Abstract
Microplastics (MP) are ubiquitous within the environment, but the approaches to analysis of this contaminant are currently quite diverse, with a number of analytical methods available. The comparability of results is hindered as even for a single analytical method such as Fourier transform infrared spectroscopy (FT-IR) the different instruments currently available do not allow a harmonized analysis. To overcome this limitation, a new free of charge software tool, allowing the systematic identification of MP in the environment (siMPle) was developed. This software tool allows a rapid and harmonized analysis of MP across FT-IR systems from different manufacturers (Bruker Hyperion 3000, Agilent Cary 620/670, PerkinElmer Spotlight 400, and Thermo Fischer Scientific Nicolet iN10). Using the same database and the automated analysis pipeline in siMPle, MP were identified in samples that were analyzed with instruments with different detector systems as well as optical resolutions and the results discussed.
Keywords: Microplastic, quantification, identification, FT-IR imaging, automated analysis, Raman, FT-IR, micro-FT-IR, wastewater
Introduction
Small plastic particles, called microplastics (MP) are currently recognized as a potential risk for environmental and human health.1,2 The near-ubiquitous contamination of the environment, from terrestrial soils and air to the freshwater and marine environments has raised the profile of this topic in recent years, leading to a wealth of methods and approaches for sampling and analyzing MP in environmental matrices.3–11 In general, these particles are defined as <5 mm in size while a lower size limit and a standard definition of MP has yet to be agreed on.7,12 Subcategories distinguishing between large MP (5 mm–500 µm) and small MP (500–1 µm)13 are often used, reflecting practical considerations during the full analytical procedure.
The analytical procedure to identify particles can be divided into three steps, starting with sampling for MP, followed by sample extraction and finally, identification and quantification. Each of these steps has its challenges (cf. Lusher et al.,3 Brander et al.,4 Primpke et al.,6 and Cowger et al.14). Additionally, there is an increasing awareness for quality assurance and quality control (QA/QC) to successfully and reliably identify MP in environmental samples.15,16 Individual steps for QA/QC are currently discussed within this overarching special issue.4 While QA/QC is important for the quality of the results, the intercomparison of studies is further hampered by a missing harmonization of the three steps: sampling, sample extraction, and identification. Especially for the chemical analysis, a plethora of different methods and software are used to interpret generated data.5,6,14 Among the spectroscopy-based techniques, Fourier transform infrared spectroscopy (FT-IR) is considered most suitable to analyze MP from 5 mm to ∼10 µm, even if it requires different acquisition modes according to the particle size.6 Micro-FT-IR (µFT-IR) imaging17–20 permits scanning of the whole sample, avoiding the manual sorting/point-and-shoot steps that are otherwise necessary, which are a source of bias as the analysis becomes operator dependent.21,22 On the other hand, FT-IR imaging for MP analysis produces a huge amount of data (large areas are scanned at a very high spatial resolution) which are difficult to manage using the commercial software provided by the instrument manufacturers.4 Challenges handling this data, together with the lack of a suitable software tool, not only impedes a reliable workflow but also makes it difficult, if not impossible, to compare between studies performed using FT-IR Imaging.14 While initial studies comparing various analytical methods have been conducted,23 a comparison between studies using the same analytical technique but on different instruments is missing for MP research. One reason is the lack of a suitable software tool to perform such a comparison. Such a study, however, is essential to reproduce and compare results within meta-studies. Moreover, it allows the harmonized analysis of MP in the future, as the advantages and disadvantages of the currently available high-throughput analysis pipelines for individual systems can be determined.
To achieve this goal, a new software tool is presented and intercalibrated with existing studies.24 Its performance is evaluated via the analysis against existing reference data sets and comparison of the achieved results. Furthermore, the tool allows the analysis of data generated by different FT-IR imaging techniques from various manufacturers. This is demonstrated through the application of the software to data sets from state of the art instruments from four major manufacturers, namely Agilent, Bruker, PerkinElmer, and ThermoFisher Scientific, with different types of detection modes ranging from focal plane array (FPA), linear arrays to single-element detectors. This analysis is followed by a short performance evaluation of the assigned spectra. The software and corresponding reference spectra databases24 are available free of charge via the internet and can be used by everyone for the analysis of FT-IR data.
