Abstract
Developing sampling programs for Culicoides can be challenging due to variation in ecology and behavior of the numerous species as well as their broad distributions and habitats. In this paper, we emphasize the need to clearly define research goals to select appropriate sampling methods. This includes not just the choice of sampling device, but also choice of attractant, site, number of traps per site, the duration and frequency of sampling, and the number of traps per unit area. Animal-baited trapping using enclosure traps and direct animal aspiration is more labor-intensive but yields information on species attracted to specific hosts as well as their biting rates. Sampling immatures is discussed with respect to choosing collection sites in semiaquatic mud, soil, and rich organic habitats. Sorting and extracting larvae using emergence traps, flotation, and Berlese funnels is also discussed.
Keywords: suction and light trapping, carbon dioxide, animal-baited traps, larval sampling
The genus Culicoides (Diptera: Ceratopogonidae) contains over 1,300 species (Borkent 2014) and occurs worldwide except for Antarctica, New Zealand, and other isolated areas (Mellor et al. 2000). Many species are important pests of humans and animals, and have the capacity to transmit a wide range of pathogens (Mellor et al. 2000, Carpenter et al. 2013), including viruses that cause highly invasive diseases such as bluetongue, epizootic hemorrhagic disease, and African horse sickness. Culicoides have a four-stage lifecycle that includes the adult, egg, larva, and pupa. Adults are the most well-studied stage due to their hematophagous habits. Blood feeding is generally required to produce batches of eggs, although some species are autogenous. Females develop eggs in batches following a bloodmeal and oviposit near the larval developmental sites. These sites vary among species and may include mud along shallow shorelines of ponds or a variety of other moist organic habitats (Purse et al. 2015). Larvae pass through four instars, pupate, then emerge as adults to complete the cycle. Slow developing larvae are often thought to be the overwintering stage in temperate areas, though the biology of immature stages has received less attention than that of the adults (Mullens et al. 2015).
A variety of methods are used for sampling Culicoides due to variation in the behavior and ecology of the main species of interest. Species of veterinary importance, such as C. sonorensis Wirth and Jones and C. obsoletus (Meigen), tend to be associated with livestock production systems but have different larval habitats, as do nuisance species that develop in coastal wetland habitats. Choice of sampling method will depend on the target species’ biology, the questions being addressed, as well as practical considerations such as commercial availability and remoteness of the habitat (McDermott and Mullens 2018). No single technique will work best for all species. When designing a Culicoides study or program, the objectives need to be clearly defined before choosing a sampling method and known biases of the sampling methods should be considered. Certainly, surveillance is a key reason for sampling, and these programs typically use a mandated sampling approach. Protocols are available for vector surveillance programs in Europe (Goffredo and Meiswinkel 2004, Medlock et al. 2018). For North America, Ruder et al. (2015) indicated there is a need for developing improved sampling and surveillance strategies for both adult and larval Culicoides. However, sampling is conducted for a variety of reasons other than surveillance. These include determining species composition for biodiversity and ecological studies, detecting the presence of a species in an area for distribution modeling and risk assessment, determining seasonal changes in abundance and age structure to indicate periods of vector activity, and determining diel activity (Walgama and Lysyk 2019). Because of their role as vectors, researchers may be interested in quantifying host-seeking or biting rates for studies of vectorial capacity (Gerry et al. 2001), or collecting virus-infected midges for studies of virus biology and transmission dynamics (Mayo et al. 2012a,b, 2014a,b). Procedures for best examining these different questions can vary.
The purpose of this paper is to guide researchers on choosing and implementing Culicoides sampling methods to achieve their study goals. We tend to focus on North American issues but draw from international studies in the hope that our suggestions will have broader utility. We outline two commonly used adult sampling methods (suction trapping and animal-baited collections) and indicate variations that can be used to answer specific questions. Adult Culicoides can also sampled using vehicle-mounted traps, but these are specialized and will not be discussed here. See Sanders et al. (2012) for a recent example of their use. We also provide some guidelines for immature sampling in various habitats, including extraction methods.
Sampling Adults—Suction Trapping
Suction traps are the staple device for sampling adult Culicoides. These are best used for either trying to capture a range of species, or when a passive approach is required, such as collecting over large areas. Suctions traps come in various designs, but common features include an electric motor that blows air and flying insects away from the trap opening into a collection device (Blackwell 1997). Capture is enhanced by adding attractants such as light or carbon dioxide. These are particularly useful for long-term surveillance or studies of population dynamics. The two most popular traps are the Onderstepoort Veterinary Institute (OVI) trap and the CDC downdraft suction trap with UV light. Both include an attached light source as an attractant, but can be operated without light. Rothamstead suction traps are a specialized trap that have been used to examine Culicoides populations (Fassotte et al. 2008; Sanders et al. 2011, 2019) but will not be discussed here.
The OVI trap is widely used in Europe (Cuéllar et al. 2018) and Africa (Venter et al. 2009b, Diarra et al. 2015), weighs 4 kg, and uses an 8-W UV tube that is 30 cm in length (Venter et al. 2009b). This trap is available in a 12-V model that can be powered by a car battery, or a 220-V model that can be powered from mains electricity or a generator. This prohibits its use in North America where mains electricity is 110–120 V. The CDC downdraft suction trap with UV light is widely used in North America, but also in some European countries. It is light, 0.8 kg, and uses a 4-W, 15-cm UV tube (Venter et al. 2009b, Hope et al. 2015). Several versions of this trap are commercially available and may have different lighting options including incandescent bulbs. Users should ensure that traps purchased include their light of choice. This trap can be powered using 6-V or 12-V batteries depending on the model. Because of its smaller fan and less powerful light, it tends to capture fewer insects and possibly fewer species than the OVI trap under certain situations (Venter et al. 2009b, Probst et al. 2015), but performs almost equally as well under other circumstances (Venter et al. 2009b, Del Río et al. 2013). Capture composition in terms of percentage nulliparous or parous and percentage males are similar. Failure to capture as many insects is perhaps a minor issue; what is most important is that a trap captures vector species when present, and that changes in numbers captured reflect changes in the ambient populations.
Attractants
Prior to setting out traps, researchers should decide what attractants will be used. Ultraviolet light, LED lights, and carbon dioxide are most used, while semiochemicals such as octenol have received relatively little attention for routine sampling (Harrup et al. 2012).
UV light
Traps using UV light-only have been used for a variety of purposes, including collections for biodiversity (Sarvašová et al. 2014), detecting species presence (Schmidtmann et al. 2011, Vigil et al. 2018), and determining seasonal changes in abundance over broad areas (Lysyk and Dergousoff 2014, Meiswinkel et al. 2014, Rádrová et al. 2016). Their popularity stems from their ease of use and portability, as they can be operated directly from batteries. Adult males are attracted to and captured more frequently using UV traps, which is important for species that can only be identified based on male genitalia. The biggest drawbacks to using UV lights alone are that nulliparous flies can be undersampled in some situations, and bluetongue virus (BTV)-infected midges are poorly sampled, perhaps repelled, by UV light (Mayo et al. 2012b, McDermott et al. 2015). Ultraviolet-baited light traps may also under sample species that are more strongly attracted to vertebrate hosts (Carpenter et al. 2008).
LED light
Traps using light-emitting diodes are becoming increasing popular and have lower power requirements and increased portability compared with fluorescent bulbs (Hope et al. 2015). CDC miniature traps or similar designs are commercially available with LED arrays (BioQuip Products, Rancho Dominguez, CA; John W. Hock, Gainesville, FL), as are LED arrays with wavelengths ranging from UV (390 nm) to red (660 nm). Culicoides generally seem not to be attracted to red LEDs. Some species are attracted to a range of wavelengths, while others seem to be preferentially attracted to blue and green wavelengths (Hope et al. 2015). Green LEDs have been adopted for monitoring C. brevitarsis Kieffer in Australia as part of the National Arbovirus Monitoring Program (Bishop et al. 2006). Ultraviolet/LED traps have proven satisfactory for Culicoides biodiversity studies in Florida (Sloyer et al. 2019). They do, however, tend to collect fewer numbers than traps baited with UV fluorescent tubes (Hope et al. 2015, González et al. 2016).
Carbon dioxide
Use of carbon dioxide as an attractant for Culicoides dates to the initial report of Nelson (1965). It has been used as a sole attractant with CDC downdraft traps during intensive studies of BTV transmission in California (Gerry and Mullens 2000; Gerry et al. 2001; Mayo et al. 2012a,b, 2014a,b). Captures in traps baited solely with carbon dioxide reflect biting rates of C. sonorensis (Mullens and Gerry 1998), and have been used to estimate host-biting rates in field studies (Gerry et al. 2001). Carbon dioxide-baited traps provide more reliable estimates of midge infection by BTV (Mayo et al. 2012b) and will detect infected midges several weeks before UV-baited traps (McDermott et al. 2015).
