Abstract
Given the wide heterogeneity of phenotypes and of the underlying pathophysiological mechanisms associated with the disorder, pregnancy and delivery in von Willebrand disease (VWD) represent a significant clinical challenge. The variable pattern of changes observed during pregnancy of von Willebrand factor (VWF) and factor VIII (FVIII), the protein carried by VWF, prompts a careful evaluation of pregnant women with VWD to plan the most appropriate treatment at the time of parturition. However, there are also instances during pregnancy (amniocentesis, vaginal bleeding associated with placental detachment, sudden abortion) that may require urgent hemostatic treatment to prevent bleeding. Thus, women with VWD should start pregnancy after being well characterized as to their type, subtype, and treatments. Women with VWD who have VWF and FVIII basal levels >30 U/dL typically normalize these levels at the end of pregnancy and specific anti-hemorrhagic prophylaxis is seldom required. On the contrary, those with basal levels <20 U/dL usually show a lesser increase and specific treatment is required. Some women with mutations associated with increased clearance can be treated with desmopressin, while those unresponsive or with contra-indications to this agent need replacement therapy. For these latter women, the risk of vaginal bleeding during pregnancy may be increased and prophylaxis with VWF concentrates required. Similarly, women with type 2 VWD who maintain reduced VWF activity throughout pregnancy require replacement therapy with FVIII/VWF concentrates. Delayed post-partum bleeding may occur when replacement therapy is not continued for some days. Tranexamic acid is useful at discharge to avoid excessive lochia.
Introduction
Von Willebrand disease (VWD), the most frequent autosomal inherited bleeding disorder, is caused by quantitative or qualitative defects of von Willebrand factor (VWF), an adhesive protein that binds platelets to exposed sub-endothelium and carries factor VIII (FVIII) in circulation1,2. As a consequence, in addition to the defect of VWF, also FVIII, the protein deficient in hemophilia A, may be variably reduced in VWD patients.
VWD is classified into 3 types: type 1, the most frequent, caused by a quantitative reduction of a normal VWF, type 2 characterized by qualitative abnormalities of VWF and divided in 4 subtypes (A,B,M, and N) and type 3, the least frequent but clinically most severe type caused by a virtual absence of VWF (Table 1). Clinical manifestations are mainly represented by muco-cutaneous and soft tissue bleeding and the severity of bleeding symptoms is variable depending on the degree of VWF and FVIII reduction, as well as other factors2. Joint and muscle bleeding may also occur in the most severe deficiencies and in general bleeding tendency is mostly severe in type 3 and mildest in type 1.
Table 1.
Classification of von Willebrand disease.
| Quantitative deficiency of VWF |
| Type 1 Partial quantitative deficiency of VWF |
| Type 3 Virtually complete deficiency of VWF |
| Qualitative deficiency of VWF |
| Type 2 Qualitative deficiency of VWF |
| - Type 2A Qualitative variants with decreased platelet-dependent function associated with the absence of high and intermediate-molecular-weight VWF multimers |
| - Type 2B Qualitative variants with increased affinity for platelet GPIb |
| - Type 2M Qualitative variants with decreased platelet-dependent function not caused by the absence of high-molecular-weight VWF multimers |
| - Type 2N Qualitative variants with markedly decreased affinity for factor VIII |
General guidelines for treatment of von Willebrand disease
According to the pathophysiological background, treatment of VWD requires the correction of the dual haemostatic defect (low FVIII and low/abnormal VWF). These goals can be achieved by increasing plasma concentrations of these factors through their release from endothelial cells with desmopressin (DDAVP) or by using replacement therapy with human plasma-derived (pd) low-purity FVIII-VWF concentrates or a high-purity VWF product.
