Externally Threaded Cryogenic Vials | www.fischersci.com | 0976171 | $390/case of 500 |
| Eppendorf Reference 2 Pipet Packs—Three adjustable pipettes, 0.5–10 μl, 10–100 μl, 100–1,000 μl | www.fischersci.com | 05412520 | $1,192.00 |
| Thermo Scientific Sabouraud Dextrose Agar, Emmons w/Chloramphenicol, Gentamicin prepoured plates | www.fischersci.com | R01772 | $128.84/pack of 10 |
| Pro Breeze Electric Mini Dehumidifier, 1,200 cubic feet (150 sq ft) | www.fischersci.com | N/A | $39.99 |
| Sartorius Polystyrene Weighing Boats | www.fischersci.com | 13-735-743 | $37.05/pack of 50 |
| Thermo Scientific Forma 8600 Series −86°C Ultra-Low Temperature Chest Freezers | www.fischersci.com | 803CA | $13,250.00 |
| Thermo Scientific LP Vortex Mixer | www.fischersci.com | 11676331 | $566.00 |
| PYREX Reusable Media Storage Bottles (500 ml) | www.fischersci.com | 06-414-1C | $157.78/case of 10 |
| Percival Scientific Incubator Model I-36VL | www.percival-scientific.com/product/i-36vl/ | N/A | $6,570.08 |
aPrices as of 31 January 2020.
The hydrophobic nature of B. bassiana conidia requires a wetting agent for adequate dispersion when working with aqueous suspensions. The most commonly used surfactant for this purpose is 0.01–0.10% solutions of Tween 80 (Mishra et al. 2013, Immediato et al. 2015, Andreadis et al. 2016). However, in a previous publication we compared several surfactants for topical applications on house fly adults and found that some other surfactants were superior to Tween with regards to spreadability over the fly thorax (Johnson et al. 2019). Solutions of 0.1–1.0% CapSil (Aquatrols, Paulsboro, NJ) provided superior spreadability over the house fly thorax with 0–5% control mortality when applied topically or injected and had no deleterious effect on conidial viability. CapSil is a nonionic, organosilicone surfactant designed to facilitate the even spreading of solutions over the surface of vegetation. Although oils are used with some other insects, topical application of oils to house flies results in unacceptably high control mortality, apparently due to oil entry into the thoracic spiracles (unpublished data).
To prepare for fungal culturing, add 500 μl of sterile 0.1% CapSil to a sterile microcentrifuge tube. Briefly remove parental stock from −80°C to thaw and use a sterile loop to transfer a small amount of stock to the microcentrifuge tube containing CapSil. Vortex the mixture for 30 s and return stock to −80°C. Inoculate plates in a biosafety cabinet by pipetting 100 μl of the above solution onto the surface of each SDYA plate and evenly disperse with a sterile L-shaped (‘hockey stick’) spreader. Plates are placed in an incubator at 26°C and held between 80 and 100% RH until sporulated (7–10 d). Sporulation is easily detected with the naked eye as a textured white cloud (Fig. 1). Under 10× magnification, spore balls are visible as white spherical structures and can be distinguished from the base of mycelium.
Fig. 1.
Plate of Beauveria bassiana fungal lawn. Magnified (10×) inset shows appearance of spore balls containing conidia at the edge of a lawn.
Harvest, Enumeration, and Viability
Plates can be harvested using either dry or wet collection methods (described below in the Protocol section). If conidia are to be harvested in a dry state, plates should be opened slightly and held in a desiccator jar/cabinet or a biosafety cabinet in a room with low relative humidity for several days. Temperature should be kept under 22°C to maintain conidia viability. We also find it useful to dry plates in a reach-in incubator at 18°C with a small electric dehumidifier.
Selection of the appropriate method depends on whether the bioassay will require dry conidia, which are most useful for incorporation into oils, as a dry food or applied as a dust. Wet harvesting with filtration results in a cleaner product with less mycelial and other debris and is used to prepare aqueous spore suspensions. Harvested conidia can then be applied either directly onto the insects (dose–response bioassays) or applied to surfaces or food (concentration–response bioassays). Plates with conidia or dry-harvested spores are stored at 4°C for up to 4 wk with virtually no decrease of viability.
