Abstract
Deer keds (Diptera: Hippoboscidae: Lipoptena Nitzsch, 1818 and Neolipoptena Bequaert, 1942) are blood-feeding ectoparasites that primarily attack cervids and occasionally bite humans, while ticks may be found on cervids, but are more generalized in host choice. Recent detection of pathogens such as Anaplasma and Borrelia in deer keds and historical infections of tick-borne diseases provides reason to investigate these ectoparasites as vectors. However, previous methods employed to sample deer keds and ticks vary, making it difficult to standardize and compare ectoparasite burdens on cervids. Therefore, we propose a standardized protocol to collect deer keds and ticks from hunter-harvested deer, which combines previous methods of sampling, including timing of collections, dividing sections of the deer, and materials used in the collection process. We tested a three-section and a five-section sampling scheme in 2018 and 2019, respectively, and found that dividing the deer body into five sections provided more specificity in identifying where deer keds and ticks may be found on deer. Data from 2018 suggested that deer keds and ticks were found on all three sections (head, anterior, posterior), while data from 2019 suggested that more Ixodes scapularis were found on the head and deer keds were found on all body sections (head, dorsal anterior, dorsal posterior, ventral anterior, and ventral posterior). The protocol provides an efficient way to sample deer for deer keds and ticks and allows researchers to compare ectoparasite burdens across geographical regions. Furthermore, this protocol can be used to collect other ectoparasites from deer or other cervids.
Keywords: deer ked, tick, deer, sampling method, hunter
Deer keds (Diptera: Hippoboscidae: Lipopteninae: Lipoptena Nitzsch, 1818 and Neolipoptena Bequaert, 1942) are hematophagous ectoparasitic flies that primarily feed on cervids. Keds have historically been considered pests of minor medical importance but can occur in high enough numbers to be a nuisance and pose an occupational hazard in rural areas of Europe (Chitiakov 1968, Reunala et al. 1980, Rantanen et al. 1982, Hackman et al. 1983, Laukkanen et al. 2005, Härkönen et al. 2009). While they are not considered major vectors of human pathogens, recent studies have detected pathogens such as Anaplasma, Bartonella, Borrelia, Ehrlichia, Trypanosoma, and Rickettsia in deer keds (summarized by Skvarla and Machtinger 2019).
Like deer keds, ticks can also be found on cervids (Artiodactyla: Cervidae). Over the past decade, the number of tick-borne disease cases have nearly tripled (Rosenberg et al. 2018). To preclude future cases, a better understanding of the ecology of tick vectors and their interactions with hosts is needed. White-tailed deer Odocoileus virginianus (Zimmerman) are primary hosts for the adult stage of Ixodes scapularis (Say) (Acari: Ixodidae), the main vector for the causative agent of Lyme disease (Borrelia burgdorferi), Powassan virus, anaplasmosis (Anaplasma phagocytophilum), and babesiosis (Babesia microti) (Spielman et al. 1979, Ebel et al. 1999). Tick bites and presumably deer ked bites can also incur negative physical effects on white-tailed deer and other cervids. For example, elk Cervus canadensis (Erxleben) and moose Alces alces (Linnaeus) extensively groom themselves when the threat of tick infestation is greater, leading to hair loss (Mooring and Samuel 1998, 1999). Because deer keds and ticks can concurrently be found on deer, combining collection efforts for both ectoparasites would be advantageous to understand vector-host relationships and disease dynamics. With increasing interest in the importance of deer keds as potential pathogen vectors and the current establishment of ticks as vectors, standardized sampling methods are required that allow researchers to compare ectoparasite burdens on deer across collection events, studies, and geographic areas.
Currently, there are no standardized methods to collect multiple ectoparasites from deer and other cervids. Instructions on sampling for deer keds in previous studies vary widely (Table 1). For example, some authors divide deer into various sections and search without a time limit, others search the whole animal for 5 min, comb the animal for 30 strokes with a flea comb, or utilize a flea comb to go through the hide and count any ectoparasites attached to the comb. However, in most studies, authors did not describe their collection methods (Supp Table S1 [online only]). While tick collections from deer and other cervids were more consistent in patterns of collection (Table 2), the purposes for collecting ticks affected how sampling occurred. For example, Arsnoe et al. (2015) sampled deer for ticks for the purpose of rearing ticks in the laboratory. Other studies (e.g., Apperson et al. 1990 and Hertz et al. 2017) used citizens or organizations to collect ticks during necropsies, which could result in variable sampling methods and effort. Another option to sample for deer keds and ticks on deer is dissolving the hide and hairs in KOH and then straining leftover materials to better visualize ectoparasites that are among the hairs (Westrom et al. 1985, Kashivakura 2013). With inconsistencies in how deer are sampled for deer keds and ticks, there is an opportunity to propose a standardized method of collection.
Table 1.
Ked collection methods used in previous studies
| Reference | Host species | Ked species (Rondani) | Deer ked collection method | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed area | Full body | Body region (single) | Body regions (multiple) | Fixed time | Full count | N/A | Other | |||
| Heine et al. 2017 | O. virginianus (Zimmerman) | L. mazamae (Rondani) | √ | |||||||
| Hermosilla et al. 2006 | Canis lupus familiaris (Linnaeus) | L. cervi | √ | |||||||
| Izenour et al. 2020 | O. virginianus | L. mazamae | √ | √ | ||||||
| Kellogg et al. 1971 | O. virginianus | L. cervi, L. mazamae | √ | |||||||
| Mertins et al. 1992 | Antilope cervicarpa (Linnaeus) | L. mazamae | √ | |||||||
| Nelder and Reeves 2005 | O. virginianus | L. mazamae | √ | √ | ||||||
| Osborn 1990 | Axis axis (Erxleben), Cervus nippon (Temminck), Dama dama (Linnaeus), O. virginianus | L. mazamae | √ | |||||||
| Paakkonen et al. 2010 | Alces alces (Linnaeus) | L. cervi | √ | √ | √ | |||||
| Russell 1967 | O. virginianus | L. depressa, N. ferrisi | √ | √ a | ||||||
| Samuel and Trainer 1972 | O. virginianus | L. mazamae | √ | |||||||
| Samuel 1979 | O. virginianus | L. mazamae | √ | |||||||
| Sokół and Gałęcki 2017 | C. lupus familiaris | Lipoptena sp. | ||||||||
| Wedincamp Jr and Durden 2016 | O. virginianus | L. mazamae | √ | √ | ||||||
| Westrom and Anderson 1992 | Odocoileus hemionus columbianus (Richardson) | L. depressa, N. ferrisi | √ | √ | ||||||
aFull count was only done for adult ticks.
Table 2.