Traps are typically baited with carbon dioxide using one of two methods. The first is to fill an insulated container with dry ice, allowing the carbon dioxide to sublimate and be released from holes in the side and bottom of the container (Mullens 1995, Gerry and Mullens 1998). Release rates from a 1.2- to 1.4-kg piece of dry ice decline over time, ranging from ca. 1,500 ml/min during the first hour to ca. 300 ml/min thereafter. The second method is to use regulated flow from a carbon dioxide cylinder through a two-stage regulator (Mullens 1995, Gerry and Mullens 2000) with the release rate measured using a calibrated glass flowmeter. Release rates of 300 and 1,000 ml/min approximate the output from a calf and full-grown heifer, respectively (Mullens 1995). Carbon dioxide output should be positioned at approximately the level of the trap entrance, so that attracted insects will quickly be drawn into the collection container by the fan. Researchers may need to operate suction traps without rain guards when using dry ice in order to properly position the containers to maximize trap efficiency.
Alternate methods of carbon dioxide generation can be considered in areas where it is difficult to obtain regular supplies of dry ice or compressed gas. These include generating carbon dioxide from yeast fermentation (Saitoh et al. 2004), chemical reactions (Burkett-Cadena et al. 2015), electrochemical reactions, or combustion (Benante et al. 2019). These methods have been evaluated for several families of biting flies, but information on their utility for sampling Culicoides is limited.
Octenol
Octenol (1-octen-3-ol) can be released from traps using commercially available sachets, or by release from glass vials. The liquid is placed into a vial that has a hole drilled in the lid and a exposed cotton or pipe cleaner wick running from the liquid inside the vial to the outside of the lid (Harrup et al. 2012). The exposed length of the wick determines the release rate, with longer wicks generating higher release rates. A 9-mm wick will release ~4–5 mg/h of octenol, which is sufficient to attract many Culicoides species (Ritchie et al. 1994, Harrup et al. 2012). The (R)-enantiomer enhances capture of host-seeking Culicoides and species composition of captures compared well with animal-baited traps (Harrup et al. 2012). Octenol may also be used in conjunction with carbon dioxide to increase capture (Ritchie et al. 1994) although this effect is not uniform across all species (Kline et al. 1994, Venter et al. 2011). Mixtures of octenol and phenols can also enhance capture of some species (Cilek and Kline 2002). In fact, a variety of host kairomones can enhance Culicoides capture, but further work is required before these can be used to make trap captures more reflective of the host-seeking population (Isberg et al. 2017).
Site Selection
Trap location can influence the number of midges captured, even more so than choice of attractant (McDermott et al. 2016). Suitable sites are numerous on farms, and traps usually are placed in relatively sheltered areas, away from light pollution if possible, or near animals or immature developmental sites. In more open, rangeland areas, traps can be placed near livestock watering sites, ponds, and natural water sources (Schmidtmann et al. 2011).
Data are somewhat conflicting concerning the effects of proximity to hosts and developmental sites on numbers captured using traps. Traps positioned near developmental sites may have greater captures than more distant traps (Mullens 1985, Gerry and Mullens 2000), while in other studies this is inconsistent (Lysyk 2007, Mayo et al. 2014b, McDermott et al. 2016). Host proximity can also influence numbers captured, either positively (Garcia-Saenz et al. 2011, Kirkeby et al. 2013a) or negatively (Gerry and Mullens 2000, McDermott et al. 2016). Fortunately, however, numbers captured within farms or traps in close proximity are often correlated (Gerry and Mullens 2000, Lysyk 2007), suggesting they measure similar changes in abundance over time. Therefore, traps should be deployed at consistent locations if temporal trends in abundance are being recorded. Kirkeby et al. (2013a) recommended that traps be placed a standard distance from hosts if sampling is done over multiple farms as abundance increases closer to animal pens. Traps should be placed out of the reach of animals, or, if in open areas, surrounded by temporary fencing. We have used three metal T-stakes placed in a triangular pattern around the traps, with three strands of barbed wire or poultry wire wrapped around the stakes. Trap position also influences composition of the catch. Traps positioned closer to livestock may have lower parity rates as these populations may be host-seeking and dispersing away from developmental sites, while traps near developmental sites may have greater parity rates due to the presence of females that recently oviposited (Mullens 1985, Gerry et al. 2001, Lysyk 2007).
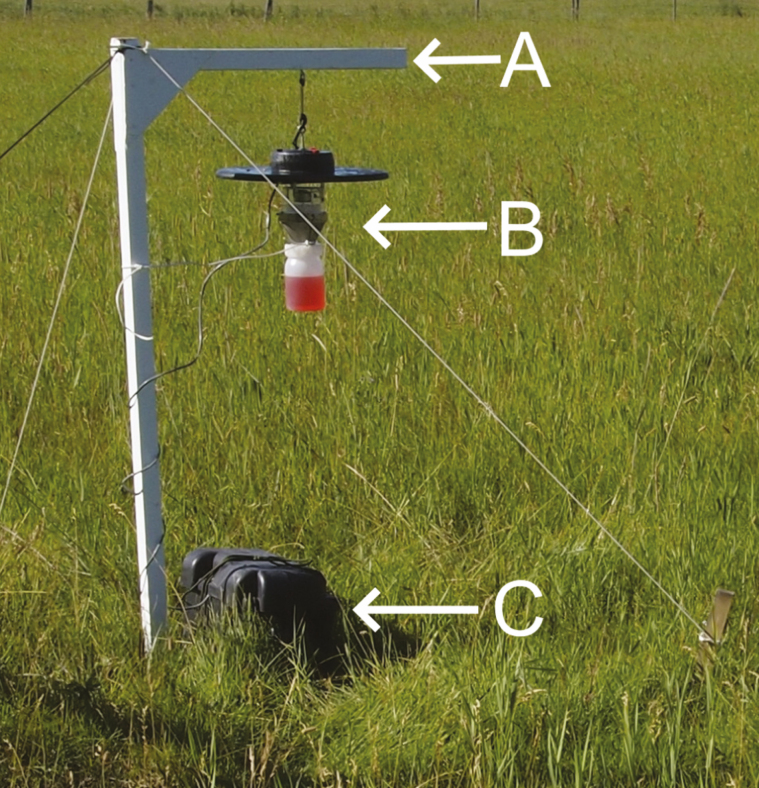
Culicoides capture can vary with trap height (Venter et al. 2009a); therefore, it is important to standardize traps to a uniform height. In most studies, traps are usually suspended with the opening from 0.7 to 2 m aboveground. For livestock feeding species, this will approximate the height of the host animal. Traps can either be suspended from existing structures or trees, or in open areas from Г-shaped brackets made from 38 × 38 mm dimensioned lumber (Fig. 1A). The vertical post is ca. 1.5 m tall and the horizontal bracket is ca. 0.6 m long. The horizontal bracket is joined to the post 2 cm from the top of the post and fastened with triangular section of plywood 0.2 m on the top and bottom. The offset from the top of the post is to allow use of a maul or sledgehammer for driving the bracket 0.3 to 0.4 m into the ground. The trap is hung from hooks driven into the underside of the bracket. Alternatively, commercial garden hooks can be used. Sampling avian species may require positioning traps at greater heights either using towers (Henry and Adkins 1975) or ropes and pulleys suspended from tree limbs (Swanson and Adler 2010, McGregor et al. 2018). These workers used various projectiles to position the ropes and pulleys in the tree canopy. Preliminary studies may be necessary to determine optimal height.
Fig. 1.
Field setup for CDC UV suction trap. A = bracket; B = trap with killing jar assembly; C = battery box. Power cables are wrapped around the support post to prevent movement. Photo supplied by Dr. S. J. Dergousoff, Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada.
Number of Traps per Site
The number of traps per site and optimal sampling strategies have received very little attention, apart from the work of Kirkeby et al. (2013a,b). Intensive studies on transmission dynamics are usually conducted at relatively few sites (1–4 sites per study) and have used from 5 to 28 traps per site (Gerry and Mullens 2000, Mayo et al. 2014a). More extensive surveys will involve a greater number of sites and travel over broader areas, with fewer traps, usually 1–3, per site (Lysyk 2006, 2007; Schmidtmann et al. 2011; Lysyk and Dergousoff 2014; Cuéllar et al. 2018). A single trap per farm will not produce a reliable estimate of abundance for that farm due to spatial heterogeneity; however, it may provide an indication of species diversity or presence of a species at the site. Schmidtmann et al. (2011) sampled 68 cattle operations for two 2-d periods in three states in 2001. Traps were operated at two locations on each of 44 operations. Disagreement between the two locations per site occurred on only 4/44 (9.1%) of these operations, suggesting a single trap per location sampled twice a year is sufficient to detect species presence. Repeated sampling at the same location throughout the year can provide an indication of seasonal changes in abundance. Relationships between environmental factors and mean abundance can be modeled if many farms are sampled using a single trap per farm and predictions are restricted to mean trap captures under a combination of environmental variables (Kirkeby et al. 2013a).