Materials and Methods
Sample Extraction and Analysis Using the Agilent System
Sample preparation started with an effluent sample (Ryaverket wastewater treatment plant [WWTP], Götheborg, Sweden) as collected using filtration with a custom filtration device containing a ø100 mm stainless steel filter (10 µm mesh size).
The material collected on the steel filter mesh was treated to extract MP using a method derived by Löder et al.13 and modified by Liu et al.22 The filter was sonicated into filtered Milli-Q water containing 5% (w/v) sodium dodecyl sulfate (SDS) to detach the solids and left stirring (100 rpm) at 50 ℃ for 48 h. The resulting suspension was then filtered onto a 47 mm steel filter (10 µm mesh). The particles trapped were re-suspended and first incubated with proteolytic enzymes (Alcalase, Novozymes, Denmark) in a Tris buffer (pH 8.2, stirring at 100 rpm, and 50 ℃ for 48 h), filtered onto a 47 mm steel filter, and the solids were then removed from the filter surface. A second enzymatic treatment was performed using cellulolytic enzymes (Cellulase blend and Viscozyme, Sigma-Aldrich) in acetate buffer (pH 4.8, stirring at 100 rpm and 50 ℃ for 48 h) to eliminate the majority of the organic fraction of the sample matrix. The remaining undigested matter was filtered onto a 47 mm steel filter, and the solids were again removed from the filter surface. The gathered solids were oxidized using Fenton reaction (hydrogen peroxide catalyzed by Fe(II) at ∼20 ℃ for 24 h). After a further filtration on a 47 mm steel filter, the solids were removed from the filter surface and recovered. MP were further separated from the inorganic particles in a zinc chloride solution (1.7 g cm−3) using a glass separatory funnel. After discharging the settled material, the supernatant was filtered (47 mm steel filter) and the material was recovered following the same procedure described for the previous steps using 50% (v/v) ethanol. The extracted MP were transferred in 10 mL glass headspace vial, the liquid was evaporated at 55˚C, and finally, 5 mL 50% (v/v) ethanol solution were added to obtain a known volume in the vial.
In order to minimize MP contamination deriving from the equipment used for sampling and sample preparation, all lab tools were flushed with filtered (1.2 µm) Milli-Q water three times before use. Tools made of glass or metal or which were coated with PTFE were used instead of plastic whenever possible. Sample containers were covered with aluminum foil to reduce airborne contamination, and steel filters were muffled at 500 ℃ before usage.
The µFT-IR analysis was performed using a FPA-based µFT-IR imaging technique provided by a Cary 620 µFT-IR microscope from Agilent Technologies (USA) coupled with a Cary 670 FT-IR spectroscope. The instrument was equipped with a 128 × 128 FPA/mercury–cadmium–telluride (MCT) imaging detector (FPA-MCT-imaging detector). The analysis was carried out in transmission mode, using a 15 × Cassegrain (visible IR) objective-condenser system which produces 5.5 µm pixel resolution on the FPA detector. An aliquot of the sample (600 µL) was deposited onto a ø13 mm × 2-mm-thick zinc selenide (ZnSe) transmission window. A background scan was collected before each sample scan on a clean window at 8 cm−1 spectral resolution, using 120 co-added scans in the spectral range of 3750–850 cm−1. Subsequently, an area of 14 × 14 tiles was scanned on the samples window, using 30 co-added scans, and the same settings as for the background scan. The analyzed area covered the entire active surface of the windows (diameter of 10 mm, area 78.5 mm2), recording the spectra of all deposited particles.
Sample Extraction and Analysis Using the PerkinElmer System
The sample represents a composite sample taken at 30-min intervals across a 24-h period, directly sampling from the effluent of a WWTP. The auto-sampler filtered this water through a woven stainless steel cylindrical filter cartridge (27.8 cm long, nominal pore size 10 µm, ∼500 cm2 filter area; Wolftechnik, Germany). The concentrated sample was transferred from the filter into dispersion for processing in the lab. Processing in order to “clean” the sample in preparation for µFT-IR analysis consisted of two steps: a Fenton's reaction to chemically degrade organic material and enzyme digestion to remove cellulosic and proteinaceous material.