Tick collection methods used in previous studies
| Reference | Host species | Tick species | Tick collection method | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed area | Full body | Body region (single) | Body regions (multiple) | Fixed time | Full count | N/A | Other | |||
| Arsnoe et al. 2015 | Odocoileus virginianus (Zimmerman) | Ixodes scapularis (Say) | √ | |||||||
| Baer-Lehman et al. 2012 | O. virginianus | I. scapularis, Dermacentor albipictus (Packard) | √ | √ | ||||||
| Bouchard et al. 2013 | O. virginianus | I. scapularis, D. albipictus | √ | √ | ||||||
| Campagnolo et al. 2018 | O. virginianus | I. scapularis | √ | |||||||
| Cortinas and Kitron 2006 | O. virginianus | I. scapularis, D. albipictus | √ | |||||||
| Daniels et al. 2009 | O. virginianus | I. scapularis | √ | |||||||
| Fantahun and Mohamed 2012 | Bos taurus indicus (Linnaeus) | Boophilus decoloratus (Koch), Amblyomma coherence (Dönitz), Rhipicephalus evertsi evertsi (Neumann), Amblyomma variegatum (Fabricius) | √ | |||||||
| Handeland et al. 2013 | Alces alces (Linnaeus), Cervus elaphus (Linnaeus), Capreolus capreolus (Linnaeus) | Ixodes ricinus (Linnaeus) | √ | |||||||
| Han et al. 2019 | O. virginianus | I. scapularis | √ | |||||||
| Han et al. 2016 | O. virginianus | I. scapularis | √ | |||||||
| Heine et al. 2017 | O. virginianus | I. scapularis, D. albipictus, A. americanum (Linnaeus) | √ | |||||||
| Hereid 2017 | C. capreolus (fawns) | I. ricinus | √ | |||||||
| Hertz et al. 2017 | Wild Turkey (Meleagris gallopavo [Linnaeus]), Feral Swine (Sus scrofa [Linnaeus]), O. virginianus | A. americanum, Amblyomma maculatum (Koch), Dermacentor variabilis (Say), I. scapularis | √ | |||||||
| Kashivakura 2013 | Alces americanus (Clinton), Rangifer tarandus caribou (Gmelin) | D. albipictus | √ | √ | √ | |||||
| Keefe et al. 2009 | O. virginianus | I. scapularis | √ | |||||||
| Kiffner et al. 2010 | C. capreolus | Ixodes spp. | √ | √ | ||||||
| Kiffner et al. 2011 | C. capreolus | I. ricinus | √ | √ | ||||||
| Lee et al. 2013 | O. virginianus | I. scapularis | √ | |||||||
| Mysterud et al. 2014 | C. elaphus | I. ricinus | √ | √ | ||||||
| Ojeda-Chi et al. 2019 | Odocoileus virginianus yucatanensis (Zimmerman), Mazama temama (Kerr) | Amblyomma mixtum (Koch), Amblyomma parvum (Aragão), Amblyomma cf. oblongoguttatum (Koch), Ixodes affinis (Neumann), Rhipicephalus microplus (Canestrini), Rhipicephalus sanguineus sensulato (Latreille), Haemaphysalis juxtakochi (Cooley) | √ | |||||||
| Raizman et al. 2013 | O. virginianus | I. scapularis | √ | |||||||
| Raizman et al. 2010 | O. virginianus | I. scapularis | √ | |||||||
| Rand et al. 2003 | O. virginianus | I. scapularis | √ | √ | √ | |||||
| Rosen et al. 2013 | O. virginianus | I. scapularis, D. albipictus, A. americanum | √ | |||||||
| Solberg et al. 2003 | O. virginianus | I. scapularis | √ | |||||||
| Teague III 2018 | O. virginianus | I. scapularis | √ | |||||||
| Vázquez et al. 2011 | C. capreolus | I. ricinus | √ | √ | ||||||
Only studies published after 2000 are included in this table.
To achieve the goal of comparing the burden of deer keds or ticks on deer and other cervids, the objectives for this paper are: 1) to develop a standardized method of collection for deer keds and ticks found on hunter-harvested deer and other cervids; 2) to present preliminary data of deer ked and tick locations on cervid hosts and; 3) to provide resources to conduct ectoparasite collections. The protocol described below combines the techniques from previous studies such as timing of collections, dividing the deer into sections for more thorough sampling, and using a flea/louse comb for greater visibility of ectoparasites.
Experimental Design
Within the United States, deer harvest reporting requirements vary by state and sometimes even within a state (Table 3). Some states require hunter-harvested deer to be checked in at deer check stations, which can provide centralized places for sampling; however, deer check stations may not be required across a whole state (i.e., hunters may not be required to visit a deer check station if the deer was hunted in a county that does not have a deer check station or require a visit). In states that do not have deer check stations, such as Pennsylvania, deer processors are another source of hunter-harvested deer. Some states require harvest information (e.g., county, township, and time of harvest) to be written on physical tags that accompany hunter-killed deer, which makes obtaining such information easy and straightforward, especially when more deer arrive at a check station or processor than can be examined in a timely fashion. Other states require only online check-in of harvested deer without physical documentation, which necessitates asking hunters where they harvested their deer, which makes documenting harvest information difficult when many deer arrive concurrently. The location of the deer check stations/deer processors, speed of the processing at the site, availability of harvest information, and timing of sampling should be considered when choosing sampling sites. Finally, permission to sample deer at deer check stations/processor facilities should be obtained days to weeks before sampling is scheduled to begin as some check stations/processors may be unwilling to host sampling teams and alternative check stations/processors will need to be contacted. Additionally, consultation from the Institutional Animal Care and Use Committee (IACUC) should be sought on whether an IACUC is necessary for sampling hunter-harvested deer. The decision from IACUC may differ per institution, so institutional rules should be followed. Once permissions from processors and the IACUC are secured as needed, teams should prepare the materials to sample for deer keds and ticks.
Table 3.