Duration and Frequency of Trapping
Duration of trapping refers to how long a trap is continuously operated. Operating traps for multiple nights increases the probability of detecting a species relative to single overnight captures (Kirkeby et al. 2013c, Walgama and Lysyk 2019) and smoothes fluctuations in capture that may arise from daily variation in environmental factors. The length of time a trap can be operated is limited by the supply of the attractant, either the amount of carbon dioxide available or available electricity to run the fan and lights. A full cylinder of carbon dioxide can last several trapping nights depending on the flow rate while about 1 kg of dry ice is required for 12-h operation (Venter et al. 2016). Because the carbon dioxide source is finite, use of carbon dioxide-baited traps is usually restricted to overnight sampling at readily accessible locations. Duration of light trap operation commonly ranges from 1 to 7 nights (Cuéllar et al. 2018) and depends on the power consumption of the trap and the length of time it is operated. Power consumption is given by amperes/hour and ranges from 0.86 A/h for a UV CDC trap to 0.35 A/h for a CDC LED trap. These can be powered for 1 and 3 nights, respectively, using a 10 ampere-hour battery (Hope et al. 2015). A nonrechargeable 6-V dry-cell battery can power a CDC miniature trap for several trap nights if the trap is operated between dusk and dawn using a photoswitch (Schmidtmann et al. 2011). Deep-cycle batteries rated from 45 to 105 ampere-hours can be used to power traps for up to a week, and can also be charged in the field using solar panels connected to the battery. A charge controller can be installed to regulate panel output to 12 V and prevent overcharging. Positive and negative leads from the battery are connected to the positive and negative leads on the trap. Batteries should be housed in plastic weather-proof boxes staked to the ground near the trap for protection from the elements (Fig. 1C).
Frequency of trapping refers to the time between samples and depends on the objective of the study. Studies on seasonal abundance may require trapping twice weekly, weekly, or biweekly (Gerry and Mullens 2000; Lysyk 2007; Mayo et al. 2012a,b, 2014a,b). Weekly samples may be preferred over biweekly samples in cooler climates with severe winters. If traps are operated continuously and emptied weekly, the batteries should be replaced with fresh ones and old batteries charged in the laboratory. In warmer climates with mild winters, sampling can be conducted less frequently during periods of low vector abundance. Hourly samples or interval samples within a night can be used to approximate diel activity.
Number of Traps per Unit Area
The number of sampling sites for area wide surveys has ranged widely, from 1 trap per 400 km2 (20 × 20 km grids; Meiswinkel et al. 2008) to 1 site per 8,576 km2 (Schmidtmann et al. 2011). Choice of sample trap density will depend on the size and heterogeneity of the area to be covered and the level of resources available. For studies testing the effect of different attractants or trap types on Culicoides collections, traps should be placed a sufficient distance apart from each other to limit the influence of treatments on each other. The physical limitations of the size and layout of the study location may limit where traps can be placed, but previous studies have utilized distances of 20–50 m between traps (Viennet et al. 2011, McDermott et al. 2016). The attraction range of OVI traps and CDC traps are estimated at 29.6 (95% CI = 26.3–31.9) m and 15.3 (95% CI = 12.7–18.3) m, respectively (Rigot and Gilbert 2012, Kirkeby et al. 2013b).
Insect Collection
Suction traps usually come equipped with fine mesh cloth tubes with a terminal container for capturing insects. The cloth tube will blow about in windy areas and can be replaced with a wire mesh killing jar assembly that is more firmly attached to the trap chassis (Fig. 1B). The collection container may have a 40 by 40 mesh screen on the bottom allow air to pass through without losing insects. A vial of 10% sucrose can be supplied if collection of live insects is desired. The collection container can be replaced with a solid container containing liquid to trap and preserve the insects if dead insects are required. Collection liquids can consist of water with an added detergent (Venter et al. 2009b), ethanol (Sloyer et al. 2019), or propylene glycol (Lysyk et al. 2006, 2007). The water and detergent combination will work well if traps are serviced daily and collections transferred to ethanol. Use of ethanol requires that traps also be serviced regularly as it will evaporate. Propylene glycol is used as a nontoxic, nonevaporating alternative to ethanol when traps are deployed for longer periods than overnight. Insects are returned to the laboratory, filtered from the propylene glycol, and stored in ethanol.
Sampling Adults—Animal-Baited Trapping
Animal-baited trapping is conducted to determine which Culicoides species are attracted to a particular host species, their diel feeding patterns, and attack or biting rates as these may not be reflected in suction trap samples (Carpenter et al. 2008, Gerry et al. 2009, Cohnstaedt et al. 2012, Meiswinkel and Elbers 2016). Host feeding patterns can be inferred from molecular identification of blood meals from engorged specimens captured in suction traps; however, these usually constitute a very small percentage of the insects captured (Garros et al. 2011) and require a large sampling effort. Animal-baited trapping directly measures the insects feeding on a particular host.
Two general animal-baited collection methods are used: enclosure traps or direct aspiration. Gerry et al. (2009) provide a summary of studies that have used both methods. Host animals are required for both methods, and the host species chosen should be relevant to the questions asked. Commonly used hosts include cattle, sheep, horses, goats, and deer (see summary in Gerry et al. 2009). Animals should be tamed and of uniform size, color, and sex, and provided with food and water throughout the study. Approved animal use protocols must be obtained. Personnel working near the animals should be trained in animal handling and wear appropriate safety clothing including steel-toed footwear.
Animal Enclosure Traps
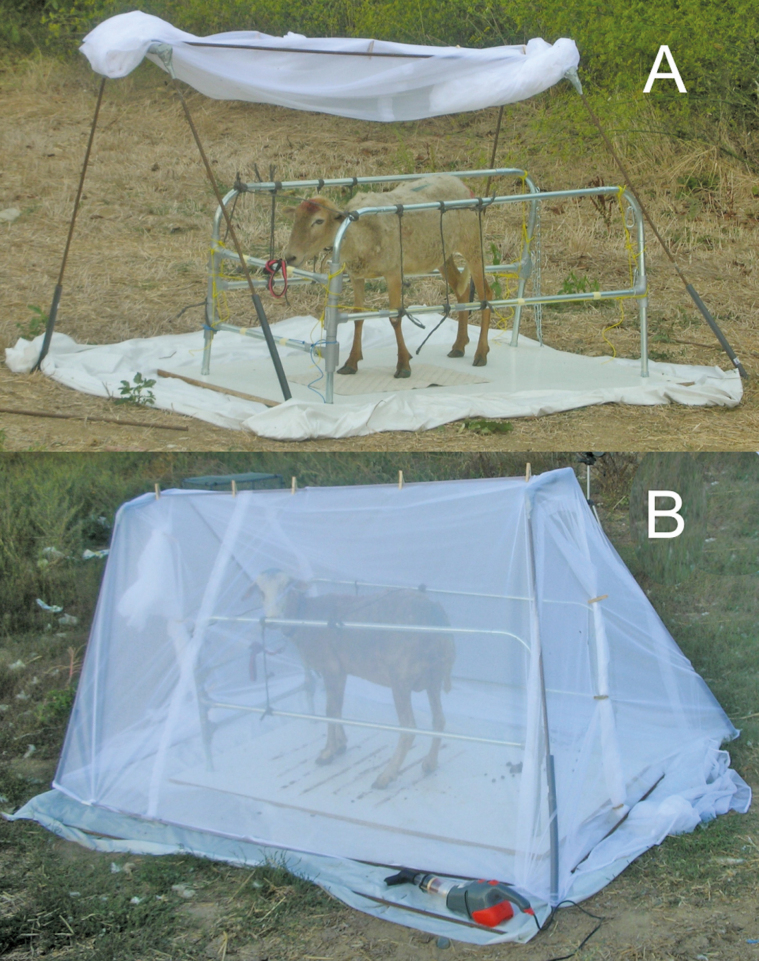
Animal enclosure traps are wooden or metal cuboidal frames that are covered with a fine mesh fabric net (Shemanchuk 1978, Mullens and Gerry 1998, Carpenter et al. 2008). Animals are led into the trap and held in either an internal corral or stanchion, or are tethered to stakes in the ground (Fig. 2A). The sides of the trap are raised for a set period, usually 10 min, so that the animals are exposed to biting flies. During this period, researchers should wait 40–100 m downwind of the trap to avoid interfering with Culicoides attraction. After the exposure period, the researchers can quickly approach the trap and lower the netting (Fig. 2B). The flies can feed to repletion for an additional 10 min, after which the researchers enter the trap and collect insects from the sides of the trap and animals using vacuum aspirators.
Fig. 2.
Field setup for animal-baited trap in the (A) open and (B) closed position. Photos by Dr. A. C. Gerry, Department of Entomology, University of California, Riverside.
Direct Animal Aspiration
Direct animal aspiration is a similar approach and avoids the need for a trap. This method has been used with a variety of host animals. Excellent examples of the use of direct animal aspiration are Schmidtmann et al. (1980), Gerry et al. (2009), Elbers and Meiswinkel (2014, 2015), Meiswinkel and Elbers (2016), and Elbers et al. (2019). The host animals are transported to a site and tethered to stakes in the ground separated by anywhere from 6 m (Schmidtmann et al. 1980) to 65 m (Elbers and Meiswinkel 2015). Aspiration during a specified period of time is used to collect Culicoides that contact the host. Researchers can use either mechanical or mouth aspirators. Mechanical vacuum aspirators are commercially available. The Prokopack aspirator is quite suitable, lightweight, and powered by batteries (Vazquez-Prokopec et al. 2009). Mouth aspirators can also be used for targeted collections (Elbers et al. 2019) but should be fitted with a HEPA filter to avoid inhaling animal dander.