DDAVP (1-deamino-8-D-arginin-vasopressin) is a synthetic analog of the antidiuretic hormone vasopressin. The drug is generally well-tolerated, safe and cheap. DDAVP can be administered intravenously (0.3 μg/kg diluted in 50–100 mL saline and infused over 30 minutes), subcutaneously (0.3 μg/kg), or intranasally (fixed doses of 300 μg in adults and 150 μg in children). This treatment increases circulating FVIII/VWF three to five times above basal levels within 30–60 minutes; high plasma FVIII/VWF concentrations last for 6–8 hours. DDAVP response is mainly dependent on genotype and phenotype3 and a test-dose infusion at the time of diagnosis is recommended to establish the pattern of response and its duration. Progressive reduced response to DDAVP can be observed due to depletion of VWF/FVIII from the storages (tachyphylaxis) after repeated treatments (every 12–24 hours). Side effects, attributable to the vasomotor effect of the molecule, may include mild tachycardia, flushing, and headache. Hyponatremia, especially in children below the age of 2, may occur due to the antidiuretic properties of DDAVP, and volume overload, both preventable by limiting fluid intake for 24 hours after the administration of DDAVP4. Although no major thrombotic events have been reported in VWD, the drug should be used carefully in elderly patients with atherosclerotic disease5.
DDAVP is usually effective in patients type 1 VWD and baseline VWF and FVIII levels higher than 10 IU/dL2,4 while variable responses are observed in type 2 VWD patients1,2. In type 2B, DDAVP is generally contraindicated because of the transient appearance or aggravation of thrombocytopenia leading to an increased risk of bleeding6. Patients with type 3 VWD are unresponsive to DDAVP.
When DDAVP is either ineffective or contraindicated, replacement therapy with pd-VWF/FVIII concentrates is the treatment of choice. Current effective intermediate and high-purity VWF-FVIII concentrates are several and all plasma-derived2, with a variable content of VWF and FVIII, as well as a heterogeneous multimer pattern (Table 2) due to the different production techniques7. However, there is no clinical evidence that the various pd-VWF/FVIII products differ regarding hemostatic efficacy and in terms of pharmacokinetics2. Recently, a recombinant VWF concentrate, with a better multimer profile, has been approved in USA after its demonstration of clinical efficacy and safety8. Several useful recommendations or national guidelines for VWD treatment are available1,2.
Table 2.
VWF/FVIII concentrates licensed for the treatment of von Willebrand disease in Europe and North America
| Product | Manufacturer | Purification | Viral inactivation | VWF:RCo/Ag# (Ratio) | VWF: RCo/FVIII# (Ratio) |
|---|---|---|---|---|---|
| Alphanate | Grifols | Heparin ligand chromatography | S/D + dry heat (80°C, 72 h) | 0.47 ± 0.1 | 0.91 ± 0.2 |
| Factor 8Y | BioProducts | Heparin/glycine precipitation | Dry heat (80°C, 72 h) | 0.29 | 0.81 |
| Fanhdi | Laboratory Grifols | Heparin ligand chromatography | S/D + dry heat (80°C, 72 h) | 0.47 ± 0.1 | 1.04 ± 0.1 |
| Haemate P/ | |||||
| Humate | CSL Behring | Multiple precipitation | Pasteurization (60°C, 10 h) | 0.59 ± 0.1 | 2.45 ± 0.3 |
| Talate | Shire | Ion exchange chromatography | S/D + vapor heat (60°C, 10 h) | 0.47 | 1.1 |
| Wilate | Octapharma | Ion exchange + size exclusion | S/D + dry heat (100°C, 2 h) | - | 0.9 |
| Wilfactin | LFB | Chromatography Ion Exchange + affinity | S/D, 35 nm filtration, dry Heat (80°C, 72 h) | ≈0.95 | ≈50 |
| VonVendi | Shire | Chinese Hamster Ovary (CHO) cell line co-expressing the VWF and FVIII genes, in absence of any animal or other human plasma proteins; purified by immune-affinity chromatography | - | - | - |
Risk of bleeding during pregnancy in normal women
Women experience naturally-occurring physiological events (menstruation, pregnancy and parturition) that may cause excessive bleeding even in absence of a specific bleeding disorder. Pregnancy is considered as a hypercoagulable condition because several hemostatic factors increase throughout. Factor VII, factor X, fibrinogen and plasminogen activator inhibitor type 1 increase, while free protein S decreases9. These changes are considered to be adaptive in preparation for the hemostatic challenge of delivery. VWF and FVIII increase significantly during pregnancy in normal women reaching the greatest level during the third trimester, with levels far exceeding 100 U/dL by the time of parturition.