The Fenton's reaction used a Fe(II) solution (0.05 M FeSO4°7 H2O, Fischer Scientific, USA, >98% purity) acidified with 0.2% sulfuric acid (H2SO4, AnaTaR, 98.07% purity) and 30% hydrogen peroxide (H2O2, Fisher Scientific). The reaction was allowed to exhaust itself overnight before the sample was filtered and re-dispersed for enzymatic digestion. The enzyme digestion steps utilized cellulase in a pH 5, phosphate-buffered saline solution (MP Biomedicals, USA) incubated at 50 ℃ for 48 h and trypsin at 37 ℃ for 30 min (Sigma-Aldrich, Germany). The final concentrated sample was dispersed and stored in 50% ethanol (Sigma-Aldrich, Germany) before depositing onto 25 mm diameter 5.0 µm pore size silver membrane filters (Sterlitech, USA) for µFT-IR analysis. All reagents were filtered through a 1.2 µm glass-fiber filter before use, and all processing took place in the Microflow Biological Safety Cabinet, fitted with HEPA filters to control for airborne microplastic contamination during the processing of samples.
For the µFT-IR analysis, a PerkinElmer Spotlight 400 FT-IR microspectrometer with MCT detector was used for the analysis of the sample. The silver filter containing the processed sample was mounted on a glass slide and held in place with a clamped stainless steel O-ring. The spectrometer collected spectra in the range between 4000 and 700 cm−1 in reflectance mode. A background spectrum was collected for each sample from a blank area of the silver filter at a spectral resolution of 8 cm−1 and pixel size of 25 µm. A total of 90 scans were taken per pixel with an interferometer speed of 2.2 cm s−1. An optical image was first collected by tiling single field of view images together to cover an area of approximately 13 mm × 13 mm. A smaller mapping area for the FT-IR spectrum of 11.6 mm × 11.6 mm was selected (92 % of the filter area), due to constraints on the file size that could be generated by the PerkinElmer SpectrumIMAGE software. The FT-IR mapping was performed with the same parameters as that of the background scan, but at four scans per pixel. Atmospheric correction was performed on the resulting .fsm file.
Sample Extraction and Measurement Using the ThermoFisher Scientific System
The sample was taken by filtering water at the effluent of a WWTP over a stainless sieve (ø20 cm, ThermoFisher Scientific) having a mesh size of 20 µm. The concentrated sample was exposed successively to SDS (one day, 5%, Serva Electrophoresis GmbH, Germany), potassium hydroxide (five days, 10%, Carl Roth GmbH, Germany), and hydrogen peroxide (two days, 32%, Carl Roth GmbH, Germany). During all steps, the sample was incubated in an oven with a temperature set at 35 ℃. In between steps, the sample was filtered over a ø47 mm stainless steel filter with a mesh size of 20 µm. Inorganic particles were removed by performing a density separation using a zinc chloride (ZnCl2, Carl Roth GmbH, Germany) solution with a density of 1.6 g cm−3. Subsequently, sample residues were filtered on an aluminum oxide filter (Anodisc 25 mm, Whatman, UK) which was then dried at 35 ℃ for several days.
In order to minimize MP contamination, all chemicals were filtered through stainless steel filters with a mesh size of 10 µm. Additionally, all lab equipment was thoroughly rinsed before usage, and the lab surfaces cleaned with ethanol (30%, Carl Roth GmbH and Co. KG, Germany) and Milli-Q water. Whenever possible, plastic equipment was reduced by tools made of glass or metal, and when finishing sample handling these were immediately covered with aluminum foil to reduce airborne contamination.
For the µFT-IR analysis, an FT-IR microscope equipped with a single MCT detector (Nicolet iN10, ThermoFisher Scientific, USA) was used. For the measurements, an Anodisc filter was placed on a calcium fluoride crystal (EdmundOptics, Germany). About one third of each filter was mapped in transmission mode, with one scan recorded per pixel, the aperture size set at 50 × 50 µm, the spectral resolution as 16 cm−1, and the spatial resolution at 20 µm. A background scan using the same settings was conducted on a blank area of the same filter.