When working with dry conidia, each harvested batch should be assessed for the number of conidia per mg of harvested product. Conidial counts are taken by suspending 10 mg of dry harvested B. bassiana conidial mass in 1 ml of 0.5% CapSil in autoclaved DI water. After mixing well, 10 μl of this suspension is loaded onto an improved Neubauer hemocytometer, and counts are made to estimate the number of conidia per ml of the suspension (Inglis et al. 2012, Moore 2018); dividing this number by 10 gives number of conidia per mg of dried conidia. An alternative to using a hemocytometer is the use of an automated cell counter such as a Cellometer X2 (Nexcelom Bioscience LLC, Lawrence, MA). This unit has the additional capability of using a two-stain system to provide viability information.
Conidial viability should be measured at the start of each experiment. The most common method for doing this is to pipette approximately 106 conidia in 100 µl of an aqueous suspension and spreading on agar plates (water agar, SDYA, or other suitable media). After 24 h at 25°C, a coverslip is placed on the surface of the plate and conidia are examined under a compound microscope to determine the percentage of conidia with germination tubes that are at least two times the diameter of individual conidia (Inglis et al. 2012). A minimum of 200 conidia should be examined and counted. An alternative method that does not require agar plates is to first incubate conidia in SDY broth (i.e., without agar) at 105 conidia per ml for 24 h at 25°C. Drops of the suspension are placed on a microscope slide with a coverslip and examined for percent germination. Samples (10 μl) of the suspension can also be loaded onto a hemocytometer, which gives the observer-defined fields in which to make germination counts. Samples with less than 90% viability should not be used in a bioassay, and viability of >95% is a good target.
Hosts and Handling
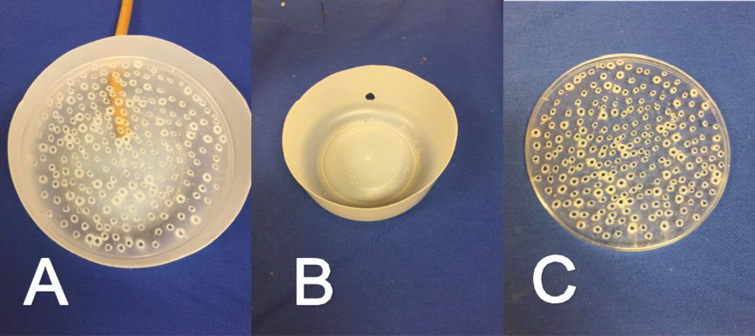
Insect hosts used in bioassays should be from healthy colonies that are free of other pathogens such as Serratia marcescens unless the investigator is interested in looking at interactions among pathogens. Young flies should be used to minimize mortality due to aging, as B. bassiana can take up to 9 d to kill the host. Because male and female insects vary in longevity and susceptibility to infection it is advisable to work with a single sex in an individual bioassay. Female house flies that are 2–3 d old are ideal for experiments because control mortality in such flies is typically below 5% for up to 9 d postexposure. Hosts are anesthetized for counting and sex determination using either CO2 or cold. A CO2 ‘knockdown device’ can be easily constructed to keep flies anesthetized for sexing and counting (Fig. 2). The bottom chamber of the device is formed by cutting away the upper walls of a 2-liter container so that only the bottom 10 cm remains, forming a shallow, circular dish with a diameter of just over 15 cm. A 1-cm hole is cut into the side of the bottom chamber to allow insertion of vinyl tubing to deliver CO2 (Fig. 2B). The upper chamber is made of a 15-cm diameter plastic Petri dish lid with numerous small perforations to allow CO2 to flow through the holes (Fig. 2C). Because CO2 is heavier than air, this creates a blanket of CO2 over the flies in the device. To avoid excess human exposure, this work should be done in a well-ventilated room. Use of an illuminated magnifier allows the researcher to count and sort sexes without needing to work at close range to the CO2.
Fig. 2.
Knockdown device with CO2 tube inserted for anesthetizing adult house flies (Musca domestica L.) for experimental setup, (A) assembled knockdown device used to anesthetize flies with CO2, (B) shallow circular dish used as the bottom chamber, (C) upper chamber made of a 15-cm Petri dish with holes for CO2 flow. Device is also appropriate for other insects that tolerate anesthesia with CO2.
In the results presented below, bioassays consisted of 20–25 individuals per treatment, and exposed flies were transferred to screen-topped 500-ml plastic cups with water and food (8:8:1 mixture of powdered milk, sucrose, and powdered egg yolk). Mortality was monitored daily. Three groups of flies were used for each concentration and strain tested, and the entire experiment was replicated three times. Complete replication in time with different batches of flies and conidia is crucial to reduce pseudoreplication and because results can vary among test runs.