However, at the time of delivery, several obstetric complications may cause bleeding with or without associated hemostatic abnormalities10. Post-partum hemorrhage (PPH) is a major cause of maternal complications and death, especially in low-income countries11. As a consequence, the use of utero-tonic agent soon after delivery is recommended in all women to reduce this risk12. A standard definition of post-partum hemorrhage (PPH) considers the threshold of >500 mL blood loss for vaginal delivery and of >1,000 mL for caesarean section13. Rates of PPH in Western Countries based on hospital discharge may range from 3 to 6%, while cases needing blood transfusion are as low as 1 %10.
Uterine atony is still the leading cause of hemorrhage at parturition accounting for approximately 75 % of all cases with post-partum hemorrhage (PPH) and causes a high rate of transfusion or even death in otherwise healthy women10,14. These complications may add to the inherent risk of bleeding in women with an inherited bleeding disorder and should not be overlooked as a potential cause of bleeding resistant to specific treatment.
Risk of bleeding during pregnancy in VWD
Whether or not women with VWD are at increased risk of spontaneous abortion is unclear from the literature. In a retrospective study of 27 women with VWD, higher rates of miscarriage and bleeding throughout pregnancy were reported9, however caution should be exercised when interpreting these results as women with VWD may bleed more after a pregnancy complication, and come to medical attention more readily. Another large study did not show an increase in miscarriage in women with type 1 VWD, the less severe type of the disorder15. These data were confirmed in a population of 182 Iranian women with type 3 VWD, the most severe form of the disease16.
In a case-control study, women with VWD were 10 times more likely to experience other ante-partum bleeding (OR 10.2; 95 % CI 7.1–14.6)17, but this latter data was not confirmed in a recent study where the risk of uterine bleeding during pregnancy was similar to that of normal women18. Interestingly, in the case-control study no increased risk of placental abruption, preterm delivery, fetal growth restriction or stillbirth was observed17. Certainly, women with VWD who experience bleeding during pregnancy should be evaluated carefully, including measurement of VWF/FVIII to determine the need for additional treatment.
Villocentesis/amniocentesis in VWD
The use of desmopressin in responsive women during the first trimester of pregnancy to cover chorionic villus sampling or amniocentesis appears to be feasible, effective and safe, without an increased risk of bleeding and miscarriage19. Usually the compound is administered intravenously or subcutaneously at 0.3 μg/kg but capped doses of either 20 or 15 μg have been suggested to have similar effects20,21 The risk of hyponatremia is remote when using a single dose in adults but caution should be used in pregnancy and fluid restriction is advisable when further dose(s) is required along with monitoring of urinary output and serum electrolytes.
Desmopressin is the treatment of choice for of type 1 VWD patients because it induces the release from endothelial stores of FVIII and VWF reaching plasma levels > 50 U/dL for several hours in the vast majority of them1,2,4,5.
Risk of bleeding at parturition in women with von Willebrand disease
There are conflicting results in the literature about the correct estimation of the risk and the severity of bleeding at parturition in women with VWD. Historical series reported percentages of VWD women with post-partum bleeding ranging from 15 to 60 %22–26. This uncertainty is also reported when women with wide range of basal FVIII and VWF are included among type 1 patients. In the MCMDM-1VWD study no difference of bleeding risk at parturition was observed between women with VWD and their normal relatives15. In a recent large case-control study from the USA including 4067 deliveries in women with VWD based on all pregnancy-related discharge codes for the years 2000–2003, these women were more likely to experience PPH (OR 1.5; 95 % CI 1.1–2.0) and had a fivefold increased risk of being transfused (OR 4.7; 95 % CI 3.2–7.0) compared with women without VWD17. However, no data about anti-hemorrhagic prophylaxis and adequacy of treatment was available and thus it is not possible to reliably quantify the risk.