Status of deer check stations in the United States and requirements for in-person check-ins for hunter-harvested deer
| State | Deer check station in the state? | Deer check-in required at station? |
|---|---|---|
| Alabama | No | |
| Alaska | Yes | Yes, but depends on the management area and species harvested. |
| Arizona | Yes | Yes, but depends on the management area to monitor where CWD is entering the state. |
| Arkansas | No | |
| California | No | |
| Colorado | Yes | Yes, mandatory testing for CWD in management areas (brain tissue). |
| Connecticut | No | |
| Delaware | No | |
| Florida | Yes | Yes, visiting deer check stations is mandatory in some wildlife management areas. |
| Georgia | No | |
| Hawaii | Yes | Yes |
| Idaho | Yes | Yes, in certain regions that change yearly. |
| Illinois | Yes | Yes, in certain counties for CWD monitoring. |
| Indiana | No | |
| Iowa | No | |
| Kansas | No | |
| Kentucky | No | |
| Louisiana | No | |
| Maine | Yes | Yes |
| Maryland | No | |
| Massachusetts | Yes | Yes, but only during the first week of shotgun deer hunting season. |
| Michigan | Yes | Yes, primarily for CWD. |
| Minnesota | Yes | Yes, in certain regions, primarily for CWD. |
| Mississippi | No | |
| Missouri | No | |
| Montana | No | |
| Nebraska | Yes | Yes |
| Nevada | Yes | No, voluntary check stations for CWD exist. |
| New Hampshire | Yes | Yes |
| New Jersey | No | |
| New Mexico | No | |
| New York | Yes | No, voluntary check stations exist (non-CWD). |
| North Carolina | No | |
| North Dakota | Yes | No, voluntary check stations for CWD exist. |
| Ohio | Yes | Yes, mandatory testing for CWD only in surveillance areas. |
| Oklahoma | No | |
| Oregon | Yes | No, voluntary check stations for CWD exist. |
| Pennsylvania | No | |
| Rhode Island | Yes | Yes, during the first 4 d of Muzzleloader Deer Season, including deer taken with archery equipment; except those deer taken on Patience, Prudence, and Block Island. |
| South Carolina | No | |
| South Dakota | Yes | Yes, CWD testing required for deer harvested in surveillance area (lymph nodes). |
| Tennessee | No | |
| Texas | Yes | No, voluntary check stations exist (non-CWD). |
| Utah | Yes | No, voluntary check stations for CWD exist. |
| Vermont | Yes | Yes |
| Virginia | Yes | No, voluntary check stations exist (non-CWD). |
| Washington | Yes | No, voluntary check stations exist (non-CWD). |
| West Virginia | No | |
| Wisconsin | No | |
| Wyoming | Yes | Yes |
To collect deer keds, ticks, and other ectoparasites from deer, the materials in Table 4 are needed. Manufacturer and vendor information is provided for each item; however, the items do not need to come from the listed manufacturer.
Table 4.
Materials used to collect ectoparasites from deer
| Item | Vendor | Catalog/item number |
|---|---|---|
| Fine-point forceps | Bioquip | 4535 |
| Flea combs | SBYURE (Amazon) | N/A |
| 2-ml microcentrifuge tubes (at least 2 colors if sampling for multiple ectoparasites) | Fisher Scientific | 05-408-137 |
| Sample boxes and box dividers | Fisher Scientific | 03-395-455 (boxes) 03-395-465 (dividers) |
| Cardstock or label paper | Cardstock Manufacturer (Amazon) | N/A |
| 70% ethanol (diluted from 100%) | Koptec | V1001 |
| Stopwatches | Champion Sports (Amazon) | N/A |
| Permanent markers | Amazon | N/A |
| Pigma Micron Pen (preferably size 01 or 02) | Sakura of America | 0 84511 30636 3 (01) 0 84511 31837 3 (02) |
| Scissors (8 in/20 cm) | Amazon | N/A |
| Laboratory gloves (latex, nitrile, etc.) | Fisher Scientific | Varies |
| Knee pads | Amazon | N/A |
| Cooler (28 qt or larger) | Coleman | 6278-703G |
| Hand sanitizer | Purell | 9652-12 |
| Paper towels | Scott Brand | 01804 |
Preliminary steps should be completed prior to arriving at the study site. Datasheets for collecting ectoparasites from deer should include a column for a unique identification number for each deer assigned by the researcher, deer hunting tag number/code to link back to the processor and hunter, hunting/harvesting time, sampling time, city/county of where the deer was hunted, age and sex of the deer, and columns for tick and deer ked sample numbers. To account for the variability in the amount of time between harvesting and sampling, deer can be sampled within a certain time period as chosen by the researchers. For example, researchers can choose to only sample deer within a 6-h period from the harvest time listed on the physical hunting tag. However, tag information may differ depending on the state that is being sampled. An example of a datasheet is provided in Supp Material 1 (online only), but the datasheet can be customized to accomplish unique research objectives.
Microcentrifuge tubes for ectoparasite collections should also be prepared. Two colors of tubes will be needed for easy identification between deer ked and tick specimens. Labels correspond to the unique identification number created by the researcher and can include the date of sampling, deer identification number, and the section from which the ectoparasites were collected (i.e., 2019-1104-001-H represents the ectoparasites collected from the head of the first deer sampled on 4 November 2019). An example for labels is provided in Supp Material 2 (online only). Labels can be printed on cardstock paper using a laser or ink printer and then cut to the size of the tube. One label will go into each tube. If deer keds and ticks are being collected, there should be ten total labels and tubes (five labels for deer ked tubes and five labels for tick tubes) for each deer sampled. In addition to the labels, 70% ethanol is also added to each tube. Before sampling, tubes with the same deer identification number can be placed around the deer’s body corresponding to each section of collection.
Protocol
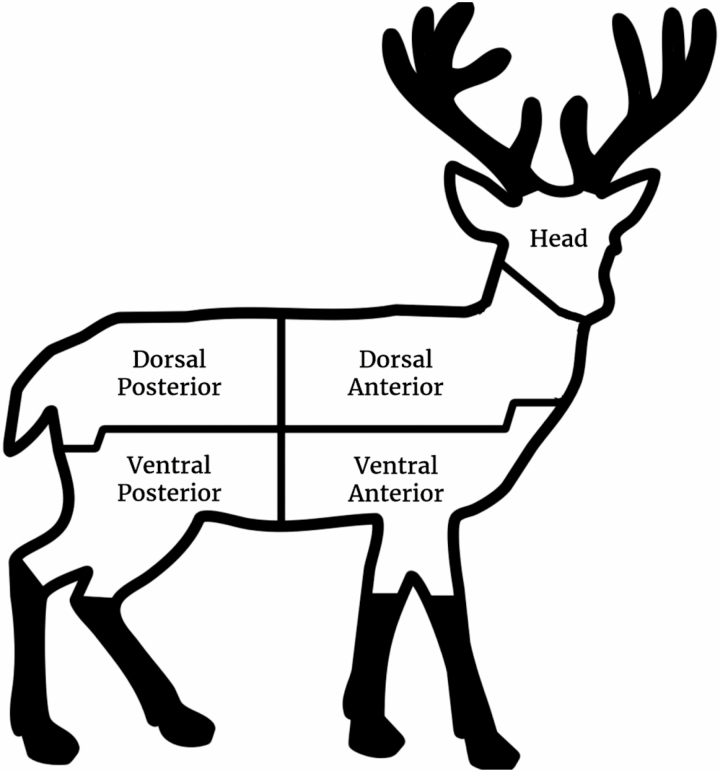
Deer are visually searched for deer keds and ticks using flea combs to separate hairs for better visibility, brushing against the direction of the hair (Fig. 1). There is evidence that ticks may be found more often on the left side of deer (Bloemer et al. 1988); however, we suggest that individual deer can be checked on either the left or right side as certain factors (e.g., large exit wound and dried blood if a deer is taken with a high-caliber rifle or which side of a deer was against the ground when it was dragged out after being harvested) can affect the ability to comb through the hair or the presence or absence of ectoparasites. The deer is divided into five general sections (Fig. 2): head, dorsal anterior, ventral anterior, dorsal posterior, and ventral posterior. The sections of deer are separated based on the natural midlines on the body. The dorsal anterior section includes the neck down to the midline separating the ventral anterior section and the midline separating the dorsal posterior section. The ventral anterior sections and the ventral posterior sections start from the lateral midline separating the dorsal anterior and dorsal posterior sections, respectively, and ends at the carpals/tarsals of the legs. Sections such as the ears and axillae should also be searched. Genitals of the deer can be checked if available or visible and can be included in the counts for the ventral posterior region. Lower legs past the carpals/tarsals were not checked for ectoparasites because there is evidence that few or no ticks were expected to be found on this section of the legs (Schmidtmann et al. 1998).