Sample Extraction and Measurement Using the Bruker System
For this comparison study, a data set of a sample investigated in previous studies25,26 was chosen. The sample was from the effluent of the WWTP Holdorf. Sample location and further sampling information are available within the previous studies.25,26 Briefly summarized, the sample was directly taken from the effluent of the WWTP. The sample was processed by the enzymatic digestion protocol of Löder et al.13 and subsequently concentrated onto an Anodisc filter (25 mm diameter, 0.2 µm pore size, GE Whatman). The sample was placed and covered by a BaF2 window prior to measurement on a custom-made sample holder as described in detail in Primpke et al.25
The µFT-IR measurement was performed using a Bruker TENSOR II spectrometer, which is connected to a Hyperion 3000 µFT-IR-microscope (Bruker Optics GmbH, Ettlingen, Germany). The spectra were collected using a 64 × 64 FPA MCT detector as described in literature.19 Prior to measurement, a visual image of the sample surface was recorded. The FT-IR measurements were performed using 15 × Cassegrain objective, in the spectral range of 3600–1250 cm−1 with 4 × 4 binning at a spectral resolution of 8 cm−1 and six coadded scans. With this setup, a pixel size of 11.1 × 11.1 µm per spectra was achieved. All data were collected using Bruker software OPUS 7.5 (Bruker Optics GmbH, Germany).
Sample Preparation of Algae Samples
Ecotoxicity tests were carried out with the microalga Raphidocelis subcapitata with modifications according to “Water Quality–Freshwater Algal Growth Inhibition Test with Unicellular Green Algae, ISO Standard 8692, 2004”. The test included five replicates of the control sample, in which algae were not exposed to the toxicant, and triplicates of algae exposed to five different concentrations of the tested toxicant. The replicates were combined after the toxicity test, and algae from all treatments were preserved with Lugol's iodine solution for their infrared analysis.
For the infrared analysis described in Kansiz et al.,27 2 mL of the preserved control sample was prepared. The cells were centrifuged, and the residues of the preservative and growth media were washed off to prevent interference with algae spectra. The purified cells were resuspended in 200 µL deionized water, and the entire volume was deposited on a 13 mm diameter, 1-mm-thick CaF2 transmission window, and dried in a vacuum desiccator.
A Cary 620 µFT-IR microscope from Agilent Technologies (USA) coupled with a Cary 670 FT-IR spectroscope was used for the FPA-based µFT-IR imaging analysis of the algae cells. The analysis was carried out in transmission mode using the 15 × Cassegrain objective in high magnification mode to create a mosaic with 1.1 μm pixel resolution on the FPA. A background scan was collected on a clean window in the range of 3750–850 cm−1 with an 8 cm−1 spectral resolution applying 256 co-added scans. An area of 6 × 6 tiles was scanned on the sample window following the background scan with the same parameters applying 240 co-added scans per pixel.
Software and Database
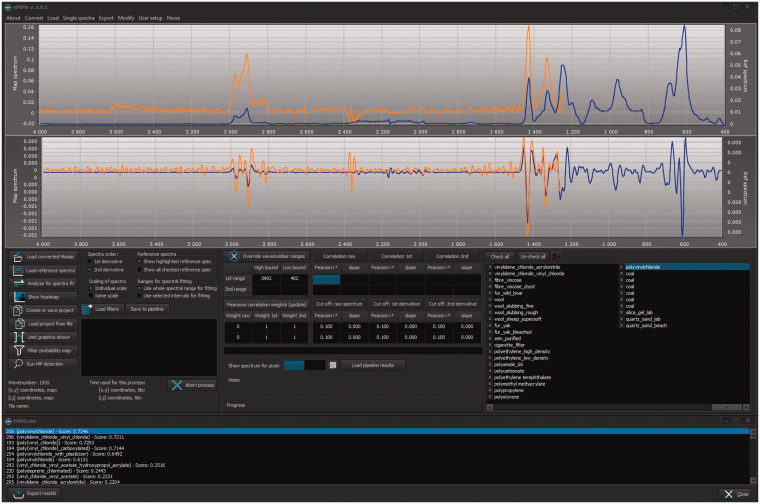
The software siMPle is the combination of the software MPhunter presented in Liu et al.22 and the automated analysis of Primpke et al.21 It is written in Delphi using Windows 10 as the operating system, which is available at www.simple-plastics.eu, where reference databases for FT-IR and Raman spectroscopy are also provided (Fig. 1).