A progressive increase of FVIII and VWF occurs in most women with type 1 VWD, the partial quantitative deficiency of the disorder, with levels reaching > 50 U/dL in the third trimester27. However, owing to the wide heterogeneity of phenotypes and genotypes underlying also this type, this general statement needs to be interpreted cautiously and careful evaluation of any pregnant woman with a diagnosis of VWD is recommended. In general, women with levels at baseline of VWF and FVIII > 30 U/dL, suggesting type 1 VWD, usually show a high likelihood to achieve normal levels at the end of pregnancy2,27. However, significant PPH, requiring blood transfusion in 22 % of cases, has also been reported in women with low VWF but normal levels at parturition28. This again suggests the need for close surveillance of these women and that probably levels around 100 U/dL and antifibrinolytic treatment should be best pursued in these women. Women with basal levels < 20 U/dL usually have a lesser increase since most of these women carry mutations associated with increased VWF clearance or decreased synthesis and secretion or are compound heterozygous for different VWF mutations which prevent the achievement of satisfactory hemostatic levels29,30. Similarly, women with compound heterozygosity for null and missense mutations, associated with clearly measurable FVIII/VWF levels do not show significant improvements during pregnancy30. Since the genetic background (which is highly predictive of the type of response to desmopressin and changes during pregnancy in most cases) is not available for most of these patients30, careful monitoring during pregnancy or at least during the third trimester is highly recommended to identify those who will need specific treatment.
General guidelines for the treatment of women with von Willebrand disease at delivery
Since pregnant women with VWD are at increased risk of postpartum hemorrhage if untreated9,17,27,31, treatment options should be planned at the beginning of pregnancy. Invasive management of delivery with ventouse or rotational forceps should be avoided because of the risk of bleeding for the potentially affected neonate2,30. Ideally, the results of a test-infusion with desmopressin should be available before pregnancy for every woman with VWD and basal level of FVIII and VWF < 30 U/dL2. However, choosing the treatment at parturition on the basis of basal levels alone, without knowledge of mutational background and/or the modifications of FVIII and VWF during pregnancy could be risky since several heterogeneous patterns are possible. In general, VWD patients should be monitored for VWF:RCo and FVIII:C at least once during the third trimester of pregnancy2. The risk of bleeding is minimal when FVIII:C and VWF:RCo levels are higher than 50 U/dL1,4,30,32.
In type 1 VWD, pregnant women with FVIII:C and/or VWF levels lower than 30 U/dL at time of parturition, the administration of desmopressin after umbilical clamping and for 3–4 days thereafter is required2,30, especially when midline episiotomy is required. The intravenous route elicits the same increase as that of the subcutaneous one, but the time to peak is generally shorter5. Some frequent VWF mutations with high penetrance and expressivity (e.g. R1205H, C1130F) are associated with increased clearance of VWF, as documented by an increased VWF propeptide/VWF:Ag ratio33, which prevents achievement of normal levels at the end of pregnancy34,35. However, it has been shown either in retrospective or prospective studies that women with these mutations are safely treated with desmopressin in this setting30,36. Monitoring of urinary output and fluid restriction are necessary to avoid the risk of hyponatremia2. The same approach, with less infusions, can be applied to those with VWF > 30 and < 50 U/dL. A recent experience suggests the possibility to start treatment immediately before delivery, without evident side-effects for the mother and the newborn37. However, alternative approaches using FVIII/VWF concentrates are also used in some Countries especially when close surveillance of the patient is not easily available. In this case 40–60 IU/kg VWF is administered during the late stage of labor and repeated once daily for at least three days, followed by oral tranexamic acid for a week.
Pregnancy in variants VWD
Type 2A VWD is characterized by the lack of high molecular weight multimers and an abnormal VWF:RCo/VWF:Ag (< 0.6)30,38. During pregnancy, multimer abnormalities usually do not correct and VWF:RCo remains markedly reduced. However, a significant increase of FVIII and VWF:Ag may occur30. These patients usually require treatment with FVIII/VWF concentrates38. Several concentrates are available, all with a satisfactory profile of efficacy and safety (Table 2)2. However, they differ as to their FVIII and VWF content. In presence of high FVIII levels, observed in variants type 2 VWD or in patients with clinical conditions triggering high FVIII levels (e.g. surgery for cancer), a concentrate with low FVIII is advisable2,38. Recombinant VWF is now available in USA but not in Canada and EU. Thus, a plasma-derived product containing very little FVIII could be considered instead. FVIII levels can increase further but not as it would be expected with a product containing equal amount of the moieties or significant concentration of FVIII. Antithrombotic prophylaxis for a few days should be considered for caesarean section until daily treatment with concentrate stopped to attenuate the risk of venous thromboembolism sometimes reported in VWD patients treated with FVIII/VWF concentrates during surgery39.