Fig. 1.
Using flea combs to search for deer keds, ticks, and other ectoparasites on deer. Flea combs are used to separate hairs and increase visibility, brushing against the direction of the hair.
Fig. 2.
Suggested five-section method for sampling deer keds and ticks on hunter-harvested deer. In the ventral posterior region, the genitals can be checked, if possible. Black sections (antlers and lower legs past the carpals/tarsals) are not included in the deer checks.
The entire deer is examined for 10 person-minutes to standardize searching techniques among samplers, with each section being examined for two person-minutes, such that if two people examine a deer, each section is checked for 60 s and if one person examines a deer, each section is checked for 120 s. Time is kept with a stopwatch and as many ectoparasites are removed as possible with forceps. After deer keds and ticks are removed from deer, they are placed in their respective microcentrifuge tubes with ethanol to preserve them for identification and/or pathogen testing. Additional prepped tubes may be necessary if a deer has a high ectoparasite load. If two people collect from the same deer, the numbers of ectoparasites collected per section can then be summed for a total number of collections in the section. Once collection is complete for the deer, the tubes should be grouped together based on the unique deer identification number and placed into a sample box. Samples can be stored at room temperature (22–25°C).
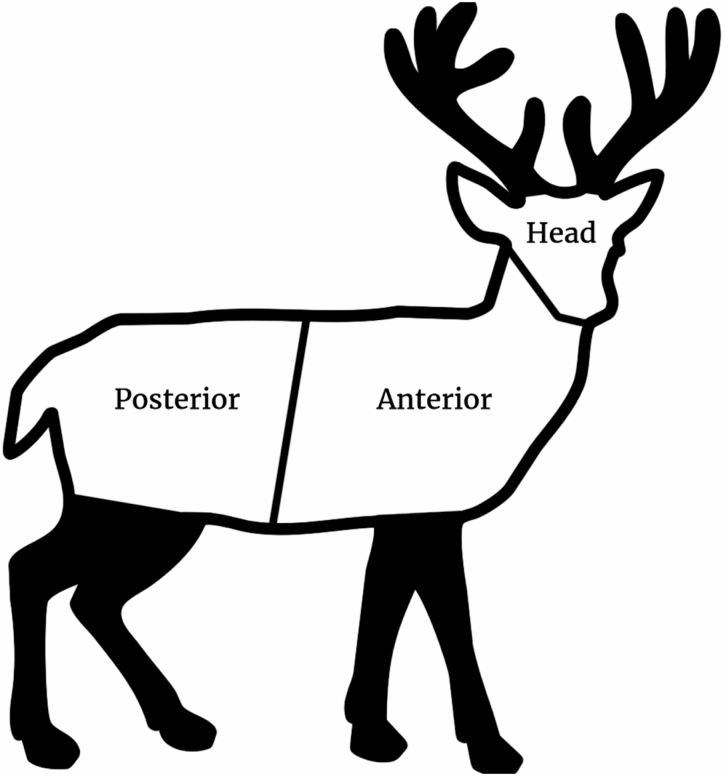
In 2018, we sampled deer at deer processors throughout Pennsylvania using a three-section technique that separates the deer into head, anterior, and posterior sections and sampled each section for 2 min using similar sampling techniques as the ones described above (Fig. 3). In 2019, we expanded our study region and sampled deer at deer processors in Indiana, Maryland, Pennsylvania, and Virginia using a five-section technique for better specificity of deer ked and tick location on the deer. All procedures were conducted according to the Pennsylvania State University Institutional Animal Use and Care Committee Protocol (PROTO201900871).
Fig. 3.
Three-section method to sample deer for deer keds and ticks used in 2018. In the posterior region, the genitals can be checked, if possible. Black sections (antlers and legs) were not included in the deer checks.
Results
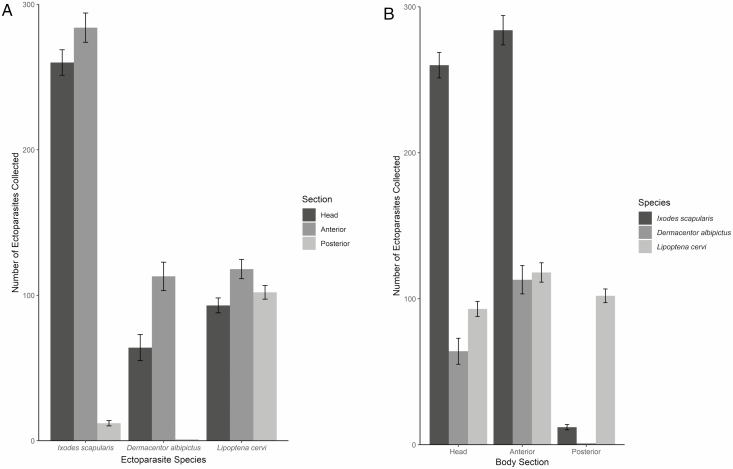
In 2018, 80 hunter-harvested deer that were examined at deer processors in Pennsylvania had ectoparasites and a total of 556 I. scapularis, 178 Dermacentor albipictus (Packard) (Acari: Ixodidae), and 313 Lipoptena cervi (Linnaeus) (Diptera: Hippoboscidae) were collected (Fig. 4). No other species of deer keds or ticks were recovered from deer. For I. scapularis, 260 ticks were recovered from the head, 284 from the anterior section, and 12 from the posterior. For D. albipictus, 64 ticks were found on the head, 113 from the anterior, and 1 from the posterior. Finally, 93 L. cervi were collected from the head, 118 from the anterior, and 102 from the posterior. Overall, more specimens were collected from the head and anterior sections. Ixodes scapularis and D. albipictus were found on the head and anterior sections of deer, while L. cervi were found throughout the body.