In the examples presented below, mortality was used as the metric for comparing strains. Sometimes it is also useful to observe/compare the proportion of dead flies that produce visible signs of mycosis. When this is the case, flies should be housed in small cages that allow the daily removal of dead flies. Cadavers can then be placed, alone or in groups, in Petri dishes with a disk of filter paper moistened with 1 ml of sterile water. High humidity is needed for cadavers to form blooms, so dishes should be checked for moisture daily, sealed with paraffin strips, or placed in locking-style plastic bags. White mycelium will be visible emerging from the cadavers by 24–36 h at 23–27°C, and conidiation will be evident within 5 d.
Sources of B. bassiana
Isolates of B. bassiana can be obtained from other researchers or repositories such as the ARS Collection of Entomopathogenic Fungal Cultures (ARSEF, https://data.nal.usda.gov/dataset/ars-collection-entomopathogenic-fungal-cultures-arsef) or the American Type Culture Collection (ATCC, https://www.atcc.org). Commercial products can be diluted and tested as formulated or used as a source from which to isolate and culture the strain of B. bassiana in the product. Examples of strains that are present in commercial products include GHA (Botaniguard, BioWorks, Victor, NY), HF23 (balEnce, JABB of the Carolinas, Pine Level, NC), and PPRI 5339 (Broadband, BASF, Florham Park, NJ). Companies may provide unformulated cultures as well, and it may be advisable from a legal standpoint to obtain a memorandum of understanding before reisolating strains from commercial products. Finally, new strains can be obtained from the field by collecting naturally infected flies and isolating the fungus from field-collected cadavers or collecting wild flies, holding them for 9 d, and transferring dead flies daily from the cages to wet filter paper to allow the cadavers of infected flies to produce conidial blooms. Fly cadavers obtained that show signs of fungal infection in the field may yield fungal strains better adapted to the host and field conditions, as opposed to fungal infections that occur only when insects are maintained under laboratory conditions.
Cadavers of laboratory-infected flies can be surface-sterilized with ethanol (70–80%) or sodium hypochlorite (we use 0.4%) before placing on filter paper to minimize growth of incidental microorganisms present on the cadavers. Cadavers of flies that are either field-infected or laboratory-infected by can then be used to isolate pure cultures (Inglis et al. 2012). It should be noted that flies handled in this manner may produce blooms of other fungal species such as Aspergillus spp., so candidate strains should be submitted for identification and curation as early as possible.
The following protocols provide step-by-step instructions for harvesting and using conidia in bioassays.
Protocols
Dry Harvest
Remove agar plates with conidial lawns from incubator (e.g., Percival I36VL).
Allow plates to dry at 18–22°C in a biosafety cabinet, desiccator cabinet, or in an incubator with a small dehumidifier at (e.g., Pro Breeze Electric Mini Dehumidifier) with incubator set to 16–18°C).
Check the plates daily for 1–3 d.
Gently scrape dry conidia from the agar surface with a sterile plastic disposable spatula (Fisherbrand Disposable Sterile Spatula) into a weigh boat or piece of aluminum foil in a biosafety cabinet.
Determine number of conidia present per mg of harvested product with a hemocytometer or automated cell counter.
Store dry conidia in a sterile container (e.g., glass scintillation vial) at 4°C until use for up to 4 wk. For longer storage periods, make sure conidial viability is not affected.
Wet Harvest
Place a sterile glass funnel (Fisherbrand Reusable Glass Short-Stem Funnels) on the top of a sterilized 250-ml Erlenmeyer flask.
Arrange a square (approximately 70 × 70 × 2 mm) of sterile glass wool (PYREX Glass Wool) inside the funnel.
Premoisten the glass wool by rinsing with 0.5% CapSil in sterile DI water, then discard the liquid collected in the flask.
Flood the fungal plate with 15 ml sterile 0.5% CapSil.
Scrape the surface of the agar with a sterile plastic disposable spatula (Fig. 3).
Carefully pour the plate containing the wet conidia onto the glass wool in the funnel above the flask.
Rinse residual conidia in the glass wool into the funnel with an additional 35 ml of 0.5% CapSil with a squeeze bottle.
Vortex flask containing the ~50 ml of conidial suspension for 30 s to homogenize.