Worsening of pre-existing thrombocytopenia is the most important change observed in type 2B VWD women during pregnancy because an increased release of abnormal multimers with enhanced affinity for glycoprotein Ib on platelet surface occurs6,40–42. However, its severity is strongly dependent on the specific mutation in the A1 domain of VWF responsible for the patient’s disease, with some mutations resulting in normal platelet counts (e.g., P1266L) while others are associated with severe thrombocytopenia (e.g. R1308C, M1316V)6. Regardless of the mutation, or if it is not known, platelet count should be also closely monitored during pregnancy in women with this type. In some women, platelet transfusion has been used with platelet count < 30,000/μL, despite the fact that pseudo-thrombocytopenia is partly responsible for platelet lowering as assessed by the presence of platelet clumps on peripheral blood smear40. While this approach seems to be reasonable when invasive procedures are needed, its true clinical benefit remains unproven because of the rarity of the disorder. Factor replacement therapy can be given either by bolus dosing or by continuous infusion, with the decision based on practical considerations (ie: availability of specialized nursing and lab monitoring) as opposed to on data favoring one strategy over the other.
Women with type 2M VWD often show a significant correction of FVIII and VWF:Ag, while VWF:RCo does not reach levels of 50 U/dL. This is similar to the pattern observed after desmopressin in these patients and means that factor replacement should be used30,43. Despite adequate substitutive treatment, uterine atonia may play still a significant role in increasing the risk of bleeding and appropriate management should be carried out in addition to the hemostatic treatment.
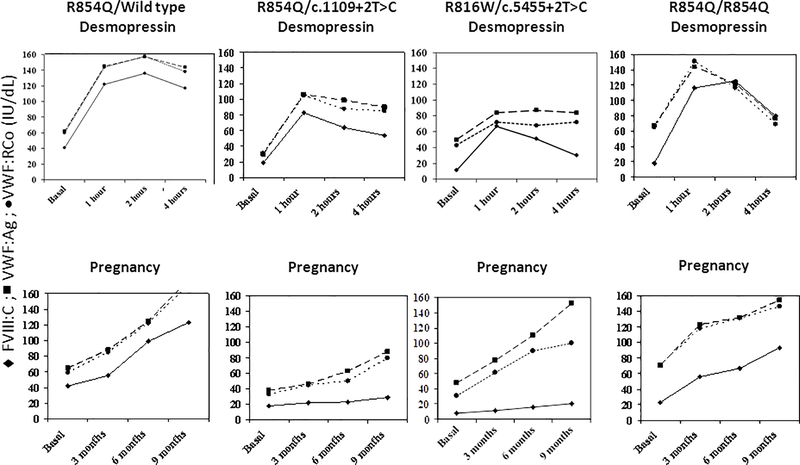
In type 2N VWD, normalization of FVIII, which is more reduced compared to VWF in this type, usually occurs during pregnancy in women heterozygous or homozygous for the most frequent mutation responsible for this type (R854Q)44, similar to what happens after desmopressin3,30,44. Usually, these women can be safely managed with desmopressin in case of bleeding complications (Figure 1). However, information about the more rare mutations is lacking and patients compound heterozygous with a null allele usually do not achieve a normal FVIII level and thus need treatment with VWF concentrates to stabilize FVIII level for a longer period (Figure 1). Again, the results of a desmopressin trial before pregnancy are helpful to predict its usefulness at parturition. Thus, in unresponsive women, during labor and before epidural anesthesia, 50 IU/kg of VWF should be administered, followed by 30–40 IU/kg/daily for at least 3 days. Daily monitoring of FVIII and VWF activity is recommended during the same period. Oral tranexamic acid is advised (15–25 mg/kg) up to 10–15 days.
Figure 1. Heterogeneity of laboratory phenotype in type 2 N von Willebrand disease.
Different pattern of response to desmopressin and of FVIII and von Willebrand factor changes in women with type 2 N von Willebrand disease according to the different mutational profile.