Figure 1.
The software graphical user interface with a loaded reference (blue) and a sample spectrum (orange) using the Match single spectrum function of siMPle on a spectrum assigned as polyvinylchloride using ESM1.csv.
In this work, the database for automated analysis24 and the release version (1.0.0) of siMPle was used to analyze all data sets. Prior to analysis, all spectra were converted from the original file format (Agilent:.dmd and PerkinElmer:.fsm) or JCAMP-DX files (Bruker and Thermo Fisher Scientific) into two siMPle file formats, namely .spe and .wno files, allowing fast data access (see How to Use siMPle.pdf Supplemental Material file for further instructions). These file formats are accompanied by an extra file, the MaschineData.ini, which contains all necessary information for size calculations and data handling. In all cases, the mosaic structure is either kept or newly generated, allowing faster data handling. After loading the data and reference spectra, the spectral fit between the two was calculated by Pearson correlation for the untreated data, the first derivative and the second derivative, resulting in their correlation factors r0, r1, r2, respectively. If not further specified, the following settings were used: omit CO2 peak (upper wavenumber: 2420 cm−1, lower wavenumber: 2200 cm−1), suppress negative correlations, and include second-order derivatives.
To investigate the performance between Bruker OPUS and siMPle, the calculation times were measured using a HP KP719AV computer (Intel Core 2 Duo Processor, 8 GB RAM, AMD Radeon HD 5450 graphic card, extra USB 3.0 Controller card and SANDISK Extreme 64 GB USB-Stick) which is the same as used in previous studies.21,24–26,28–34 Further calculations were performed on a HP Z440 workstation (Intel Xeon E5-2630 v.3 CPU, 64 GB RAM, NVidia Quadro M2000 graphics card) for all other purposes.
The automated analysis pipeline (AAP)21 identifies the recorded spectra based on the results of the Pearson correlation factors (r) calculated for the respective untreated spectra r0 and the first derivatives r1. Only if maximum values of r0 and r1 are assigned to the same polymer entry, then the spectra are counted as identified and the polymer type added to the list of analyzed pixels together with the x,y coordinates and the summarized hit quality index (HQI, Eq. 1):
| (1) |
This type of data represents a false color image which was then in silico treated by Image Analysis as described in Primpke et al.21 by a pixel hole closing mechanism prior to the size determination and particle quantification. For further calculations, the data thresholds described in Lorenz et al.32 were used. To avoid confusion for the reader, we did not apply the second analysis pipeline1,22,35 available in siMPle for the scope of this study.
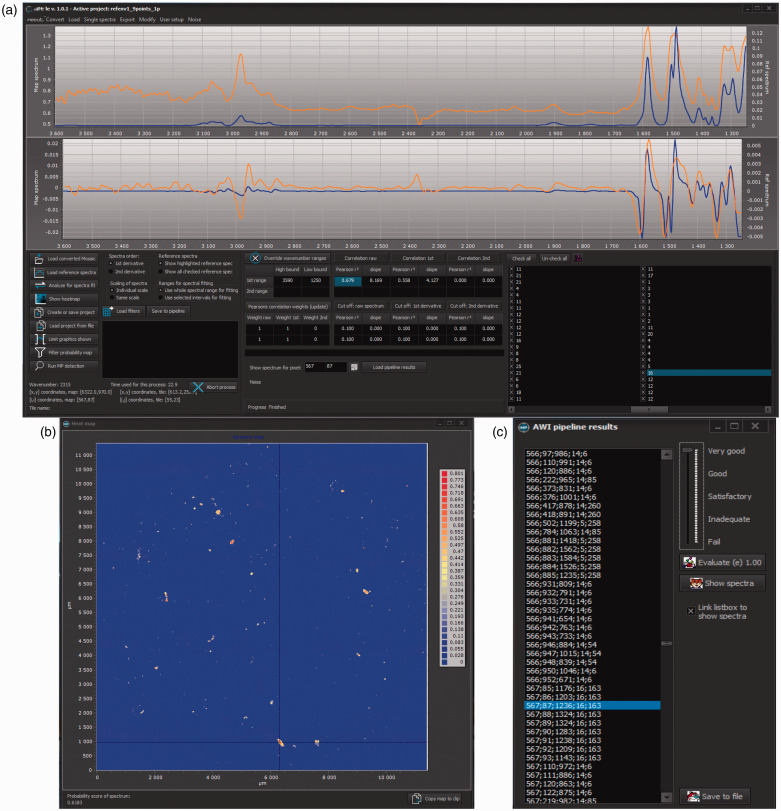
The siMPle software allows rapid QA/QC for the assigned polymer hits. During image analysis, a designated file is generated named “_forqc.csv,” which contains x,y coordinates of the measured spectra together with the hit quality, the assigned polymer type, and a reference spectrum identifier. Via the button “Load Pipeline Results,” the QA/QC process will be started (Fig. 2).