The areas of the host sampled can be initially brushed to remove feeding midges, then the aspirator moved over the animal in a systematic fashion for a fixed period to determine attack per unit time. Gerry et al. (2009) vacuumed animals along the dorsum from head to tail, moving progressively toward the belly, completing first one side them the other. Collections can be separated by body region (Schmidtmann et al. 1980, Elbers et al. 2019). Five-minute collections can be made, the animals rested for 15 min, then the process repeated. Collected insects are placed in alcohol vials and later identified to species and blood-fed status. Attack rate is defined as the total number of female insects collected per unit time while biting rate defined as the number of blood-fed insects collected per unit time (Elbers and Meiswinkel 2015).
Sampling Considerations
Multiple host animals should be available and rotated or assigned randomly to the collection periods. Host animals should be also sampled throughout the insect’s daily activity periods. This may require initial studies for up to 24 h if the insect has both morning and evening activity periods or is a daytime flying species. However, sampling about 5 h before sunset up to 2–3 h after sunset should suffice for most species (Elbers and Meiswinkel 2014). Researchers should consider that diel activity patterns of typically crepuscular species may shift earlier to the late afternoon during the fall and winter (Lillie et al. 1987), a consideration that applies to suction trap collections as well as animal sampling. Headlamps fitted with red lights can be used to improve visibility during darkness as red is not generally attractive to Culicoides (Hope et al. 2015). Sampling should also be conducted throughout the season as species composition may change over time.
Sampling Larvae
Although the majority of Culicoides field sampling is based on collecting adults, the immature stages can also be collected from development substrate for studies on habitat use, resource partitioning, larval behavior, physiology, and pathology, among others. Compared to trapping for adult Culicoides, sampling for eggs, larvae, and pupae can be more labor-intensive, and can require a more in-depth knowledge of species ecology. Researchers know what types of microhabitats in which their target species are likely to be found, and then sort through substrate samples to remove individual larvae. For many species, habitat preferences and larval development sites are not well characterized, adding to the difficulty of collecting sufficient numbers of larvae for studies. Here, we discuss identifying and sampling larval Culicoides habitat, and outline the three most used methods for sorting immatures from field-collected substrate samples.
Choosing a Collection Site
Culicoides spp. utilize a wide variety of habitats for oviposition and larval development. The species that are of the highest concern for veterinary health are typically associated with livestock production, and can be found in intact dung pats (e.g., C. brevitarsis), composted manure or soil (e.g., C. obsoletus), or organically enriched semiaquatic habitats, like wastewater ponds (e.g., C. sonorensis) (Purse et al. 2015). Species associated with wildlife, including captive Cervid populations, may also be found in naturally occurring, organically enriched standing water, like puddles (e.g., C. stellifer Coquillett), or from stream or pond margins (e.g., C. haematopotus (Malloch)) (Erram et al. 2019). Many anthropophilic nuisance pests are associated with coastal habitats, including mangrove swamps (e.g., C. furens Poey), salt marshes (e.g., C. hollensis Melander and Brues), and sandy intertidal zones (e.g., C. melleus Coquillett) (Blanton and Wirth 1979). Beyond these examples, essentially any moist, organic substrate can support Culicoides development, including tree holes, rotting vegetation, and soil (Jones 1961, Blanton and Wirth 1979). The broad range of substrates from which Culicoides immatures can be collected has necessitated the development of several sampling methods depending on the target species and habitat. Once the target habitat has been identified, researchers must choose how to subsample that habitat, as larvae are unlikely to be evenly distributed throughout the area. The goals of the study should inform how many samples are taken, and how far apart these samples should be. When the purpose of collecting eggs, larvae, or pupae is for later use in laboratory assays, samples need not necessarily be taken methodically, but it is best to target high-density areas to ensure that enough individuals are collected.
Semiaquatic mud habitats (e.g., puddles, wastewater ponds, stream margins)
The majority of what is known about Culicoides in semiaquatic mud habitats comes from work on C. sonorensis. Culicoides larvae in standing or still water can be found in the mud at the waterline (Mullens 1989, González et al. 2013, Pfannenstiel and Ruder 2015), and eggs and pupae will be found just above waterline (Wong et al. 2017). When choosing where to sample from in the habitat, researchers may find performing a field check of the substrate before making collections useful. A small amount of mud can be washed into a shallow tray or container to observe larvae (Fig. 3). Samples should be collected from banks with a gentle slope, fine substrate, and little to no vegetation, as steep banks, course substrate, and heavy vegetation are unlikely to support larval development (Mullens 1989, Pfannenstiel and Ruder 2015). Areas in the direct sun are more likely to have higher C. sonorensis larval density (Mullens and Rodriguez 1985), though other species may exhibit a negative phototaxis and be found in higher densities in shaded areas (Hrirbar 1990). It is only necessary to collect the top 2–3 cm of substrate, as larvae do not typically burrow deeply (Mullens and Rodriguez 1992). Researchers wishing to collect eggs should remove mud no more than 5 cm above the waterline, as this is where the majority of C. sonorensis eggs are laid (Wong et al. 2017). Field studies on the oviposition behavior of other Culicoides spp. in these types of habitats are lacking, but egg positioning is likely to be similar to C. sonorensis, as larvae cannot move freely in the drier sand above waterline. A gardening trowel can be used to remove larger sections of substrate, and a Scoopula style spatula can be used for more precise sample removal (Wong et al. 2017). Samples can be transported back to the laboratory in waterproof, sealed containers, like plastic freezer bags, on ice.
Fig. 3.
Sampling larval Culicoides at the edge of a wastewater lagoon in southern California.
Tree-hole breeding Culicoides spp., such as C. debilipalpis Lutz, can be sampled by collecting both standing water from the habitat, as well as any detritus in the base of the tree hole. Little information is available on whether Culicoides spp. show a preference for particular tree species, but they may be fairly generalist in this regard. For example, C. debilipalpis has been collected in Salix spp. willows (Ronderos et al. 2010) and bamboo stumps, as well as other nontree hole habitats (Williams 1964). What may be more important is whether a tree hole contains standing water or not. Certain species, including C. paraensis (Goeldi) (a vector of Oropouche virus), are more commonly found in dry (detritus but no standing water) tree holes, while others, like C. guttipennis (Coquillett), may be more common in wet tree holes (Kruger et al. 1990). Water can be collected using disposable transfer pipettes or turkey basters (Yanoviak and Fincke 2005, Ronderos et al. 2010), depending on the volume of liquid to be collected, and samples can be stored in any number of leak-proof containers, including Whirl-Pak bags (Nasco, Fort Atkinson, WI) and screw top vials. Glass jars (e.g., Mason jars) can be used for larger samples. Detritus can be manually removed using forceps if there is a large amount of leaf litter material (Yanoviak and Fincke 2005) or the insides of the tree hole can be scraped to remove residual material (Brickle et al. 2008). Researchers should always visually inspect tree holes for animals and biting/stinging arthropods before reaching inside, and the removal of detritus with hands is not recommended (Yanoviak and Fincke 2005).
Soil habitats (e.g., marsh, swamp, peat)
Culicoides spp. inhabiting soil habitats may be more difficult to target, as development sites are not marked by distinct landscape features, like ponds. Some species-specific studies have been conducted on Culicoides microhabitat markers which may guide researchers in selecting sampling sites. For instance, C. impunctatus Goetghebeur larval density is positively correlated with the presence of Juncus spp. rushes, Sphagnum spp. mosses, and soil wetness (Blackwell et al. 1994, 1999), and negatively correlated with Pteridium aquilinum ferns and non-Sphagnum moss (Blackwell et al. 1999). However, the dearth of information on the ecology of most Culicoides spp. means that it may be difficult to specifically target larval development sites beyond sampling in a general habitat type. A variety of soil core sampling tools and augers are available and can be used to collect uniform samples to the desired depth. Fencepost hole diggers have also been used to collect larger volumes of soil (Kline et al. 1981). As with the semiaquatic species, soil-dwelling Culicoides larvae are also typically not found at depths greater than ~3 cm (Blackwell and King 1997, Uslu and Dik 2006). Egg placement in terrestrial soil habitats is unknown for most species. Laboratory assays have shown that C. impunctatus preferentially oviposit on live Sphagnum moss (Carpenter et al. 2001), and so researchers looking to collect C. impunctatus eggs from the field should consider collecting moss, rather than soil, samples to examine for eggs. It may be beneficial to collect plant material adjacent to soil samples when surveying for immature stages of other soil-dwelling Culicoides to identify oviposition sites.
Rich organic habitats (e.g., manure, composted organic material)
Culicoides inhabiting rich organic habitats, like manure, may be targeted more easily by the defined nature of these resources. Much of the work on manure breeding Culicoides spp. has focused on C. brevitarsis, which primarily develops in cattle dung. Culicoides brevitarsis does not oviposit on very fresh (≤1 d old) dung pats, so researchers should target collections to 2- to 4-d-old pats (Bishop et al. 1996) to increase the odds of collecting larvae. Oviposition site selection by gravid females appears to rely at least partially on visual cues. Dung pats that are distinct from the surrounding area, and those that are intact, with rounded sides are more likely to contain larvae (Campbell and Kettle 1976). Eggs are most likely to be laid in the center of the pat and are deposited on the outer surface (Campbell and Kettle 1976), though larvae may be evenly distributed throughout the pat (Bishop et al. 1996). To standardize subsamples of dung for collecting Culicoides, core samples can be taken from each pat using a length of PVC pipe, split in two and hinged on one side, as described by Bishop et al. (1996). Other Culicoides spp., like C. obsoletus and C. imicola Kieffer, prefer to oviposit in composted manure, like dung heaps, rather than intact pats. Culicoides obsoletus complex larvae have been found in the highest densities in the outer layer of dung heaps, closer to the ground, though the effect of height may be an artifact of fresh dung being placed on top of the pile (Lühken et al. 2014). Dung heap samples can be taken in the same manner as dung pat cores.