Fig. 4.
Deer keds and ticks collected from hunter-harvested deer (n = 80) at deer processors in Pennsylvania in 2018. (A) Number of deer keds and ticks collected per species. (B) Number of deer keds and ticks collected per body section.
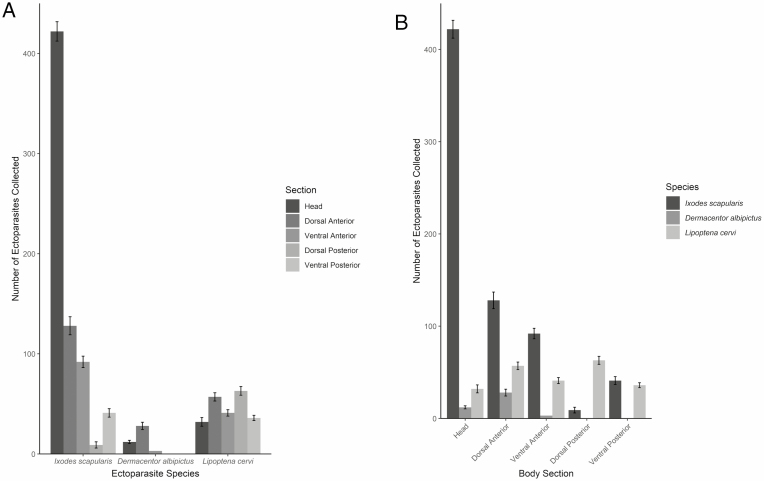
In 2019, a total of 692 I. scapularis, 43 D. albipictus, and 229 L. cervi were collected from 112 deer examined at processors in Indiana, Maryland, Pennsylvania, and Virginia (Fig. 5). No other species of ticks were recovered from deer. Ixodes scapularis were mostly found on the head of deer, totaling 422 ticks from this section. The dorsal and ventral anterior sections of the deer (128 and 92, respectively) had greater numbers of I. scapularis compared to the posterior sections (9 and 41, respectively). While only 12 D. albipictus were collected from the head of deer, the dorsal and ventral anterior sections had 28 and 3 ticks, respectively. No D. albipictus were found on either posterior section. Lipoptena cervi were found on all regions of deer, with the majority of keds from the dorsal posterior section (63 keds), followed by the dorsal anterior (57), ventral anterior (41), ventral posterior (36), and the head (32).
Fig. 5.
Deer keds and ticks collected from hunter-harvested deer (n = 112) at deer processors in Indiana, Maryland, Pennsylvania, and Virginia. (A) Number of deer keds and ticks collected per species. (B) Number of deer keds and ticks collected per body section.
Discussion
We presented a standardized protocol to sample hunter-harvested deer and other cervids for deer keds, ticks, and possibly other ectoparasites combining methods from previous studies to maximize productivity during collection. The protocol divides the deer into sections for easier management, provides a short time limit to search each section for time management, and employs the use of a flea comb for enhanced visibility of ectoparasites. Standardizing the method to collect deer keds and ticks from deer allows for the comparison of burdens across counties or states. Because the deer is partitioned into sections, we can also determine where deer keds and ticks can be found, if they utilize the same space at the same time, if there is competition or exclusion to infest a certain area of the deer, or if there are host sex differences.
Our results for 2018 indicate that deer keds and ticks can be found on all three sections of deer, with more I. scapularis found on the head and anterior sections of the deer and L. cervi found throughout the body. Results were mirrored in 2019 in that I. scapularis were concentrated on the head while L. cervi were present throughout all sections of the deer body. Overall, more I. scapularis were found in both anterior sections than the posterior sections in 2019. Lipoptena cervi moves quickly across host hairs, which could explain how deer keds can be found on almost all sections of deer. As ticks initiate blood-feeding, however, they become immobile and may elicit pheromones that attract conspecific ticks for reproduction and survival (Sonenshine 2006).
Previous studies that surveyed deer keds and ticks on deer and other cervids have identified various body sections with high infestations. For keds, a study of L. cervi on moose found that keds were more likely to be found on the anterior back compared to four other sections (Paakkonen et al. 2010). This coincided with our 2018 findings, where more L. cervi were found on the anterior section; however, our 2019 data indicate that more deer keds were found on the dorsal posterior section rather than dorsal anterior. Haarløv (1964) found that L. cervi were found on the neck, groin, and flank sections of red deer Cervus elaphus (Linnaeus) when the body was divided into eight regions. An additional study reported that more Lipoptena depressa (Say) and Neolipoptena ferrisi (Bequaert) were found on the posterior ventral section and the head, respectively (Westrom and Anderson 1992).
A study of I. scapularis and D. albipictus from white-tailed deer revealed that more ticks were found on the body of the deer rather than the head, which followed similar patterns with our 2018 data, but contradicts our 2019 data where we found more ticks on the head than the other four sections (Baer-Lehman et al. 2012); however, this could be an artifact of separating the number of collected specimens from both anterior sections in 2019. Ixodes scapularis were also likely to be found on the neck followed by the head (Schmidtmann et al. 1998) or the neck and shoulders (Watson and Anderson 1976) of deer. Dermacentor albipictus did not show predilection for any one section on moose or caribou (Kashivakura 2013). Inconsistent results could be attributed to the differences in sampling style and behaviors of different keds and ticks from diverse sections or ectoparasite competitive interactions.
We utilized the five-section method proposed here specifically to search for deer keds and ticks on deer; however, the method can also be used to search other cervids or animals for ectoparasites. Slight modifications may be required depending on the species as well as its status as a host for the ectoparasites.
Another method of surveying ectoparasites from deer, is to dissolve the hide and hair using KOH. While this method is likely to find more keds and ticks and potentially eliminate biases introduced by the difficulty of searching through longer or shorter pelage, it is labor-intensive since it requires separating the hide from deer, partitioning the hide, and then removing fat and other material before putting the hide into boiling KOH solution. Boiling large quantities of KOH is also potentially dangerous due to the corrosive properties of the solution. Finally, the KOH method may be difficult to implement using hunter-harvested deer as the hunters or processors may choose to keep the hide. On the other hand, active sampling of deer using the proposed technique could save time and effort on sampling deer.