Women with type 3 VWD typically do not show any increase of FVIII and VWF during pregnancy because their endothelial VWF stores are lacking. Thus VWF/FVIII concentrates may be required during pregnancy to control intermittent vaginal bleeding and at delivery or for Cesarean section2,4,30,32. Some women with type 3 VWD may be on prophylaxis with a VWF concentrate prior to pregnancy, and this is generally continued throughout, with increasing doses appropriate for weight gain. Cesarean section should be reserved only for the usual obstetrical indications. Replacement therapy is as suggested above, but it usually should be prolonged up to 5–7 days to maintain FVIII:C (and possibly VWF) level > 50 U/dL4,32. The successful use of a continuous infusion of VWF concentrate has also been reported45.
Usual thrombo-prophylactic treatment with LMWH should be considered in patients at high risk of venous thrombosis during replacement therapy for caesarean section, especially if high FVIII levels are anticipated by using replacement treatment2.
Management of post-partum delayed risk of bleeding
FVIII and VWF fall to baseline levels soon after delivery27,30,46 and thus oral antifibrinolytic agents (e.g., tranexamic acid 1 g every 8 hours up to two weeks) can be used during this period to prevent delayed postpartum bleeding due to heavy lochia. Tranexamic acid appears to decrease the risk of delayed PPH45 and appears to be safe during lactation46. However, significant delayed bleeding may occur, especially in the more severe cases treated for a short period, requiring treatment with desmopressin or FVIII/VWF concentrates3,30.
Conclusions
Management of pregnancy is relatively easy in patients with type 1 VWD because usually a significant increase of FVIII and VWF does occur. However, close surveillance is always advisable because bleeding may sometimes occur even with normalization of the moieties. Qualitative type 2 variants need to be followed carefully because discrepant increase of FVIII and VWF occurs according to the unique peculiar pathophysiology of each variant. Type 3 VWD, the most clinically severe type, needs close follow-up because no increase of these moieties is observed and replacement treatment should be planned well before the end of pregnancy. The presence of VWD is not a reason to prefer caesarean section. In addition to desmopressin and FVIII/VWF concentrates, antifibrinolytics play a significant role as an ancillary treatment and during late post-partum to prevent excessive bleeding with lochia.
Acknowledgments
G.C. received grant supports directly to his Institution from CSL Behring and Pfizer. He participated in Advisory Boards or received speaker fees from CSL Behring, Shire, Kedrion outside the present work
P.D.J. received Research funding from CSL Behring, Bayer and Shire outside the present work
REFERENCES
- 1.Leebeek FWG, Eikenboom JCJ.Von Willebrand’s Disease. N Engl J Med 2016; 375: 2067–2080. [DOI] [PubMed] [Google Scholar]
- 2.Castaman G, Goodeve A, Eikenboom J, on behalf of the European Group on von Willebrand disease (EUVWD). Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica 2013; 98: 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castaman G, Lethagen S, Federici AB, Tosetto A, Goodeve A, Budde U, et al. Response to desmopressin is influenced by the genotype and phenotype in type 1 von Willebrand disease (VWD): results from the European Study MCMDM-1VWD. Blood 2008; 111: 3531–9. [DOI] [PubMed] [Google Scholar]
- 4.Rodeghiero F, Castaman G, Tosetto A. How I treat von Willebrand disease. Blood. 2009; 114: 1158–65. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci PM.Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years.Blood. 1997; 90(7):2515–2115. [PubMed] [Google Scholar]
- 6.Federici AB, Mannucci PM, Castaman G, Baronciani L, Bucciarelli P, Canciani MT, Pecci A, Lenting PJ, De Groot PG. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: a cohort study of 67 patients. Blood. 2009; 113: 526–34. [DOI] [PubMed] [Google Scholar]
