Despite extensive laboratory work-up, no underlying coagulation defect is found in many patients with mucocutaneous bleeding (MCB) symptoms, and they are categorized as having bleeding of unknown cause (BUC). The prevalence of BUC in these patients ranges from 59-72%1,2. The underlying pathophysiology has yet to be determined, which creates difficulties for management.
In general, evaluation of bleeding symptoms is a recognized challenge due to the subjective nature of bleeding histories.3 Bleeding Assessment Tools (BATs) have been developed to better quantify the bleeding history.4 BATs translate bleeding symptoms into a summative bleeding score (BS), alerting physicians to which patients have bleeding symptoms outside the range of normal.5 Many studies in different cohorts of patients support the use of BATs as a screening tool in the diagnosis of mild bleeding disorders.4,6,7 Currently, the ISTH-BAT (International Society on Thrombosis and Haemostasis) is the tool endorsed by that society.8
The main utility of BATs to date has been as a screening tool for patients presenting with MCB. Studies have also used them to describe bleeding severity in patients with mild bleeding disorders. Recently, the utility of BATs has been extended to predict the risk of future bleeding in patients with von Willebrand Disease (VWD). A prospective study of 796 Italian patients with all types of VWD showed that a BS>10 was the strongest predictor of bleeding events severe enough to require treatment with desmopressin (DDAVP), and/or von Willebrand factor (VWF)/FVIII concentrates.9 Therefore, the BS may be useful to inform treatment protocols and replacement therapies.9 No data currently exist regarding the predictive role of BS in other populations, including BUC. With this background, we aimed to determine if the BS is predictive of future spontaneous bleeding events in patients with BUC.
We conducted a single center retrospective chart review that aims to determine if the BS derived from the ISTH-BAT predicts risk of future spontaneous bleeding events in patients with BUC. We reviewed patients ≥18 years of age categorized as BUC after referral for hemostatic evaluation at a Hematology clinic at Kingston Health Sciences Centre (Ontario, Canada). Patients with a history of bleeding symptoms were classified as BUC after standard hemostatic testing revealed no diagnostic laboratory abnormality. The Molecular and Clinical Markers for the Diagnosis and Management of VWD type 1 (MCMDM-1) BS was generated at the time of initial hematological consultation.7 Bleeding scores from the MCMDM-1 were then converted to an ISTH-BS. A study by Elbatarny et al.5 showed that there is a 90% overlap between BATs, providing evidence that merging bleeding scores derived from different bleeding questionnaires is acceptable in this study. Number and site of future spontaneous bleeding events was collected from medical records.
To be included in the study, patients must have had a positive BS at the time of diagnosis, defined as an ISTH-BS ≥ 4 in adult males and ≥ 6 in adult females.5 Exclusion criteria included patients diagnosed with an inherited bleeding disorder, patients <18 years of age, active anticoagulation, and patients referred less than 6 months prior to data collection.5 The following laboratory tests were collected retrospectively from patient charts: (i) complete blood count, ABO blood type, coagulation profile (INR, PT, PTT, TT, fibrinogen), ferritin, iron, transferrin, transferrin saturation; (ii) VWF antigen level, VWF functional assay and factor VIII level; (iii) platelet function studies; and (iv) additional factor assays if performed. Data was imported into IBM SPSS and analyzed using t-tests, chi-squared tests and logistic regression.
A total of 90 patients were included in the study. The mean age was 46 years, and the sample was 98% female. There was a wide range of BSs among the study population (6-20). The mean (SD) bleeding score among all BUC patients included in the study was 10 (3.22) (Figure 1). In this cohort, 57.8% of patients (52/90) had at least one spontaneous bleeding event following designation as BUC. Heavy menstrual bleeding (HMB) was the most common type of bleeding event, while muscle hematoma was the least common. There were no hemarthrosis nor central nervous system bleeds reported. The mean (SD) BS of BUC patients with at least one bleeding event was 10.42 (2.88), which is not statistically significantly higher than those that did not have any bleeds [9.55 (3.62), p=0.208] (Table 1).