Figure 2.
Quality assurance/quality control for siMPle for results of the image analysis of sample RefEnv124 via the AWI pipeline allowing a direct comparison (a) between sample spectrum (orange) and database spectrum (blue) by clicking on the determined pixel (c). The process allows checking other spectra by clicking on another database entry as well as the individual Pearson correlation factors. The heatmap (b) allows the user to locate the assigned spectra on the map.
Using a designated window, each spectrum can be individually assessed and rated from a perfect assignment down to a full misassignment with values ranging from 1 to 0.01. Following the approach of Primpke et al.,21 the spectra were rated with five different values (1.00, 0.75, 0.5, 0.25, and 0.01) ranging from best match to a full misassignment, respectively. To screen the data of the different instruments and evaluate the successful assignment of polymer identify to particles, a randomly selected number (n = 100) of spectra were manually reanalyzed for each instrument data set.
For matching single spectra, the score used within siMPle was first described in Liu et al.22 In short, prior to the quantification of particles, the score (Sd) for the identification of the polymer type was calculated using Eq. 2:22
| (2) |
Each result (r0,r1,r2) was squared and multiplied by a weight (k0,k1,k2, respectively) for the respective correlation factor, which can be assigned by the user.
Results and Discussion
The software allows two types of data analyses: first, the matching of single spectra and second, the analysis of large filter areas. To interpret a single spectrum, the reference database must first be loaded and then a single spectrum in a defined file format (Paragraph S1; ESM1.csv, Supplemental Material) must be loaded (Fig. 1). In this example, the spectrum was assigned to polyvinylchloride (PVC) with a Sd of 0.7246 using the default options of siMPle (k0 = 0, k1 = 1, k2 = 1). By assigning weights, the user can decide which correlation method should be represented by Sd, e.g., for comparison with studies using Bruker OPUS28,30–33 where only the first derivative was used for single spectrum analysis. For the chosen example, values of k0 = k2 = 0, k1 = 1 (first derivative only) were used. In this case, the Sd decreased to 0.6689. If the user decides to include all correlation results (k0 = k1 = k2 = 1), the Sd was further decreased to 0.6435. Therefore, it is mandatory to state the weights k0, k1, k2 within the material and methods section for comparison of studies if siMPle is used. In general, this analysis is independent from the instrumental source of the data. All types of data in the described file format (see Paragraph S1) can be processed independently from manufacturer and measurement method. An example of this using preprocessed Raman spectra is shown in Fig. S1. Future data processing steps for single spectra or lists of spectra will be introduced in future releases. All releases will be accompanied via a change log and a living manual on the website.
During this process, QA/QC is easily available, because the spectrum with the highest hit is indicated at the end of the analysis together with a hit list for all other database entries (Fig. 1). Together with this, the hit result can be exported for further use.
Analysis of Chemical Imaging Files
For larger data sets, the siMPle software allows a time-efficient loading of data using a harmonized file system for data storage which is independent from the original file format. The file formats introduced were optimized for the fast use within the software. The commonly shared file format by the International Union of Pure and Applied Chemistry (IUPAC) J-CAMPdx file format was limited to the import of data, due to long loading times of such text type based files. Currently, siMPle is able to convert native data from Agilent and PerkinElmer systems using the file import function while for Bruker and ThermoFisher Scientific systems extra steps are necessary (see How to Use siMPle.pdf, Supplemental Material).