Other alternatives to time-limited visual counting include taking skin samples and shaving or clipping the hair before counting (MacIvor et al. 1987, Matthee et al. 1997, Mysterud et al. 2014). Skin sampling from deer or other cervids requires cutting a small portion of the deer and looking for ectoparasites (MacIvor et al. 1987, Mysterud et al. 2014). While this would reduce the amount of time spent at the study site since no timed sampling is required, sampling a small portion of a deer would not provide a representative amount of ectoparasites found on the whole deer. In addition, hunters may want to keep the deer hide, thereby making skin sampling prohibitive. Removing hair from skin samples prior to counting provides an advantage to see ectoparasites more clearly along the skin. However, clipping hair from the animal can result in artifacts such as excess hair that would make it difficult to identify ectoparasites, but this method could be combined with chemically digesting the artifacts, as mentioned previously (Matthee et al. 1997). While both methods are not limited by time, they are limited by other factors such as the ambiguity associated with sampling the skin from a small portion of deer, the hunters’ desire to keep the hide/skin, and artifacts present on the skin sample.
Actively trapping and anesthetizing deer is another way to acquire deer to survey for deer keds and ticks. Anesthetizing deer allows time to collect ectoparasites; however, samplers are limited to the time that the deer is anesthetized. Furthermore, cocktails used to anesthetize deer may be considered controlled substances that require certain authorization to access and use and deer can react poorly to being anesthetized. Deer may also experience capture myopathy due to stress caused by trapping and anesthesia, leading to premature death (Beringer et al. 1996). Finally, actively catching deer is labor-intensive, time-consuming, and may not yield a large number of deer.
Using hunter-harvested deer from deer check stations or deer processors is a cost-effective method to detecting changes in the distribution of ticks (Centers for Disease Control and Prevention 2018). Furthermore, statewide coverage and comparisons of deer ked or tick burden on deer are possible if check stations or processors are available throughout the state (Table 2) and because hunters often travel throughout the state to hunt before taking their deer to local processors.
Searching for ectoparasites on hunter-harvested deer within a specified time limit has its limitations. If a deer is heavily infested, 2 min per section may not be enough time to find and collect all possible ectoparasites. Time becomes a limiting factor, making it difficult to compare the ectoparasite loads of deer that are the most heavily infested. Furthermore, searching for ectoparasites for two person-minutes per section is arbitrary and the collecting plateau encountered on heavily infested deer due to time-limited collecting may not be biologically relevant. However, keeping the examination time brief allows deer processors or check station staff to quickly process the deer and can increase the likelihood that hunters participate in the study. The short examination time also allows the research team to process a larger number of deer in a single day if enough deer continuously arrive at the processor.
While this proposed standardized protocol provides a general guideline on sampling deer and other cervids for ectoparasites, this may not fit with all research objectives. The protocol can still be utilized, but it can also be modified to achieve unique research questions. However, it should be noted that if sampling techniques change, such as the amount of time to sample per section or the limits of each body section, then it may not be possible to compare results across other studies.
In summary, we developed a standardized method of collection for deer keds and ticks found on hunter-harvested deer, presented preliminary data on where deer keds and ticks may be found on cervid hosts, and provided resources to conduct ectoparasite collections. With the emergence of pathogens detected in deer keds and the increase in tick-borne disease reporting in the United States, it is critical to utilize a standard method of sampling nationwide to compare ectoparasitic load on cervids across the country.
Supplementary Material
Acknowledgments
We extend our gratitude to the deer processors and hunters of Indiana, Maryland, Pennsylvania, and Virginia that participated in our study and allowed us to search their deer for deer keds and ticks. We also thank Jessica Brown, Carley Lionetto, Taylor Miller, Alex Pagac, Hannah Tiffin, and our field volunteers for dedicating their time and effort to streamlining the protocol and their assistance with deer ked and tick collections from deer. This project was funded by the Penn State Extension Multistate and Integrated Grant Program.
References Cited
- Apperson C S, Levine J F, and Nicholson W L. . 1990. Geographic occurrence of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) infesting white-tailed deer in North Carolina. J. Wildl. Dis. 26: 550–553. [DOI] [PubMed] [Google Scholar]
- Arsnoe I M, Hickling G J, Ginsberg H S, McElreath R, and Tsao J I. . 2015. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One 10: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer-Lehman M L, Light T, Fuller N W, Barry-Landis K D, Kindlin C M, and Stewart R L Jr. 2012. Evidence for competition between Ixodes scapularis and Dermacentor albipictus feeding concurrently on white-tailed deer. Exp. Appl. Acarol 58: 301–314. [DOI] [PubMed] [Google Scholar]
- Beringer J, Hansen L P, Wilding W, Fischer J, and Sheriff S L. . 1996. Factors affecting capture myopathy in white-tailed deer. J. Wildl. Manage. 60: 373–380. [Google Scholar]
- Bloemer S R, Zimmerman R H, and Fairbanks K. . 1988. Abundance, attachment sites, and density estimators of lone star ticks (Acari: Ixodidae) infesting white-tailed deer. J. Med. Entomol. 25: 295–300. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Leighton P A, Beauchamp G, Nguon S, Trudel L, Milord F, Lindsay L R, Bélanger D, and Ogden N H. . 2013. Harvested white-tailed deer as sentinel hosts for early establishing Ixodes scapularis populations and risk from vector-borne zoonoses in southeastern Canada. J. Med. Entomol. 50: 384–393. [DOI] [PubMed] [Google Scholar]