Fig. 3.
Setup for wet harvesting of Beauveria bassiana from agar plates using 0.5% CapSil. The left side of the plate has been scraped with the sterile plastic disposable spatula shown.
Before bioassay setup, conidia in the suspension should be enumerated and samples set up for viability determination. The suspension is then diluted to obtain the desired concentrations. In the examples below, these are 1 × 106 to 1 × 109 conidia per ml. If the conidial concentration in the wash suspension is lower than 109 conidia per µl, use a centrifuge at 600 × g for 9 min to form a conidial pellet and resuspend in an appropriate amount of CapSil to achieve the desired concentration.
Dose–Response Bioassay (Topical Applications)
Apply 1 µl of the suspension onto the dorsal thorax of a CO2-immobilized fly with a 0.5- to 10-µl Eppendorf pipette.
Place treated insects in a screen-lidded 500-cm3 (16 oz) plastic cup with food and water.
Record mortality daily for 9 d including day 0 to account for mortality caused by fly handling (2 h after exposure).
Concentration–Response Bioassay (Treated Filter Paper Application)
Prepare Petri dishes by labeling the bottoms of the dishes with the fungal strain being tested, the date, concentration, and replicate number (e.g., 1–3).
Place a filter paper disk in the lid of the dish.
Apply 1 ml of each concentration evenly to the filter paper with a pipette. Pipette proceeding from the lowest to the highest fungal concentration.
Anesthetize insect hosts and place in groups of 20 or 25 on the treated filter paper while it is still damp.
Cover the dish with the Petri dish bottom.
Lightly shake the dish to disperse hosts across the paper. Alternatively, flies can be placed in the untreated Petri dish bottom until they recover, then invert the dish.
Leave the hosts in the dish with treated filter paper for 2 h.
After the 2-h exposure, briefly anesthetize with CO2 and move to screen-lidded 500-cm3 plastic cup with food and water.
Record mortality daily for 9 d including day 0 (2 h after exposure).
Limitations
Perhaps the greatest challenge is maintaining fungal cultures that are free of contamination with bacteria and other fungi. Practicing sterile technique is critical and standard operating procedures should always be followed to mitigate the risk of contamination. This includes, but is not limited to, correctly labeling and handling all samples, sterilizing and disinfecting tools and workspaces, using personal protection equipment, using sterile disposable supplies when possible, and proper use of the biosafety cabinet. If practical, only one fungal strain should be subcultured on any given day to help prevent cross-contamination, although careful use of sterile equipment and materials should prevent this. Contaminated fungal plates should be discarded immediately and replaced with pure cultures from frozen parental stocks.
Contamination may occur despite efforts to maintain sterile procedures. If cultures appear to be contaminated with bacteria, SDYA plates containing the antibiotics gentamicin sulfate and chloramphenicol can be made for reisolation. The antibiotics will inhibit the growth of most microbial contaminants.
To make SDYA antibiotic plates, first follow the same procedure discussed above to make 500 ml of SDYA. After autoclaving the SDYA media, allow the contents of the flask to drop to approximately 60°C before adding the antibiotics. Once the SDYA liquid reaches 60°C add 1.25 ml of gentamicin sulfate and 1 ml of chloramphenicol. Mix thoroughly using a sterile magnetic stir rod for 2 min, then pour into plates as before. Once cooled and set, plates can be labeled, sealed, and stored at 4°C until needed. If bacterial contamination persists, double the amount of antibiotics in the recipe (2.5 ml of gentamicin sulfate and 2 ml of chloramphenicol/500 ml SDYA). Prepoured plates with gentamycin and chloramphenicol are also available from commercial sources (e.g., BD # 296359, Beckton Dickinson, Franklin Lakes, NJ). Other selective media include CTC (PDYA with chloramphenicol, thiabendazole, and cycloheximide; Fernandes et al. 2010) and dodine-based media (Rangel et al. 2010).
Repeated subculturing of entomopathogenic fungi on culture media can lead to attenuation of virulence, although this is not always the case (Brownbridge et al. 2001, Butt et al. 2006, Nahar et al. 2008). As a precaution, a subculture should be plated a predetermined maximum of passages (we routinely use three maximum passages) on culture media before reculturing from the frozen parental stock.