- 7.Batlle J, Lopez-Fernandez MF. Von Willebrand factor/factor VIII concentrates in the treatment of von Willebrand disease. Blood Coagul Fibrinolysis 2009; 20:89–100. [DOI] [PubMed] [Google Scholar]
- 8.Gill JC, Castaman G. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood 2015; 126 (17): 2038–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CA, Kadir RA, Kouides PA. Inherited bleeding disorders in women. Wiley-Blackwell, Oxford, 2009. [Google Scholar]
- 10.McLintock C, James AH. Obstetric haemorrhage. J Thromb Haemost 2011; 9: 1141–51. [DOI] [PubMed] [Google Scholar]
- 11.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2(6): e323–33. [DOI] [PubMed] [Google Scholar]
- 12.Sentilhes L, Merlot B, Madar H, Sztark F, Brun S, Deneux-Tharaux C. Postpartum haemorrhage: prevention and treatment. Expert Rev Hematol 2016; 9:1043–1061. [DOI] [PubMed] [Google Scholar]
- 13.ACOG. ACOG Practice Bulletin: Clinical Management Guidelines for Obstetrician-Gynecologists. Number 76, October 2006: postpartum haemorrhage. Obstet Gynecol. 2006; 108: 1039–47. [DOI] [PubMed] [Google Scholar]
- 14.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum haemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010; 110: 1368–73. [DOI] [PubMed] [Google Scholar]
- 15.Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Budde U, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost 2006; 4: 766–73. [DOI] [PubMed] [Google Scholar]
- 16.Lak M, Peyvandi F, Mannucci PM. Clinical manifestations and complications of childbirth and replacement therapy in 385 Iranian patients with type 3 von Willebrand disease. Br J Haematol 2000; 111: 1236–9. [DOI] [PubMed] [Google Scholar]
- 17.James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost 2007; 5: 1165–9. [DOI] [PubMed] [Google Scholar]
- 18.Siboni SM, Spreafico M, Calò L, Maino A, Santagostino E, Federici AB, Peyvandi F. Gynaecological and obstetrical problems in women with different bleeding disorders. Haemophilia 2009; 15: 1291–9. [DOI] [PubMed] [Google Scholar]
- 19.Mannucci PM. Use of desmopressin (DDAVP) during early pregnancy in factor VIII-deficient women. Blood 2005; 105: 3382. [DOI] [PubMed] [Google Scholar]
- 20.Association of Hemophilia Clinic Directors of Canada. Hemophilia and von Willebrand’s disease: 2. Management. Association of Hemophilia Clinic Directors of Canada. Cmaj. 1995; 153:147–57. [PMC free article] [PubMed] [Google Scholar]
- 21.Siew DA, Mangel J, Laudenbach L, Schembri S, Minuk L. Desmopressin responsiveness at a capped dose of 15 mug in type 1 von Willebrand disease and mild hemophilia A. Blood Coagul Fibrinolysis 2014; 25: 820–823. [DOI] [PubMed] [Google Scholar]
- 22.Kirtava A, Drews C, Lally C, Dilley A, Evatt B. Medical, reproductive and psychosocial experiences of women diagnosed with von Willebrand’s disease receiving care in haemophilia treatment centres: a case–control study . Haemophilia 2003; 9: 292–7. [DOI] [PubMed] [Google Scholar]
- 23.James AH. More than menorrhagia: a review of the obstetric and gynaecological manifestations of bleeding disorders. Haemophilia 2005; 11: 295–307. [DOI] [PubMed] [Google Scholar]
- 24.Kouides PA, Phatak PD, Burkart P, Braggins C, Cox C, Bernstein Z, Belling L, Holmberg P, MacLaughlin W, Howard F.Gynaecological and obstetrical morbidity in women with type I von Willebrand disease: results of a patient survey . Haemophilia 2000; 6: 643–8. [DOI] [PubMed] [Google Scholar]
- 25.Chi C, Shiltagh N, Kingman CE, Economides DL, Lee CA, Kadir RA. Identification and management of women with inherited bleeding disorders: a survey of obstetricians and gynaecologists in the United Kingdom . Haemophilia 2006; 12: 405–12. [DOI] [PubMed] [Google Scholar]
- 26.Silwer J von Willebrand’s disease in Sweden. Acta Paediatr Scand Suppl 1973; 238: 1–159. [PubMed] [Google Scholar]