- Campagnolo E R, Tewari D, Farone T S, Livengood J L, and Mason K L. . 2018. Evidence of Powassan/deer tick virus in adult black-legged ticks (Ixodes scapularis) recovered from hunter-harvested white-tailed deer (Odocoileus virginianus) in Pennsylvania: a public health perspective. Zoonoses Public Health 65: 589–594. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2018. Surveillance for Ixodes scapularis and pathogens found in this tick species in the United States. CDC, Fort Collins, CO. [Google Scholar]
- Chitiakov A F. 1968. Skin lesions in people due to bites of Lipoptena cervi. Vestn. Dermatol. Venerol. 42: 59. [PubMed] [Google Scholar]
- Cortinas M R, and Kitron U. . 2006. County-level surveillance of white-tailed deer infestation by Ixodes scapularis and Dermacentor albipictus (Acari: Ixodidae) along the Illinois River. J. Med. Entomol. 43: 810–819. [DOI] [PubMed] [Google Scholar]
- Daniels T J, Falco R C, McHugh E E, Vellozzi J, Boccia T, Denicola A J, Pound J M, Miller J A, George J E, and Fish D. . 2009. Acaricidal treatment of white-tailed deer to control Ixodes scapularis (Acari: Ixodidae) in a New York Lyme disease-endemic community. Vector Borne Zoonotic Dis. 9: 381–387. [DOI] [PubMed] [Google Scholar]
- Ebel G D, Foppa I, Spielman A, and Telford S R 2nd. 1999. A focus of deer tick virus transmission in the northcentral United States. Emerg. Infect. Dis. 5: 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantahun B, and Mohamed A. . 2012. Survey on the distribution of tick species in and around Assosa town, Ethiopia. Res. J. Vet. Sci. 5: 32–41. [Google Scholar]
- Haarløv N. 1964. Life cycle and distribution pattern of Lipoptena cervi (L.)(Dipt., Hippobosc.) on Danish deer. Oikos. 15: 93–129. [Google Scholar]
- Hackman W, Rantanen T, and Vuojolahti P. . 1983. Immigration of Lipoptena cervi (Diptera, Hippoboscidae) in Finland, with notes on its biology and medical significance. Not. Entomol. 63: 53–59. [Google Scholar]
- Han S, Hickling G J, and Tsao J I. . 2016. High prevalence of Borrelia miyamotoi among adult blacklegged ticks from white-tailed deer. Emerg. Infect. Dis. 22: 316–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lubelczyk C, Hickling G J, Belperron A A, Bockenstedt L K, and Tsao J I. . 2019. Vertical transmission rates of Borrelia miyamotoi in Ixodes scapularis collected from white-tailed deer. Ticks Tick. Borne. Dis. 10: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handeland K, Qviller L, Vikøren T, Viljugrein H, Lillehaug A, and Davidson R K. . 2013. Ixodes ricinus infestation in free-ranging cervids in Norway–a study based upon ear examinations of hunted animals. Vet. Parasitol. 195: 142–149. [DOI] [PubMed] [Google Scholar]
- Härkönen S, Laine M, Vornanen M, and Reunala T. . 2009. Deer ked (Lipoptena cervi) dermatitis in humans–an increasing nuisance in Finland. Alces A J. Devoted to Biol. Manag. Moose. 45: 73–79. [Google Scholar]
- Heine K B, DeVries P J, and Penz C M. . 2017. Parasitism and grooming behavior of a natural white-tailed deer population in Alabama. Ethol. Ecol. Evol. 29: 292–303. [Google Scholar]
- Hereid S W.The effect of ticks (Ixodes ricinus) on early survival of roe deer (Capreolus capreolus) fawns. M.S. thesis, Norwegian University of Life Sciences, Ås, Norway. 2017.
- Hermosilla C, Pantchev N, Bachmann R, and Bauer C. . 2006. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet. Rec. 159: 286–287. [DOI] [PubMed] [Google Scholar]
- Hertz J C, Ferree Clemons B C, Lord C C, Allan S A, and Kaufman P E. . 2017. Distribution and host associations of ixodid ticks collected from wildlife in Florida, USA. Exp. Appl. Acarol 73: 223–236. [DOI] [PubMed] [Google Scholar]
- Izenour K, Zikeli S, Kalalah A, Ditchkoff S D, Starkey L A, Wang C, and Zohdy S. . 2020. Diverse Bartonella spp. detected in white-tailed deer (Odocoileus virginianus) and associated keds (Lipoptena mazamae) in the Southeastern United States. J. Wildl. Dis. 56 https://www.jwildlifedis.org/ 10.7589/2019-08-196. [DOI] [PubMed] [Google Scholar]
- Kashivakura C K. 2013. Detecting Dermacentor albipictus, the winter tick, at the northern extent of its distribution range: Hunter-based monitoring and serological assay development. M.S. thesis, University of Calgary, Calgary, Canada. [Google Scholar]
- Keefe L M, Moro M H, Vinasco J, Hill C, Wu C C, and Raizman E A. . 2009. The use of harvested white-tailed deer (Odocoileus virginianus) and geographic information system (GIS) methods to characterize distribution and locate spatial clusters of Borrelia burgdorferi and its vector Ixodes scapularis in India. Vector Borne Zoonotic Dis. 9: 671–680. [DOI] [PubMed] [Google Scholar]
- Kellogg F E, Kistner T P, Strickland R K, and Gerrish R R. . 1971. Arthropod parasites collected from white-tailed deer. J. Med. Entomol. 8: 495–498. [DOI] [PubMed] [Google Scholar]
- Kiffner C, Lödige C, Alings M, Vor T, and Rühe F. . 2010. Abundance estimation of Ixodes ticks (Acari: Ixodidae) on roe deer (Capreolus capreolus). Exp. Appl. Acarol 52: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffner C, Lödige C, Alings M, Vor T, and Rühe F. . 2011. Attachment site selection of ticks on roe deer, Capreolus capreolus. Exp. Appl. Acarol 53: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen A, Ruoppi P, and Mäkinen-Kiljunen S. . 2005. Deer ked-induced occupational allergic rhinoconjunctivitis. Ann. Allergy. Asthma Immunol. 94: 604–608. [DOI] [PubMed] [Google Scholar]
- Lee X, Hardy K, Johnson D H, and Paskewitz S M. . 2013. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J. Med. Entomol. 50: 632–639. [DOI] [PubMed] [Google Scholar]
- MacIvor K M, Horak I G, Holton K C, and Petney T N. . 1987. A comparison of live and destructive sampling methods of determining the size of a parasitic tick populations. Exp. Appl. Acarol 3: 131–143. [DOI] [PubMed] [Google Scholar]
- Matthee S, Meltzer D G, and Horak I G. . 1997. Sites of attachment and density assessment of ixodid ticks (Acari:Ixodidae) on impala (Aepyceros melampus). Exp. Appl. Acarol 21: 179–192. [DOI] [PubMed] [Google Scholar]
- Mertins J W, Schlater J L, and Corn J L. . 1992. Ectoparasites of the blackbuck antelope (Antilope cervicapra). J. Wildl. Dis. 28: 481–484. [DOI] [PubMed] [Google Scholar]
- Mooring M S, and Samuel W M. . 1998. Tick-removal grooming by elk (Cervus elaphus): testing the principles of the programmed-grooming hypothesis. Can. J. Zool. 76: 740–750. [Google Scholar]