Sorting for Larvae
Emergence traps
For studies where the goal is to assess various habitats for larval development suitability, emergence traps are likely to be the most efficient means of sorting midges from the substrate. Rather than removing the larvae from the samples, a measure of larval density can be assessed by counting and identifying the emerged adults. Various designs for emergence traps have been used to collect adult Culicoides from field-collected substrate. At their simplest, substrate samples can be placed directly into clear containers with fine mesh or organza cloth lids and monitored daily for emergence. An access port covered by a plug or tape door allows for emerged midges to be aspirated out. Erram et al. (2019) used a variation on this method, placing substrate samples into 50 ml Petri dishes with Tanglefoot (The Tanglefoot Co., Grand Rapids, MI) covered lids to trap emerged adults. Funnel-based emergence containers can also be used, where an inverted funnel is placed over the top of an opaque substrate container, and a collection jar or vial is affixed to the neck of the funnel, such that light is only visible through the funnel. Emerged insects then move up toward the light, through the funnel and into the jar for easy collection (Kline et al. 1975). Adult Culicoides can be stored in ethanol for identification. A potential disadvantage of this method is that a large amount of laboratory space needs to be dedicated for an extended period, as depending on the species, it may take several months for adults to emerge (Kline et al. 1975). Additionally, because larval mortality may occur during the emergence period, this is not an exhaustive extraction method. Significantly fewer individuals may be collected when using emergence traps versus active larval extraction methods (Steinke et al. 2014).
Flotation
For studies that require larvae, rather than emerged adults, immature Culicoides must obviously be separated from the field-collected substrate before they emerge. One of the most commonly used techniques for separating out Culicoides larvae is larval flotation. This technique involves mixing aliquots of the substrate with a solution that creates a surface layer with a higher specific gravity than the larvae, forcing them to the top of the solution, where they can be removed with a transfer pipette. Several readily available compounds have been used for Culicoides flotation, including saturated sugar, sodium chloride (uniodized table salt; NaCl), and magnesium sulfate (Epsom salt; MgSO4) solutions (Hribar 1990), and the grocery store versions of these compounds are just as effective as reagent grade materials. Sodium chloride and MgSO4 have been shown to have similar efficacy for extracting C. sonorensis larvae, though early instars may not survive well after exposure to saturated NaCl solutions (Mullens and Rodriguez 1984). Culicoides pupae will float to the surface when submerged to avoid drowning, and Culicoides sonorensis eggs have also been successfully removed from mud samples using MgSO4 flotation (Wong et al. 2017), so this method can be used to collect all three immature stages. Polyacrylamide flocculants can be added to the solution to speed substrate settling (Byrd et al. 1966). Mullens and Rodriguez (1984) found that only a few drops (~50 μl) of 0.5% Separan NP10 (no longer on the market; Dow Chemical Company, Midland, MI) was sufficient to speed settling in 70 ml of solution. One disadvantage of this technique is that it may be difficult to process an entire mud sample at once. A portion of the sample should be placed into a jar or beaker, and sufficient flotation solution to cover the sample should be added. The contents of the jar are then stirred, and the surface of the solution is observed for larvae. Samples often need to be re-stirred several times to extract all of the larvae, and processing multiple samples by this method may take considerable time.
Larval flotation works best for substrate samples with low vegetation content that can be well homogenized by mixing (Kline et al. 1975), such as mud collected from pond edges or intertidal zones. However, flotation can be used in combination with sand extraction techniques to remove larvae from other more complex soil substrates. Sand extraction is particularly useful for collecting larvae of species such as C. furens from marsh samples. The soil sample is placed into a container and covered with clean sand, which is then inundated with tap water, and left for at least 48 h (Kline et al. 1975), during which time larvae will move from the original substrate into the sand layer. The sand can then be removed and used for larval flotation as described above. Alternatively, the sand layer can be washed through a series of sieves to remove larvae (Bidlingmayer 1957). Sand can be commercially purchased from home improvement stores and warehouses for extractions.
Berlese funnel
Although less commonly employed, Berlese funnel extraction can also be used to separate Culicoides larvae from substrate. Steinke et al. (2014) used a modified Berlese device, without the bottom funnel, to collect C. chiopterus (Meigen) and C. dewulfi (Goetghebuer) larvae from cow dung samples. Homogenized dung was spread into a ~1 cm thick layer onto a coarse mesh screen, and larvae were collected into a dish of water below. The majority of larvae can be recovered from a sample within 2 d. This method was found to be as effective as sugar flotation (Steinke et al. 2014). Attempts to process larger samples (layers >1 cm) in Berlese funnels may not be as successful (Kline et al. 1975), and so researchers may need numerous devices to process multiple samples. Similar to emergence trap methods, Berlese funnel extraction requires significant amounts of dedicated laboratory space; however, it may be a useful method for collecting larvae from heterogeneous substrates such as composted manure or soil samples with high vegetation content (Blackwell et al. 1999).
Conclusions
Culicoides biting midges are a group of ecologically and behaviorally diverse biting flies. Their role as nuisance pests and pathogen vectors drives the majority of collection efforts, whether they are aimed at population monitoring, pathogen detection, or intervention assessment. However, differences in host preference, diel activity, and habitat necessitate a variety of tools and strategies for sampling adult and larval Culicoides populations. The techniques discussed here are the most commonly used in the field, though many creative modifications on these methods developed by researchers to fit their specific needs can be found in the literature. Each Culicoides collection method has advantages and limitations that should be considered by researchers when deciding on a study design.
No one technique will be appropriate in all circumstances, though some methods will be better suited to a given study than others. Light-baited suction traps are efficient and low cost, but may underrepresent host-seeking or virus-infected midges. They can provide reasonable measures of abundance and species composition at a site if operated for several consecutive days or longer, provided they are adequately powered. Continuous operation throughout the season provides useful measures of seasonal abundance that can be related to changes in environmental variables. They have also been particularly useful for extensive studies aimed at determining species’ distributions. Carbon dioxide-baited traps are necessary for sampling host-seeking or virus-infected midges but have a short period of operation and are impractical in remote or tropical locations. They can be used to measure seasonal changes in abundance at specific locations and have even been calibrated with animal-baited trap data to estimate C. sonorensis biting rates (Mullens and Gerry 1998). It would be most useful to see if this can be expanded to a wider range of species, possibly incorporating semiochemicals such as octenol into the practice. Animal-baited aspirations provide the most accurate picture of the species attracted to a given host as well as biting rates, particularly useful when attempting to incriminate vector species or estimate vectorial capacity. These are logistically challenging, expensive, and require significant safety considerations for both collector and animal. For larvae, emergence traps are a passive means to associate development sites with species, but cannot be used when immatures are needed for experiments. Flotation can be used to collect individual eggs, larvae, and pupae from samples, but is labor-intensive. For each of these techniques, researchers must also choose when and where collections will be made, both of which will affect the size and species, parity, and sex composition of collections. In all cases, the goals of the study should be considered first and foremost before sampling begins to ensure that the methods used will be appropriate to answer the question at hand.