Figure 1:
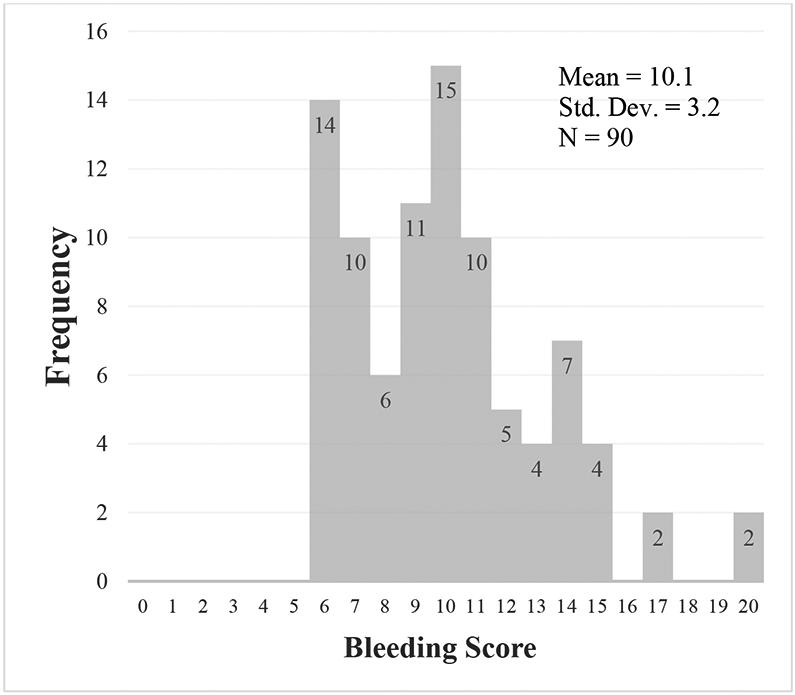
Frequency of the ISTH-BAT BS values among all 90 BUC patients included in the study.
Table 1:
Comparison of patient number, BS, and age at first visit, and ABO blood type between patients with and without bleeding events in a univariate analysis.
| All bleeding events | ||||
|---|---|---|---|---|
| № | Bleeding Score, mean (SD) |
Age at first visit, mean (SD) |
ABO Type | |
| Pts w/ bleeding event(s) | 52 | 10.4 (2.8) | 38.9 (15.9) | Type O: 43.9% Non-O: 56.1% |
| Pts w/out bleeding event(s) | 38 | 9.6 (3.6) | 44.9 (17.6) | Type O: 46.2% Non-O: 53.8% |
| p* | 0.208 | 0.075 | 0.59 | |
A secondary analysis excluding all HMB was performed, as there was concern that determination of an accurate event number of HMB may be difficult in this retrospective design. In the analysis including all bleeding events except HMB, the BS was higher, 11.20 (3.34) and 9.62 (3.09) for BUC patients with and without spontaneous bleeding events, but it also fell short of statistical significance (p=0.076).
Laboratory investigation for VWD was normal for all patients. Platelet function studies were done in 77.7% of patients (70/90), all of which were within the normal range. No significant differences in hemoglobin, platelet count, iron studies, PT, or PTT were detected between patients with and without bleeding events. The frequency of blood group O was not significantly different between those with bleeding events (43.9%) and those without (46.2%, p=0.59).
In a multivariable logistic regression model, an increase in the BS was significantly associated with an increased risk of a future bleeding event (OR=1.25, 95% CI 1.05, 1.49, p=0.013). Higher platelet count was associated with lower but non-significant risk of bleeding (OR=0.99, 95% CI 0.99, 1.00, p=0.38). Age was negatively associated with the odds of bleeding (OR=0.95, 95% CI 0.92, 0.99 p=0.008). Age was also significantly associated with higher levels of VWF:Ag, VWF:RCo, and FVIII:C assay (p=0.001, p=0.01, and p=0.002, respectively).