- Mooring M S, and Samuel W M. . 1999. Premature loss of winter hair in free-ranging moose (Alces alces) infested with winter ticks (Dermacentor albipictus) is correlated with grooming rate. Can. J. Zool. 77: 148–156. [Google Scholar]
- Mysterud A, Hatlegjerde I L, and Sørensen O J. . 2014. Attachment site selection of life stages of Ixodes ricinus ticks on a main large host in Europe, the red deer (Cervus elaphus). Parasites and Vectors 7: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder M P, and Reeves W K. . 2005. Ectoparasites of road-killed vertebrates in northwestern South Carolina, USA. Vet. Parasitol. 129: 313–322. [DOI] [PubMed] [Google Scholar]
- Ojeda-Chi M M, Rodriguez-Vivas R I, Esteve-Gasent M D, Pérez de León A, Modarelli J J, and Villegas-Perez S. . 2019. Molecular detection of rickettsial tick-borne agents in white-tailed deer (Odocoileus virginianus yucatanensis), mazama deer (Mazama temama), and the ticks they host in Yucatan, Mexico. Ticks Tick. Borne. Dis. 10: 365–370. [DOI] [PubMed] [Google Scholar]
- Osborn D A. 1990. Physical condition evaluation of axis, fallow, sika, and white-tailed deer in Central Texas. M.S. thesis, Texas Tech University, Lubbock, TX. [Google Scholar]
- Paakkonen T, Mustonen A M, Roininen H, Niemelä P, Ruusila V, and Nieminen P. . 2010. Parasitism of the deer ked, Lipoptena cervi, on the moose, Alces alces, in eastern Finland. Med. Vet. Entomol. 24: 411–417. [DOI] [PubMed] [Google Scholar]
- Raizman E A, Holland J D, Keefe L M, and Moro M H. . 2010. Forest and surface water as predictors of Borrelia burgdorferi and its vector Ixodes scapularis (Acari: Ixodidae) in Indiana. J. Med. Entomol. 47: 458–465. [DOI] [PubMed] [Google Scholar]
- Raizman E A, Holland J D, and Shukle J T. . 2013. White-tailed deer (Odocoileus virginianus) as a potential sentinel for human Lyme disease in Indiana. Zoonoses Public Health 60: 227–233. [DOI] [PubMed] [Google Scholar]
- Rand P W, Lubelczyk C, Lavigne G R, Elias S, Holman M S, Lacombe E H, and Smith R P Jr. 2003. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 40: 179–184. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Reunala T, Vuojolahti P, and Hackman W. . 1982. Persistent pruritic papules from deer ked bites. Acta Derm. Venereol. 62: 307–311. [PubMed] [Google Scholar]
- Reunala T, Rantanen T, Vuojolahti P, and Hackman W. . 1980. [Deer ked dermatitis]. Duodecim 96: 897–902. [PubMed] [Google Scholar]
- Rosen M E, Hamer S A, Gerhardt R R, Jones C J, Muller L I, Scott M C, and Hickling G J. . 2013. Borrelia burgdorferi not detected in widespread Ixodes scapularis (Acari: Ixodidae) collected from white-tailed deer in Tennessee. J. Med. Entomol. 49: 1473–1480. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey N P, Fischer M, Gregory C J, Hinckley A F, Mead P S, Paz-Bailey G, Waterman S H, Drexler N A, Kersh G J, . et al. 2018. Vital Signs: trends in reported vectorborne disease cases — United States and territories, 2004–2016. Morb. Mortal. Wkly. Rep. 67: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L J. 1967. The parasites of the whitetail deer (Odocoileus virginianus ochrourus) of British Columbia. M.S. thesis, The University of British Columbia, Vancouver, Canada. [Google Scholar]
- Samuel W M. 1979 Procedures for collecting parasites from white-tailed deer of the Welder Refuge, pp. 260–267. In Proceedings, First Welder Wildlife Foundation Symposium. [Google Scholar]
- Samuel W M, and Trainer D O. . 1972. Lipoptena mazamae Rondani, 1878 (Diptera: Hippoboscidae) on white-tailed deer in southern Texas. J. Med. Entomol. 9: 104–106. [DOI] [PubMed] [Google Scholar]
- Schmidtmann E T, Carroll J F, and Watson D W. . 1998. Attachment-site patterns of adult blacklegged ticks (Acari: Ixodidae) on white-tailed deer and horses. J. Med. Entomol. 35: 59–63. [DOI] [PubMed] [Google Scholar]
- Skvarla M J, and Machtinger E T. . 2019. Deer Keds (Diptera: Hippoboscidae: Lipoptena and Neolipoptena) in the United States and Canada: New State and County Records, Pathogen Records, and an Illustrated Key to Species. J. Med. Entomol. 56: 744–760. [DOI] [PubMed] [Google Scholar]
- Sokół R, and Gałęcki R. . 2017. Prevalence of keds on city dogs in central Poland. Med. Vet. Entomol. 31: 114–116. [DOI] [PubMed] [Google Scholar]
- Solberg V B, Miller J A, Hadfield T, Burge R, Schech J M, and Pound J M. . 2003. Control of Ixodes scapularis (Acari: Ixodidae) with topical self-application of permethrin by white-tailed deer inhabiting NASA, Beltsville, Maryland. J. Vector Ecol. 28: 117–134. [PubMed] [Google Scholar]
- Sonenshine D E. 2006. Tick pheromones and their use in tick control. Annu. Rev. Entomol. 51: 557–580. [DOI] [PubMed] [Google Scholar]
- Spielman A, Clifford C M, Piesman J, and Corwin M D. . 1979. Human babesiosis on Nantucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n. sp. (Acarina: Ixodidae). J. Med. Entomol. 15: 218–234. [DOI] [PubMed] [Google Scholar]
- Teague J L, III, 2018. Assessment of Entomological Risk for Lyme borreliosis along a north-to-south gradient from southern Virginia into North Carolina. M.S. thesis, The University of North Carolina at Greensboro, Greensboro, NC. [Google Scholar]
- Vázquez L, Panadero R, Dacal V, Pato F J, López C, Díaz P, Arias M S, Fernández G, Díez-Baños P, and Morrondo P. . 2011. Tick infestation (Acari: Ixodidae) in roe deer (Capreolus capreolus) from northwestern Spain: population dynamics and risk stratification. Exp. Appl. Acarol 53: 399–409. [DOI] [PubMed] [Google Scholar]
- Watson T G, and Anderson R C. . 1976. Ixodes scapularis Say on white-tailed deer (Odocoileus virginianus) from Long Point, Ontario. J. Wildl. Dis. 12: 66–71. [DOI] [PubMed] [Google Scholar]
- Wedincamp J Jr, and Durden L A. . 2016. Ectoparasites of white-tailed deer (Artiodactyla: Cervidae) in southeastern Georgia, USA. J. Entomol. Sci. 51: 113–121. [Google Scholar]
- Westrom D R, and Anderson J R. . 1992. The distribution and seasonal abundance of deer keds (Diptera: Hippoboscidae) on Columbian black-tailed deer (Odocoileus hemionus columbianus) in northern California. Bull. Soc. Vector Ecol. 17: 57–69. [Google Scholar]
- Westrom D R, Lane R S, and Anderson J R. . 1985. Ixodes pacificus (Acari: Ixodidae): population dynamics and distribution on Columbian black-tailed deer (Odocoileus hemionus columbianus). J. Med. Entomol. 22: 507–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.