Acknowledgments
The authors are grateful to Dr. S. J. Dergousoff and Ms. C. Himsl-Rayner, Lethbridge Research and Development Centre, Agriculture and Agri-Food Canada for advice and consultation, and for supplying photographs used in Fig. 1. Dr. Alec Gerry, University of California Riverside, supplied photographs used in Fig. 2. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
References Cited
- Benante J P, Fox J, Lawrence K, Fansiri T, Pongsiri A, Ponlawat A, and Chaskopoulou A. . 2019. A comparative study of mosquito and sand fly (Diptera: Psychodidae: Phlebotominae) sampling using dry ice and chemically generated carbon dioxide from three different prototype CO2 generators. J. Econ. Entomol. 112: 494–498. [DOI] [PubMed] [Google Scholar]
- Bidlingmayer W L. 1957. Studies on Culicoides furens (Poey) at Vero Beach. Mosq. News. 17: 292–294. [Google Scholar]
- Bishop A L, McKenzie H J, Barchia I M, Murison R, and Spohr L J. . 1996. Positions of juvenile stages of Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) and of four other flies in bovine dung. Austral. J. Entomol. 35: 209–212. [Google Scholar]
- Bishop A L, Bellis G A, McKenzie H J, Spohr L J, Worrall R J, Harris A M, and Melville L. . 2006. Light trapping of biting midges Culicoides spp. (Diptera: Ceratopogonidae) with green light-emitting diodes. Aust. J. Entomol. 45: 202–205. [Google Scholar]
- Blackwell A. 1997. Diel flight periodicity of the biting midge Culicoides impunctatus and the effects of meteorological conditions. Med. Vet. Entomol. 11: 361–367. [DOI] [PubMed] [Google Scholar]
- Blackwell A, and King F C. . 1997. The vertical distribution of Culicoides impunctatus larvae. Med. Vet. Entomol. 11: 45–48. [DOI] [PubMed] [Google Scholar]
- Blackwell A, Young M R, and Mordue W. . 1994. The microhabitat of Culicoides impunctatus (Diptera: Ceratopogonidae) larvae in Scotland. Bull. Entomol. Res. 84: 295–301. [Google Scholar]
- Blackwell A, Lock K A, Marshall B, Boag B, and Gordon S C. . 1999. The spatial distribution of larvae of Culicoides impunctatus biting midges. Med. Vet. Entomol. 13: 362–371. [DOI] [PubMed] [Google Scholar]
- Blanton F S, and Wirth W W. . 1979. Arthropods of Florida and neighboring land areas, vol. 10: the sand flies (Culicoides) of Florida (Diptera: Ceratopogonidae). Florida Department of Agriculture and Consumer Services, Gainesville, FL. [Google Scholar]
- Borkent A. 2014. The pupae of the biting midges of the world (Diptera: Ceratopogonidae), with a generic key and analysis of the phylogenetic relationships between genera. Zootaxa. 3879: 1–327. [DOI] [PubMed] [Google Scholar]
- Brickle D S, McKeever S, and Hagan D V. . 2008. Bionomics of bloodsucking midges (Diptera: Ceratopogonidae: Culicoides) on the coastal plain of Georgia, USA. Russ. Entomol. J. 17: 41–61. [Google Scholar]
- Burkett-Cadena N D, Blosser E M, Young R M, Toé L D, and Unnasch T R. . 2015. Carbon dioxide generated from carbonates and acids for sampling blood-feeding arthropods. Acta Trop. 149: 254–261. [DOI] [PubMed] [Google Scholar]
- Byrd D W, Nusbaum C J, and Barker K R. . 1966. A rapid flotation-sieving technique for extracting nematodes from soil. Plant Dis. Rep. 50: 954–957. [Google Scholar]
- Campbell M M, and Kettle D S. . 1976. Number of adult Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) emerging from bovine dung exposed under different conditions in the field. Austral. J. Zool. 24: 75–85. [Google Scholar]
- Carpenter S, Mordue (Luntz) A J, and Mordue W. . 2001. Ovipostion in Culicoides impunctatus under laboratory conditions. Entomol. Exper. App. 101: 123–129. [Google Scholar]
- Carpenter S, Szmaragd C, Barber J, Labuschagne K, Gubbins S, and Mellor P. . 2008. An assessment of Culicoides surveillance techniques in northern Europe: have we underestimated a potential bluetongue virus vector? J. Appl. Ecol. 45: 1237–1245. [Google Scholar]
- Carpenter S, Groschup M H, Garros C, Felippe-Bauer M L, and Purse B V. . 2013. Culicoides biting midges, arboviruses and public health in Europe. Antivir. Res. 100: 102–113. [DOI] [PubMed] [Google Scholar]
- Cilek J E, and Kline D L. . 2002. Adult biting midge response to trap type, carbon dioxide, and an octenol-phenol mixture in northwestern Florida. J. Am. Mosq. Cont. Assoc. 18: 228–231. [PubMed] [Google Scholar]
- Cohnstaedt L W, Rochon K, Duehl A J, Anderson J F, Barrera R, Su N Y, Gerry A C, Obenauer P J, Campbell J F, Lysyk T J, . et al. 2012. Arthropod surveillance programs: basic components, strategies, and analysis. Ann. Entomol. Soc. Am. 105: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuéllar A C, Kjær L J, Kirkeby C, Skovgard H, Nielsen S A, Stockmarr A, Andersson G, Lindstrom A, Chirico J, Lühken R, . et al. 2018. Spatial and temporal variation in the abundance of Culicoides biting midges (Diptera: Ceratopogonidae) in nine European countries. Parasit. Vectors. 11: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río R, Monerris M, Miquel M, Borràs D, Calvete C, Estrada R, Lucientes J, and Miranda M A. . 2013. Collection of Culicoides spp. with four light trap models during different seasons in the Balearic Islands. Vet. Parasitol. 195: 150–156. [DOI] [PubMed] [Google Scholar]
- Diarra M, Fall M, Lancelot R, Diop A, Fall A G, Dicko A, Seck M T, Garros C, Allène X, Rakotoarivony I, . et al. 2015. Modelling the abundances of two major Culicoides (Diptera: Ceratopogonidae) species in the Niayes area of Senegal. PLoS One. 10: e0131021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers A R W, and Meiswinkel R. . 2014. Culicoides (Diptera: Ceratopogonidae) host preferences and biting rates in the Netherlands: comparing cattle, sheep and the black-light suction trap. Vet. Parasitol. 205: 330–337. [DOI] [PubMed] [Google Scholar]
- Elbers A R W, and Meiswinkel R. . 2015. Culicoides (Diptera: Ceratopogonidae) and livestock in the Netherlands: comparing host preference and attack rates on a Shetland pony, a dairy cow, and a sheep. J. Vector Ecol. 40: 308–317. [DOI] [PubMed] [Google Scholar]
- Elbers A R W, Pacholewicz E, and Gonzales J L. . 2019. Comparison of a mouth aspirator and a mechanical vacuum aspirator for the collection of Culicoides midges (Diptera: Ceratopogonidae) in equids. J. Appl. Entomol. 143: 902–910. [Google Scholar]
- Erram D, Blosser E M, and Burkett-Cadena N. . 2019. Habitat associations of Culicoides species (Diptera: Ceratopogonidae) abundant on a commercial cervid farm in Florida, USA. Parasit. Vectors. 12: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassotte C, Delécolle J C, Cors R, Defrance T, De Deken R, Haubruge E, and Losson B. . 2008. Culicoides trapping with Rothamsted suction traps before and during the bluetongue epidemic of 2006 in Belgium. Prev. Vet. Med. 87: 74–83. [DOI] [PubMed] [Google Scholar]
- Garcia-Saenz A, McCarter P, and Baylis M. . 2011. The influence of host number on the attraction of biting midges, Culicoides spp., to light traps. Med. Vet. Entomol. 25: 113–115. [DOI] [PubMed] [Google Scholar]
- Garros C, Gardès L, Allène X, Rakotoarivony I, Viennet E, Rossi S, and Balenghien T. 2011. Adaptation of a species-specific multiplex PCR assay for the identification of blood meal source in Culicoides (Ceratopogonidae: Diptera): applications on Palaearctic biting midge species, vectors of Orbiviruses. Infect. Genet. Evol. 11: 1103–1110. [DOI] [PubMed] [Google Scholar]
- Gerry A C, and Mullens B A. . 1998. Response of male Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) to carbon dioxide and observations of mating behavior on and near cattle. J. Med. Entomol. 35: 239–244. [DOI] [PubMed] [Google Scholar]
- Gerry A C, and Mullens B A. . 2000. Seasonal abundance and survivorship of Culicoides sonorensis (Diptera: Ceratopogonidae) at a southern California dairy, with reference to potential bluetongue virus transmission and persistence. J. Med. Entomol. 37: 675–688. [DOI] [PubMed] [Google Scholar]
- Gerry A C, Mullens B A, Maclachlan N J, and Mecham J O. . 2001. Seasonal transmission of bluetongue virus by Culicoides sonorensis (Diptera: Ceratopogonidae) at a southern California dairy and evaluation of vectorial capacity as a predictor of bluetongue virus transmission. J. Med. Entomol. 38: 197–209. [DOI] [PubMed] [Google Scholar]
- Gerry A C, Sarto I Monteys V, Moreno Vidal J O, Francino O, and Mullens B A. . 2009. Biting rates of Culicoides midges (Diptera: Ceratopogonidae) on sheep in northeastern Spain in relation to midge capture using UV light and carbon dioxide-baited traps. J. Med. Entomol. 46: 615–624. [DOI] [PubMed] [Google Scholar]
- Goffredo M, and Meiswinkel R. . 2004. Entomological surveillance of bluetongue in Italy: methods of capture, catch analysis and identification of Culicoides biting midges. Vet. Ital. 40: 260–265. [PubMed] [Google Scholar]