The use of any anesthesia method can have attendant risks for the test subjects, and insects vary in their sensitivity to CO2. House flies tolerate CO2 well, but should be administered the minimum dose and exposure time of CO2 necessary to keep them immobilized. To minimize control mortality, flies should be exposed for no more than 15 min. Cold can be used instead of CO2 for counting groups of insects for treated surface, aerosol, or bait bioassays but should not be used for topical applications because low temperatures reduce the fluidity and hence spreadability of the applied CapSil solution.
Results
Examples of typical results are presented for bioassays with topical applications (Fig. 3) and treated filter papers (Figs. 4 and 5). Little mortality is observed with either method until day 4 or 5, with high mortality on days 6 and 7. Data on different strains can be analyzed with Probit analysis to determine the conidial dose/concentration that produces 50% mortality (LD50/LC50) on different days postexposure, and to determine the time to 50% mortality (LT50) at different doses/concentrations. However, reliable estimates for LC50s and LT50s require several data points in the range of 20–80% mortality. Data sets sometimes do not include sufficient data points in that range and therefore will not fit the Probit model. When the data do not permit Probit analysis, strains can be compared by ANOVA at the same dose/concentration on different days or on the same day at different doses/concentrations. A repeated-measure component is needed in the statistical model if the same groups of flies are counted on successive days.
Fig. 4.
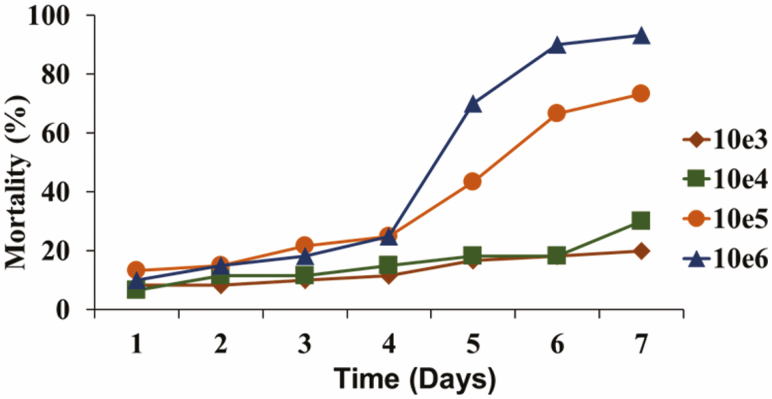
Mortality of adult house flies, Musca domestica, for 7 d after topical application (dose–response assay) of 1 µl of 0.5% CapSil containing 103 through 106 Beauveria bassiana conidia (strain L90). Raw data from study reported in Johnson et al. (2019).
Fig. 5.
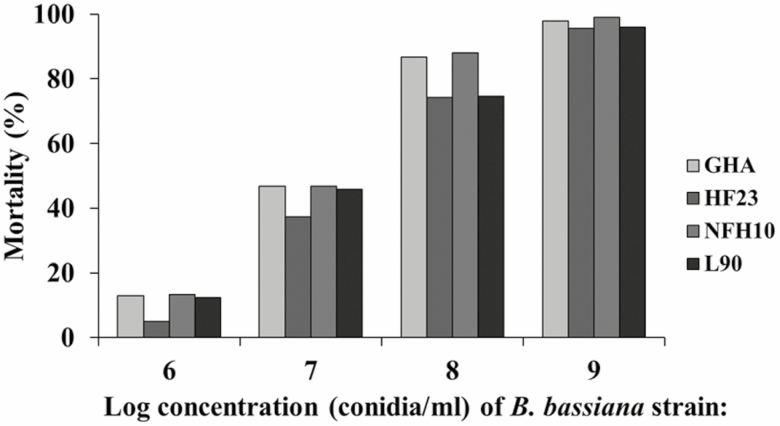
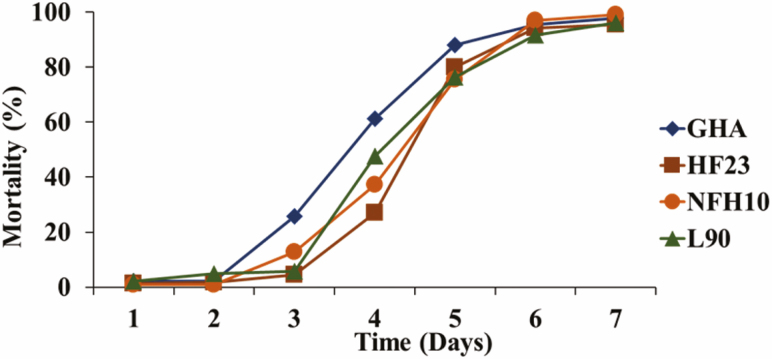
Mean % mortality of adult house flies, Musca domestica, 9 d after forced contact exposure (concentration–response assay) to 1 ml of a solution containing 0.1% CapSil and 1 × 106 through 1 × 109 conidia of Beauveria bassiana strains GHA, HF23, NFH10, and L90.