Even though BUC patients have clinically similar bleeding phenotypes as those with known mild bleeding disorders, the management of bleeding symptoms in BUC remains controversial. There is evidence suggesting that in patients with unclassified bleeding disorders, prophylaxis with TXA +/− DDAVP prior to major and minor procedures was successful at ameliorating bleeding in nearly all patients.10 In our study TXA alone was often prescribed to patients prior to surgery to prevent abnormal bleeding, the efficacy of which was not explored in the current study.
Our study has a number of limitations. It was assumed that if the PT and aPTT were normal, then factors levels within those pathways were adequate for hemostasis. Another limitation is attributable to the retrospective design, which resulted in large variability in follow-up time between study subjects depending on the date of initial hematology consultation. The length of follow up in some patients may have been inadequate to capture the occurrence the spontaneous bleeding events. This concern was mitigated by excluding patients who were referred in the last 6 months prior to data collection.
Future research should consider evaluating the presence of familial bleeding in patients with BUC, which may provide information to discern whether bleeding tendency is congenital or acquired. Investigating the effectiveness of prophylactic therapy, such as TXA, in BUC patients prior to invasive procedures would be useful to develop evidence-based perioperative management guidelines for this population.
In conclusion, a higher BS in BUC patients is associated with a significantly higher risk of future spontaneous bleeding events. Thus, the BS may be a simple, inexpensive parameter that is valuable in the assessment of patients with MCB, as a high BS may indicate increased likelihood of future bleeding events necessitating ongoing hematology follow-up and/or prophylactic therapy before invasive procedures. These results should be prospectively validated to confirm the relationship between the BS and future spontaneous bleeding events in patients with BUC.
Footnotes
Declaration of interests:
NR, SK, JG, and WH have no potential conflicts of interest. PJ receives research funding from CSL Behring, Bayer and Shire.
References
- 1.Quiroga T, Goycoolea M, Panes O, et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92(3):357–365. doi: 10.3324/haematol.10816. [DOI] [PubMed] [Google Scholar]
- 2.Gebhart J, Hofer S, Panzer S, et al. High proportion of patients with bleeding of unknown cause in persons with a mild-to-moderate bleeding tendency: Results from the Vienna Bleeding Biobank (VIBB). Haemophilia. 2018;24(3):405–413. doi: 10.1111/hae.13422. [DOI] [PubMed] [Google Scholar]
- 3.Rydz N, James PD. Why Is My Patient Bleeding Or Bruising? Hematol Oncol Clin North Am. 2012;26(2):321–344. doi: 10.1016/j.hoc.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Rodeghiero F, Castaman G, Tosetto A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. JThrombHaemost. 2005;3(1538-7933 (Print)):2619–2626. doi: 10.1111/j.1538-7836.2005.01663.x. [DOI] [PubMed] [Google Scholar]
- 5.Elbatarny M, Mollah S, Grabell J, et al. Normal range of bleeding scores for the ISTH-BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831–835. doi: 10.1016/j.immuni.2010.12.017.Two-stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosetto A, Castaman G, Plug I, Rodeghiero F, Eikenboom J. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. J Thromb Haemost. 2011;9(6):1143–1148. doi: 10.1111/j.1538-7836.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 7.Tosetto a, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost. 2006;4(4):766–773. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 8.Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: A standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063–2065. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 9.Federici AB, Bucciarelli P, Castaman G, et al. The bleeding score predicts clinical outcomes and replacement therapy in adults with von Willebrand disease. Blood. 2014;123(26):4037–4044. doi: 10.1182/blood-2014-02-557264.There. [DOI] [PubMed] [Google Scholar]
- 10.Obaji S, Alikhan R, Rayment R, Carter P, Macartney N, Collins P. Unclassified bleeding disorders: Outcome of haemostatic challenges following tranexamic acid and/or desmopressin. Haemophilia. 2016;22(2):285–291. doi: 10.1111/hae.12811. [DOI] [PubMed] [Google Scholar]