- González M, López S, Mullens B A, Baldet T, and Goldarazena A. . 2013. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet. Parasitol. 191: 81–93. [DOI] [PubMed] [Google Scholar]
- González M, Alarcón-Elbal P M, Valle-Mora J, and Goldarazena A. . 2016. Comparison of different light sources for trapping Culicoides biting midges, mosquitoes and other dipterans. Vet. Parasitol. 226: 44–49. [DOI] [PubMed] [Google Scholar]
- Harrup L E, Logan J G, Cook J I, Golding N, Birkett M A, Pickett J A, Sanders C, Barber J, Rogers D J, Mellor P S, . et al. 2012. Collection of Culicoides (Diptera: Ceratopogonidae) using CO2 and enantiomers of 1-octen-3-ol in the United Kingdom. J. Med. Entomol. 49: 112–121. [DOI] [PubMed] [Google Scholar]
- Henry L G, and Adkins T R. 1975. Vertical distribution of biting midges in coastal South Carolina. Ann. Entomol. Soc. Am. 68: 321–324. [Google Scholar]
- Hope A, Gubbins S, Sanders C, Denison E, Barber J, Stubbins F, Baylis M, and Carpenter S. . 2015. A comparison of commercial light-emitting diode baited suction traps for surveillance of Culicoides in northern Europe. Parasit. Vectors. 8: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hribar L J. 1990. A review of methods for recovering biting midge larvae (Diptera: Ceratopogonidae) from substrate samples. J. Agric. Entomol. 7: 71–77. [Google Scholar]
- Isberg E, Bray D P, Hillbur Y, and Ignell R. . 2017. Evaluation of host-derived volatiles for trapping Culicoides biting midges (Diptera: Ceratopogonidae). J. Chem. Ecol. 43: 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R H. 1961. Observations on the larval habitats of some North American species of Culicoides (Diptera: Ceratopogonidae). Ann. Entomol. Soc. Am. 54: 702–710. [Google Scholar]
- Kirkeby C, Bødker R, Stockmarr A, and Lind P. . 2013a. Spatial abundance and clustering of Culicoides (Diptera: Ceratopogonidae) on a local scale. Parasit. Vectors. 6: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby C, Græsbøll K, Stockmarr A, Christiansen L E, and Bødker R. . 2013b. The range of attraction for light traps catching Culicoides biting midges (Diptera: Ceratopogonidae). Parasit. Vectors. 6: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeby C, Stockmarr A, Bødker R, and Lind P. . 2013c. Spatio-temporal optimization of sampling for bluetongue vectors (Culicoides) near grazing livestock. Parasit. Vectors. 6: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D L, Dukes J C, and Axtell R C. . 1975. Salt marsh Culicoides (Diptera: Ceratopogonidae): comparison of larval sampling methods. Mosq. News. 35: 147–150. [Google Scholar]
- Kline D L, Roberts R H, and Focks D A. . 1981. Extraction of larvae of the Ceratopogonid biting midge, Culicoides mississippiensis from salt marsh soil with a new agar technique. Mosq. News. 41: 94–98. [Google Scholar]
- Kline D L, Hagan D V, and Wood J R. . 1994. Culicoides responses to 1-octen-3-ol and carbon dioxide in salt marshes near Sea Island, Georgia, U.S.A. Med. Vet. Entomol. 8: 25–30. [DOI] [PubMed] [Google Scholar]
- Kruger E L, Pappas L G, and Pappas C D. . 1990. Habitat and temporal partitioning of tree hole Culicoides (Diptera: Ceratopogonidae). J. Am. Mosq. Control Assoc. 6: 390–393. [PubMed] [Google Scholar]
- Lillie T H, Kline D L, and Hall D W. . 1987. Diel and seasonal activity of Culicoides spp. (Diptera: Ceratopogonidae) near Yankeetown, Florida, monitored with a vehicle-mounted insect trap. J. Med. Entomol. 24: 503–511. [DOI] [PubMed] [Google Scholar]
- Lühken R, Kiel E, and Steinke S. . 2014. Culicoides biting midge density in relation to the position and substrate temperature in a cattle dung heap. Parasitol. Res. 113: 4659–4662. [DOI] [PubMed] [Google Scholar]
- Lysyk T J. 2006. Abundance and species composition of Culicoides (Diptera: Ceratopogonidae) at cattle facilities in southern Alberta, Canada. J. Med. Entomol. 43: 840–849. [DOI] [PubMed] [Google Scholar]
- Lysyk T J. 2007. Seasonal abundance, parity, and survival of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in southern Alberta, Canada. J. Med. Entomol. 44: 959–969. [DOI] [PubMed] [Google Scholar]
- Lysyk T J, and Dergousoff S J. . 2014. Distribution of Culicoides sonorensis (Diptera: Ceratopogonidae) in Alberta, Canada. J. Med. Entomol. 51: 560–571. [DOI] [PubMed] [Google Scholar]
- Mayo C E, Gardner I A, Mullens B A, Barker C M, Gerry A C, Guthrie A J, and MacLachlan N J. . 2012a. Anthropogenic and meteorological factors influence vector abundance and prevalence of bluetongue virus infection of dairy cattle in California. Vet. Microbiol. 155: 158–164. [DOI] [PubMed] [Google Scholar]
- Mayo C E, Mullens B A, Gerry A C, Barker C M, Mertens P P, Maan S, Maan N, Gardner I A, Guthrie A J, and MacLachlan N J. . 2012b. The combination of abundance and infection rates of Culicoides sonorensis estimates risk of subsequent bluetongue virus infection of sentinel cattle on California dairy farms. Vet. Parasitol. 187: 295–301. [DOI] [PubMed] [Google Scholar]
- Mayo C E, Mullens B A, Reisen W K, Osborne C J, Gibbs E P, Gardner I A, and MacLachlan N J. . 2014a. Seasonal and interseasonal dynamics of bluetongue virus infection of dairy cattle and Culicoides sonorensis midges in northern California–implications for virus overwintering in temperate zones. PLoS One. 9: e106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo C E, Osborne C J, Mullens B A, Gerry A C, Gardner I A, Reisen W K, Barker C M, and Maclachlan N J. . 2014b. Seasonal variation and impact of waste-water lagoons as larval habitat on the population dynamics of Culicoides sonorensis (Diptera: Ceratpogonidae) at two dairy farms in northern California. PLoS One. 9: e89633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott E G, and Mullens B A. . 2018. The dark side of light traps. J. Med. Entomol. 55: 251–261. [DOI] [PubMed] [Google Scholar]
- McDermott E G, Mayo C E, Gerry A C, Laudier D, MacLachlan N J, and Mullens B A. . 2015. Bluetongue virus infection creates light averse Culicoides vectors and serious errors in transmission risk estimates. Parasit. Vectors. 8: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott E G, Mayo C E, Gerry A C, and Mullens B A. . 2016. Trap placement and attractant choice affect capture and create sex and parity biases in collections of the biting midge, Culicoides sonorensis. Med. Vet. Entomol. 30: 293–300. [DOI] [PubMed] [Google Scholar]
- McGregor B. L., Runkel A. E, Wisely S. M., and Burkett-Cadena N. D.. 2018. Vertical stratification of Culicoides biting midges at a Florida big game preserve. Parasites Vector. 11: 505. doi: 10.1186/s13071-018-3080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock J, Balenghien T, Alten B, Versteirt V, and Schaffner F. . 2018. Field sampling methods for mosquitoes, sandflies, biting midges and ticks: VectorNet project 2014–2018. EFSA Support. Publ. 15: 1435E. [Google Scholar]
- Meiswinkel R, and Elbers A R. . 2016. The dying of the light: crepuscular activity in Culicoides and impact on light trap efficacy at temperate latitudes. Med. Vet. Entomol. 30: 53–63. [DOI] [PubMed] [Google Scholar]
- Meiswinkel R, Goffredo M, Leijs P, and Conte A. . 2008. The Culicoides ‘snapshot’: a novel approach used to assess vector densities widely and rapidly during the 2006 outbreak of bluetongue (BT) in the Netherlands. Prev. Vet. Med. 87: 98–118. [DOI] [PubMed] [Google Scholar]
- Meiswinkel R, Scolamacchia F, Dik M, Mudde J, Dijkstra E, Van Der Ven I J, and Elbers A R. . 2014. The Mondrian matrix: Culicoides biting midge abundance and seasonal incidence during the 2006-2008 epidemic of bluetongue in the Netherlands. Med. Vet. Entomol. 28: 10–20. [DOI] [PubMed] [Google Scholar]
- Mellor P S, Boorman J, and Baylis M. . 2000. Culicoides biting midges: their role as arbovirus vectors. Annu. Rev. Entomol. 45: 307–340. [DOI] [PubMed] [Google Scholar]
- Mullens B A. 1985. Age-related adult activity and sugar feeding by Culicoides variipennis (Diptera: Ceratopogonidae) in southern California, USA. J. Med. Entomol. 22: 32–37. [Google Scholar]
- Mullens B A. 1989. A quantitative survey of Culicoides variipennis (Diptera: Ceratopogonidae) in dairy wastewater ponds in southern California. J. Med. Entomol. 26: 559–565. [DOI] [PubMed] [Google Scholar]
- Mullens B A. 1995. Flight activity and response to carbon dioxide of Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) in southern California. J. Med. Entomol. 32: 310–315. [DOI] [PubMed] [Google Scholar]
- Mullens B A, and Gerry A C. . 1998. Comparison of bait cattle and carbon dioxide-baited suction traps for collecting Culicoides variipennis sonorensis (Diptera: Ceratopogonidae) and Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 35: 245–250. [DOI] [PubMed] [Google Scholar]