The small amount of liquid that can be applied to an individual fly (1 µl) limits the number of conidia that can be applied, with 106 conidia being the highest dose that can be applied in a practical manner. One ml of this same concentration, containing 109 conidia, is the amount that was used in the filter paper assays. Comparison of Figs. 4 and 6 indicates that this concentration of conidia produced a similar mortality response whether the fly was treated directly with 1 µl of the suspension or spent 2 h walking on a filter paper disk treated with 1,000 times the volume (1 ml) of the same suspension.
Fig. 6.
Mean % mortality female house flies, Musca domestica, after forced contact exposure (concentration–response assay) to 1 ml of a solution containing 0.1% CapSil and 1 × 109 conidia of Beauveria bassiana strains GHA, HF23, NFH10, and L90.
Discussion and Conclusions
Beauveria bassiana can be applied in a variety of formulations and delivery methods that includes sprays, dusts, and baits (Jackson et al. 2010). The techniques discussed in this paper combined several methodologies and were modified to utilize the laboratory resources available to us. There are other methods of growing this pathogen, including liquid culture in fermentation tanks and the use of semisolid substrates such as cooked rice (Feng et al. 1994, Jaronski and Jackson 2012, Mascarin and Jaronski 2016, Sala et al. 2019). For most laboratory applications, the method of growing fungal lawns in Petri dishes provides a simple way to produce adequate quantities of B. bassiana for testing. Our description of the two methods of harvesting conidia, especially the wet method, will benefit those utilizing B. bassiana for different applications. Dry-harvested conidia are most useful for formulations as baits and dust, while wet-harvested conidia are useful in sprays, aerosols, and for treating surfaces.
It is our hope that these methods and protocols will be helpful for investigators with little prior experience in culturing, harvesting, and handling B. bassiana. In some cases, we made modifications of published methods. For example, deciding on a method for wet harvesting conidia from cultures presented some challenges. Some studies describe a wet-harvesting method that involves vacuum filtration to collect a wet paste that can be used as-is or dried to produce dry conidia (Pereira and Roberts 1990, Moslim et al. 2005, Lopes and Faria 2019). Others describe flooding agar plates but do not suggest filtering mycelial debris (Arcas et al. 1999, Leemon and Jonsson 2008, Sun et al. 2013). Mishra et al. (2013) used small volumes of surfactant to wash slant cultures and passed the rinsate through 8-µm membrane filters. The method described here is most similar to that outlined in Kirkland et al. (2004), with additional details provided on the amount of liquid used to flood the plates, the sequence of washes, and details on use of a glass wool filter.
The dry harvest method is ideal for mixing B. bassiana with oils or other dry products and affords the convenience of stockpiling preenumerated conidia in a ready-to-use form for future experiments. The major liability of this method is that it produces a product that includes highly variable amounts of mycelial debris depending on the fungal strain and the production/harvesting technique. Mycelial debris can limit visibility when making conidial counts and make it difficult to mill into a homogeneous product with other dry ingredients. Dry-harvested conidia can be passed through a sieve to remove some of the debris (Lopes and Faria 2019), but such a process presents a serious risk of contaminating the workspace.
In summary, this paper presents simple protocols for producing, harvesting, and using B. bassiana in bioassays for those who are new to working with this pathogen and house flies. The methods are applicable to other Beauveria spp. and Metarhizium spp. Although house fly is presented as the test insect, the protocols should be applicable to other pests of agricultural, medical, and veterinary importance after making allowances for innate differences in size, mobility, longevity, sensitivity to CO2, and susceptibility to entomopathogenic fungi.