- 27.Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. Pregnancy in women with von Willebrand’s disease or factor XI deficiency. Br J Obstet Gynaecol 1998; 105: 314–21. [DOI] [PubMed] [Google Scholar]
- 28.Lavin M, Aguila S, Dalton N et al. Significant gynecological bleeding in women with low von Willebrand factor. Blood Adv 2018; 2(14);1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castaman G, Tosetto A, Rodeghiero F. Shortened von Willebrand Factor survival: pathophysiologic and clinical relevance. J Thromb Haemost 2009; 7 (Suppl 1): 71–4. [DOI] [PubMed] [Google Scholar]
- 30.Castaman G, Tosetto A, Rodeghiero F. Pregnancy and delivery in women with von Willebrand’s disease and different von Willebrand factor mutations. Haematologica 2010; 95: 963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodeghiero F, Castaman G, Tosetto A, Batlle J, Baudo F, Cappelletti A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost 2005; 3: 2619–26. [DOI] [PubMed] [Google Scholar]
- 32.Mannucci PM. Treatment of von Willebrand’s Disease. N Engl J Med. 2004; 351: 683–94. [DOI] [PubMed] [Google Scholar]
- 33.Haberichter SL, Castaman G, Budde U et al. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD). Blood. 2008; 111: 4979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castaman G, Eikenboom JCJ, Contri A, Rodeghiero F. Pregnancy in women with type 1 von Willebrand disease caused by heterozygosity for von Willebrand factor mutation C1130F. Thromb Haemost. 2000; 84: 351–2. [PubMed] [Google Scholar]
- 35.Castaman G, Federici AB, Bernardi M, Moroni B, Bertoncello K, Rodeghiero F. Factor VIII and von Willebrand factor changes after desmopressin and during pregnancy in type 2M von Willebrand disease Vicenza: a prospective study comparing patients with single (R1205H) and double (R1205H-M740I) defect. J Thromb Haemost. 2006; 4: 357–60. [DOI] [PubMed] [Google Scholar]
- 36.Castaman G, Tosetto A, Federici AB, Rodeghiero F. Bleeding tendency and efficacy of anti-haemorrhagic treatments in patients with type 1 von Willebrand disease and increased von Willebrand factor clearance. Thromb Haemost. 2011;105(4): 647–54. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Luceros A, Meschengieser SS, Turdó K, Arizó A, Woods AI, Casais P, et al. Evaluation of the clinical safety of desmopressin during pregnancy in women with a low plasmatic von Willebrand factor level and bleeding history. Thromb Res. 2007; 120: 387–90. [DOI] [PubMed] [Google Scholar]
- 38.Tosetto A, Castaman G. How I treat type 2 variant forms of von Willebrand disease. Blood. 2015;125:907–14. [DOI] [PubMed] [Google Scholar]
- 39.Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost. 2002; 88: 378–9. [PubMed] [Google Scholar]
- 40.Kruse-Jarres R, Johnsen JM. How I treat type 2B von Willebrand disease. Blood. 2018; 131(12): 1292–1300 [DOI] [PubMed] [Google Scholar]
- 41.Giles AR, Hoogendoorn H, Benford K. Type IIB von Willebrand’s disease presenting as thrombocytopenia during pregnancy. Br J Haematol. 1987; 67: 349–53. [DOI] [PubMed] [Google Scholar]
- 42.Rick ME, Williams SB, Sacher RA, McKeown LP. Thrombocytopenia asscociated with pregnancy in a patient with type IIB von Willebrand’s disease. Blood. 1987; 69: 786–9. [PubMed] [Google Scholar]
- 43.Federici AB, Mazurier C, Berntorp E, Lee CA, Scharrer I, Goudemand J, et al. Biologic response to desmopressin in patients with severe type 1 and type 2 von Willebrand disease: results of a multicenter European study. Blood. 2004; 103: 2032–8. [DOI] [PubMed] [Google Scholar]
- 44.Castaman G, Bertoncello K, Bernardi M, Rodeghiero F. Pregnancy and delivery in patients with homozygous or heterozygous R854Q 2N VWD. J Thromb Haemost 2005; 3: 391–2. [DOI] [PubMed] [Google Scholar]
- 45.Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia 2016; 22: 906–11. [DOI] [PubMed] [Google Scholar]
- 46.Gilad O, Merlob P, Stahl B, Klinger G. Outcome following tranexamic acid exposure during breastfeeding. Breastfeed Med. 2014; 9: 407–W2154/*-+10. [DOI] [PubMed] [Google Scholar]