- Mullens B A, and Rodriguez J L. . 1984. Efficiency of salt flotation for extraction of immature Culicoides variipennis (Ceratopogonidae) from mud substrates. Mosq. News. 44: 207–211. [Google Scholar]
- Mullens B A, and Rodriguez J L. . 1985. Effect of experimental habitat shading on the distribution of Culicoides variipennis (Diptera: Ceratopogonidae) larvae. Environ. Entomol. 14: 749–754. [Google Scholar]
- Mullens B A, and Rodriguez J L. . 1992. Survival and vertical distribution of larvae of Culicoides variipennis (Diptera: Ceratopogonidae) in drying mud habitats. J. Med. Entomol. 29: 745–749. [DOI] [PubMed] [Google Scholar]
- Mullens B A, McDermott E G, and Gerry A C. . 2015. Progress and knowledge gaps in Culicoides ecology and control. Vet. Ital. 51: 313–323. [DOI] [PubMed] [Google Scholar]
- Nelson R L. 1965. carbon dioxide as an attractant for Culicoides. J. Med. Entomol. 2: 56–57. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel R S, and Ruder M G. . 2015. Colonization of bison (Bison bison) wallows in a tallgrass prairie by Culicoides spp (Diptera: Ceratopogonidae). J. Vector Ecol. 40: 187–190. [DOI] [PubMed] [Google Scholar]
- Probst C, Gethmann J M, Kampen H, Werner D, and Conraths F J. . 2015. A comparison of four light traps for collecting Culicoides biting midges. Parasitol. Res. 114: 4717–4724. [DOI] [PubMed] [Google Scholar]
- Purse B V, Carpenter S, Venter G J, Bellis G, and Mullens B A. . 2015. Bionomics of temperate and tropical Culicoides midges: knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu. Rev. Entomol. 60: 373–392. [DOI] [PubMed] [Google Scholar]
- Rádrová J, Mračková M, Galková Z, Lamka J, Račka K, Barták P, and Votýpka J. . 2016. Seasonal dynamics, parity rate, and composition of Culicoides (Diptera: Ceratopogonidae) occurring in the vicinity of wild and domestic ruminants in the Czech Republic. J. Med. Entomol. 53: 416–424. [DOI] [PubMed] [Google Scholar]
- Rigot T, and Gilbert M. . 2012. Quantifying the spatial dependence of Culicoides midge samples collected by Onderstepoort-type blacklight traps: an experimental approach to infer the range of attraction of light traps. Med. Vet. Entomol. 26: 152–161. [DOI] [PubMed] [Google Scholar]
- Ritchie S A, van Essen P H, Kemme J A, Kay B H, and Allaway D. . 1994. Response of biting midges (Diptera: Ceratopogonidae) to carbon dioxide, octenol, and light in southeastern Queensland, Australia. J. Med. Entomol. 31: 645–648. [DOI] [PubMed] [Google Scholar]
- Ronderos M M, Cazorla C G, and Spinelli G R. . 2010. The immature stages of the biting midge Culicoides debilipalpis Lutz (Diptera: Ceratopogonidae). Zootaxa. 2716: 42–52. [Google Scholar]
- Ruder M G, Lysyk T J, Stallknecht D E, Foil L D, Johnson D J, Chase C C, Dargatz D A, and Gibbs E P. . 2015. Transmission and epidemiology of bluetongue and epizootic hemorrhagic disease in North America: current perspectives, research gaps, and future directions. Vector Borne Zoonotic Dis. 15: 348–363. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Hattori J, Chinone S, Nihei N, Tsuda Y, Kurahashi H, and Kobayashi M. . 2004. Yeast-generated CO2 as a convenient source of carbon dioxide for adult mosquito sampling. J. Am. Mosq. Cont. Assoc. 20: 261–264. [PubMed] [Google Scholar]
- Sanders C J, Shortall C R, Gubbins S, Burgin L, Gloster J, Harrington R, Reynolds D R, Mellor P S, and Carpenter S. . 2011. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J. Appl. Ecol. 48: 1355–1364. [Google Scholar]
- Sanders C J, Gubbins S, Mellor P S, Barber J, Golding N, Harrup L E, and Carpenter S T. . 2012. Investigation of diel activity of Culicoides biting midges (Diptera: Ceratopogonidae) in the United Kingdom by using a vehicle-mounted trap. J. Med. Entomol. 49: 757–765. [DOI] [PubMed] [Google Scholar]
- Sanders C J, Shortall C R, England M, Harrington R, Purse B, Burgin L, Carpenter S, and Gubbins S. . 2019. Long‐term shifts in the seasonal abundance of adult Culicoides biting midges and their impact on potential arbovirus outbreaks. J. Appl. Ecol. 56: 1649–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvašová A, Kočišová A, Halán M, Delécolle J-C, and Mathieu B. . 2014. Morphological and molecular analysis of the genus Culicoides (Diptera: Ceratopogonidae) in Slovakia with five new records. Zootaxa. 3872: 541. [DOI] [PubMed] [Google Scholar]
- Schmidtmann E T, Jones C J, and Gollands B. . 1980. Comparative host-seeking activity of Culicoides (Diptera: Ceratopogonidae) attracted to pastured livestock in central New York state, USA. J. Med. Entomol. 17: 221–231. [Google Scholar]
- Schmidtmann E T, Herrero M V, Green A L, Dargatz D A, Rodriquez J M, and Walton T E. . 2011. Distribution of Culicoides sonorensis (Diptera: Ceratopogonidae) in Nebraska, South Dakota, and North Dakota: clarifying the epidemiology of bluetongue disease in the northern Great Plains region of the United States. J. Med. Entomol. 48: 634–643. [DOI] [PubMed] [Google Scholar]
- Shemanchuk J A. 1978. A bait trap for sampling the feeding populations of blood-sucking Diptera on cattle. Quaest. Entomol. 14: 433–439. [Google Scholar]
- Sloyer K E, Wisely S M, and Burkett-Cadena N D. . 2019. Effects of ultraviolet LED versus incandescent bulb and carbon dioxide for sampling abundance and diversity of Culicoides in Florida. J. Med. Entomol. 56: 353–361. [DOI] [PubMed] [Google Scholar]
- Steinke S, Lühken R, and Kiel E. . 2014. Assessment of the abundance of Culicoides chiopterus and Culicoides dewulfi in bovine dung: a comparison of larvae extraction techniques and emergence traps. Vet. Parasitol. 205: 255–262. [DOI] [PubMed] [Google Scholar]
- Swanson D A, and Adler P H. 2010. Vertical distribution of haematophagous Diptera in temperate forests of the southeastern U.S.A. Med. Vet. Entomol. 24: 182–188. [DOI] [PubMed] [Google Scholar]
- Uslu U, and Dik B. . 2006. Vertical distribution of Culicoides larvae and pupae. Med. Vet. Entomol. 20: 350–352. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prokopec G M, Galvin W A, Kelly R, and Kitron U. . 2009. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J. Med. Entomol. 46: 1256–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter G J, Hermanides K G, Boikanyo S N, Majatladi D M, and Morey L. . 2009a. The effect of light trap height on the numbers of Culicoides midges collected under field conditions in South Africa. Vet. Parasitol. 166: 343–345. [DOI] [PubMed] [Google Scholar]
- Venter G J, Labuschagne K, Hermanides K G, Boikanyo S N, Majatladi D M, and Morey L. . 2009b. Comparison of the efficiency of five suction light traps under field conditions in South Africa for the collection of Culicoides species. Vet. Parasitol. 166: 299–307. [DOI] [PubMed] [Google Scholar]
- Venter G J, Labuschagne K, Boikanyo S N B, Majatladi D M, and Morey L. . 2011. The effect of 1-octen-3-ol and 4-methylphenol on Culicoides midge numbers collected with suction light traps in South Africa. Vet. Parasitol. 175: 182–186. [DOI] [PubMed] [Google Scholar]
- Venter G J, Boikanyo S N, Majatladi D M, and Morey L. . 2016. Influence of carbon dioxide on numbers of Culicoides midges collected with suction light traps in South Africa. Med. Vet. Entomol. 30: 117–122. [DOI] [PubMed] [Google Scholar]
- Viennet E, Garros C, Lancelot R, Allène X, Gardès L, Rakotoarivony I, Crochet D, Delécolle J C, Moulia C, Baldet T, . et al. 2011. Assessment of vector/host contact: comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasit. Vectors. 4: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil S L, Ruder M G, Shaw D, Wlodkowski J, Garrett K, Walter M, and Corn J L. . 2018. Apparent range expansion of Culicoides (Hoffmania) insignis (Diptera: Ceratopogonidae) in the Southeastern United States. J. Med. Entomol. 55: 1043–1046. [DOI] [PubMed] [Google Scholar]
- Walgama R S, and Lysyk T J. . 2019. Evaluating the addition of CO2 to black light traps for sampling Culicoides (Diptera: Ceratopogonidae) in Alberta. J. Med. Entomol. 56: 169–180. [DOI] [PubMed] [Google Scholar]
- Williams R W. 1964. Observations on habitats of Culicoides larvae in Trinidad W.I. (Diptera: Ceratopogonidae). Ann. Entomol. Soc. Am. 57: 462–466. [Google Scholar]
- Wong N D, McDermott E G, Murillo A C, and Mullens B A. . 2017. Field distribution and density of Culicoides sonorensis (Diptera: Ceratopogonidae) eggs in dairy wastewater habitats. J. Med. Entomol. 55: 392–397. [DOI] [PubMed] [Google Scholar]
- Yanoviak S P, and Fincke O M. . 2005. Sampling methods for water-filled tree holes and their artificial analogues, pp. 168–185. In Leather S. (ed.), Insect sampling in forest ecosystems. Blackwell Publishing, Oxford, United Kingdom. [Google Scholar]