Acknowledgments
This research was supported by the U.S. Department of Agriculture, Agricultural Research Service. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
References Cited
- Acharya N, Rajotte E, Jenkins N, and Thomas M. . 2015. Potential for biocontrol of house flies, Musca domestica, using fungal biopesticides. Biocontrol Sci. Technol. 25: 513–524. [Google Scholar]
- Andreadis S, Cloonan K, Bellicanta G S, and Jenkins N E. . 2016. Efficacy of Beauveria bassiana formulations against the fungus gnat Lycoriella ingenua. Biol. Control. 103:165–171. doi: 10.1016/j.biocontrol.2016.09.003. [DOI] [Google Scholar]
- Arcas J A, Díaz B M, and Lecuona R E. . 1999. Bioinsecticidal activity of conidia and dry mycelium preparations of two isolates of Beauveria bassiana against the sugarcane borer Diatraea saccharalis. J. Biotechnol. 67: 151–158. [Google Scholar]
- Barbarin A M, Jenkins N E, Rajotte E G, and Thomas M B. . 2012. A preliminary evaluation of the potential of Beauveria bassiana for bed bug control. J. Invertebr. Pathol. 111: 82–85. [DOI] [PubMed] [Google Scholar]
- Brownbridge M, Costa S, and Jaronski S T. . 2001. Effects of in vitro passage of Beauveria bassiana on virulence to Bemisia argentifolii. J. Invertebr. Pathol. 77: 280–283. [DOI] [PubMed] [Google Scholar]
- Butt T M, Wang C, Shah F A, and Hall R. . 2006. Degeneration of entomogenous fungi, pp. 213–226. In Eilenberg J and Hokkanen H M T (eds.), An ecological and societal approach to biological control, vol. 2 Springer, Dordrecht, The Netherlands. [Google Scholar]
- Chandler D. 1994. Cryopreservation of fungal spores using porous beads. Mycol. Res. 98: 525–526. [Google Scholar]
- Fargues J, and Remaudiere G. . 1977. Consideration on the specificity of entomopathogenic fungi. Mycopathologia. 62: 31–37. [Google Scholar]
- Feng M G, Poprawski T J, and Khachatourians G G. . 1994. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci. Technol. 4: 3–34. [Google Scholar]
- Fernandes É K K, Keyser C A, Rangel D E N, Foster R N, and Roberts D W. . 2010. CTC medium: a novel dodine-free selective medium for isolating entomopathogenic fungi, especially Metarhizium acridum, from soil. Biol. Control. 54: 197–205. [Google Scholar]
- Hasaballah A J, Fouda M A, Hassan M I, and Omar G M. . 2017. Pathogenicity of Beauveria bassiana and Metarhizium anisopliae on adult housefly, Musca domestica L. Egypt Acad. J. Biol. Sci. 10: 79–86. [Google Scholar]
- Humber R A. 2012. Preservation of entomopathogenic fungal cultures, pp. 317–328. In Lacey L A. (ed.), Manual of techniques in invertebrate pathology. Academic Press, New York. [Google Scholar]
- Immediato D, Camardaa A, Iatta R, Puttilli M R, Ramosa R A N, Di Paolaa G, Giangasperoc A, Otrantoa D, and Cafarchia C. . 2015. Laboratory evaluation of a native strain of Beauveria bassiana for controlling Dermanyssus gallinae (De Geer, 1778) (Acari: Dermanyssidae). Vet. Parasitol. 212: 478–482. [DOI] [PubMed] [Google Scholar]
- Inglis G D, Enkerli J, and Goettel M S. . 2012. Laboratory techniques used for entomopathogenic fungi: Hypocreales, pp. 189–253. In Lacey L A. (ed.), Manual of techniques in invertebrate pathology. Academic Press, New York. [Google Scholar]
- Jackson M A, Dunlap C A, and Jaronski S T. . 2010. Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl. 55: 129–145. [Google Scholar]
- Jaronski S T, and Jackson M A. . 2012. Mass production of entomopathogenic Hypocreales, pp. 255–282. In Lacey L A. (ed.), Manual of techniques in invertebrate pathology. Academic Press, New York. [Google Scholar]
- Johnson D M, Weeks E N I, LoVullo E D, Shirk P D, and Geden C J. . 2019. Mortality effects of three bacterial pathogens and Beauveria bassiana when topically applied or injected into house flies, Musca domestica (Diptera: Muscidae). J. Med. Entomol. 56: 774–783. [DOI] [PubMed] [Google Scholar]
- Kaufman P E, Reaser C, Rutz D A, Ketzis J K, and Arends J J. . 2005. Evaluation of Beauveria bassiana applications against adult house fly, Musca domestica, in commercial caged-layer poultry facilities in New York state. Biol. Control. 33: 360–367. [Google Scholar]
- Kirkland B H, Westwood G S, and Keyhani N O. . 2004. Pathogenicity of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J. Med. Entomol. 41: 705–711. [DOI] [PubMed] [Google Scholar]
- Leemon D M, and Jonsson N N. . 2008. Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J. Invertebr. Pathol. 97: 40–49. [DOI] [PubMed] [Google Scholar]
- Lopes R B, and Faria M. . 2019. Influence of two formulation types and moisture levels on the storage stability and insecticidal activity of Beauveria bassiana. Biocontrol Sci. Technol. 29: 437–450. [Google Scholar]
- Mascarin G M, and Jaronski S T. . 2016. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 32: 177. [DOI] [PubMed] [Google Scholar]
- Mishra S, Kumar P, and Malik A. . 2013. Preparation, characterization, and insecticidal activity evaluation of three different formulations of Beauveria bassiana against Musca domestica. Parasitol. Res. 112: 3485–3495. [DOI] [PubMed] [Google Scholar]
- Moloinyane S, and Nchu F. . 2019. The effects of endophytic Beauveria bassiana inoculation on infestation level of Planococcus ficus, growth and volatile constituents of potted greenhouse grapevine (Vitis vinifera L.). Toxins. 11: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T C. 2018. Counting cells with hemocytometer. Protocols.io ( 10.17504/protocols.io.nxsdfne) [DOI] [Google Scholar]
- Moslim R, Hamid N H, Wahid M B, Kamarudin N, and Ali S R A. . 2005. Mass production of Metarhizium anisopliae using solid-state fermentation and wet harvesting methods, pp. 928–943. In Proceedings of the PIPOC 2005 International Palm Oil Congress (Agriculture, Biotechnology and Sustainability) Malaysian Palm Oil Board, Kuala Lumpur, Malaysia. [Google Scholar]
- Nahar P B, Kulkarni S A, Kulye M S, Chavan S B, Kulkarni G, Rajendran A, Yadav P D, Shouche Y, and Deshpande M V. . 2008. Effect of repeated in vitro subculturing on the virulence of Metarhizium anisopliae against Helicoverpa armigera (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 18: 337–355. [Google Scholar]
- Pereira R M, and Roberts D W. . 1990. Dry mycelium preparations of entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. J. Invertebr. Pathol. 56: 39–46. [Google Scholar]
- Rangel D E N, Dettenmaier S J, Fernandes E K K, and Roberts D W. . 2010. Susceptibility of Metarhizium spp. and other entomopathogenic fungi to dodine-based selective media. Biocontrol Sci. Technol. 20: 375–389. [Google Scholar]
- Roberts D W, Fuxa J R, Gaugler R, Goettel M, Jaques R, and Maddox J. . 1991. Use of pathogens in insect control, pp. 243–278. In Pimentel D. (ed.), Handbook of pest management in agriculture, 2nd ed. CRC Press, Boca Raton, FL. [Google Scholar]
- Sala A, Barrena R, Artola A, and Sánchez A. . 2019. Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Crit. Rev. Environ. Sci. Technol. 49: 655–694. [Google Scholar]
- Smith D. 1993. Long-term preservation of test strains (fungus). Intern. Biodeteriol. Biodegrad. 31: 227–230. [Google Scholar]
- Smith D, and Onions A H S. . 1994. The preservation and maintenance of living fungi, 2nd ed. IMI Technical Handbook No. 2, International Mycological Institute, CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- Sun M, Ren Q, Guan G, Li Y, Han X, Ma C, Yin H, and Luo J. . 2013. Effectiveness of Beauveria bassiana sensu lato strains for biological control against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in China. Parasitol. Int. 62: 412–415. [DOI] [PubMed] [Google Scholar]
- Uma Devi K, Padmavathi J, Uma Maheswara Rao C, Khan A A, and Mohan M C. . 2008. A study of host specificity in the entomopathogenic fungus Beauveria bassiana (Hypocreales, Clavicipitaceae). Biocontrol Sci. Technol. 18: 975–989. [Google Scholar]
- Watson D W, Geden C J, Long S J, and Rutz D A. . 1995. Efficacy of Beauveria bassiana for controlling the house fly and stable fly (Diptera: Muscidae). Biol. Control. 5: 405–411. [Google Scholar]