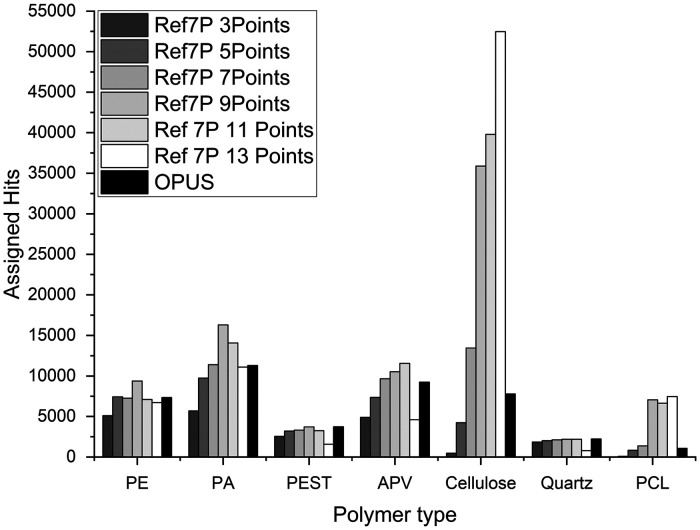
To validate the performance of siMPle, it was tested against existing reference data sets from literature. These reference sets consist of materials of known origin (Ref7P) or from environmental samples (RefEnv1 and RefEnv2). These three samples were analyzed using siMPle and the results were compared to the automated analysis via Bruker OPUS. Starting with Ref7P, we started to analyze the performance on an artificial sample only containing MP (polyethylene (PE), polyester (PEST), polyamide (PA) and polyurethane (PU)), cellulose, quartz, and diatomaceous earth. Out of these seven particle types, only diatomaceous earth could not be detected within the wavenumber range of Anodisc. In comparison to OPUS, siMPle identified almost twice the number of spectra on the specific polymer types (Fig. 3). Especially, cellulose (plant fiber in the database) and polycaprolactone (PCL, not originally introduced as a material) were affected by factors larger than four (Fig. 3, Ref7P 9Points and OPUS).
Figure 3.
Assigned polymer types for sample Ref7P24 using different smoothing factors by siMPle (3 to 13) versus OPUS with an unknown fixed value for polyethylene (PE), polyamide (PA), polyester (PEST), acrylates/polyurethanes/varnish (APV), cellulose, quartz and polycaprolactone (PCL).
This difference was rather striking and the main difference between both kinds of software was found in the data handling for the calculation of the first derivative. In the default settings, siMPle adds a nine data points smoothing to reduce the noise. For Bruker OPUS, it is not documented if smoothing is applied. To test for a better comparison of the results, a range of this value from 3 to 13 data points was investigated for siMPle (Fig. 3).
By screening the number of data points for smoothing, it was found that an optimal hit was reached with nine data points for most polymer type assignment (Fig. 3). Only cellulose kept an increase in assigned polymer hits while PCL reached a constant level. The data determined by OPUS could not be assigned to a smoothing factor applied by siMPle. Still, for this particular sample, the high number of assignments to PCL started using this number of data points for smoothing. To avoid any misassignment issues, a manual reanalysis on the assigned spectra to PCL was performed.
Through quality assurance, it was found that the spectra assigned to PCL were caused by a misinterpretation of the measured PEST spectrum. This spectrum has a high similarity with PCL in transmission, which was not visible by using Bruker OPUS during cluster performance analysis.24 In Fig. S2, one of the assigned spectra from Ref7P is plotted against the assigned reference spectrum and the spectra of the original material. The original database states this material as a pure PEST, but via an extended material research, it was found that the material was meanwhile relabeled to copolyester by the manufacturer. Due to these differences, the material could not be assigned to the original PEST cluster, as no pure PEST spectrum was yielded. This issue will be addressed in the future by a database update including more materials and using siMPle for cluster performance analysis. All samples in the following were analyzed using the default nine data points smoothing.
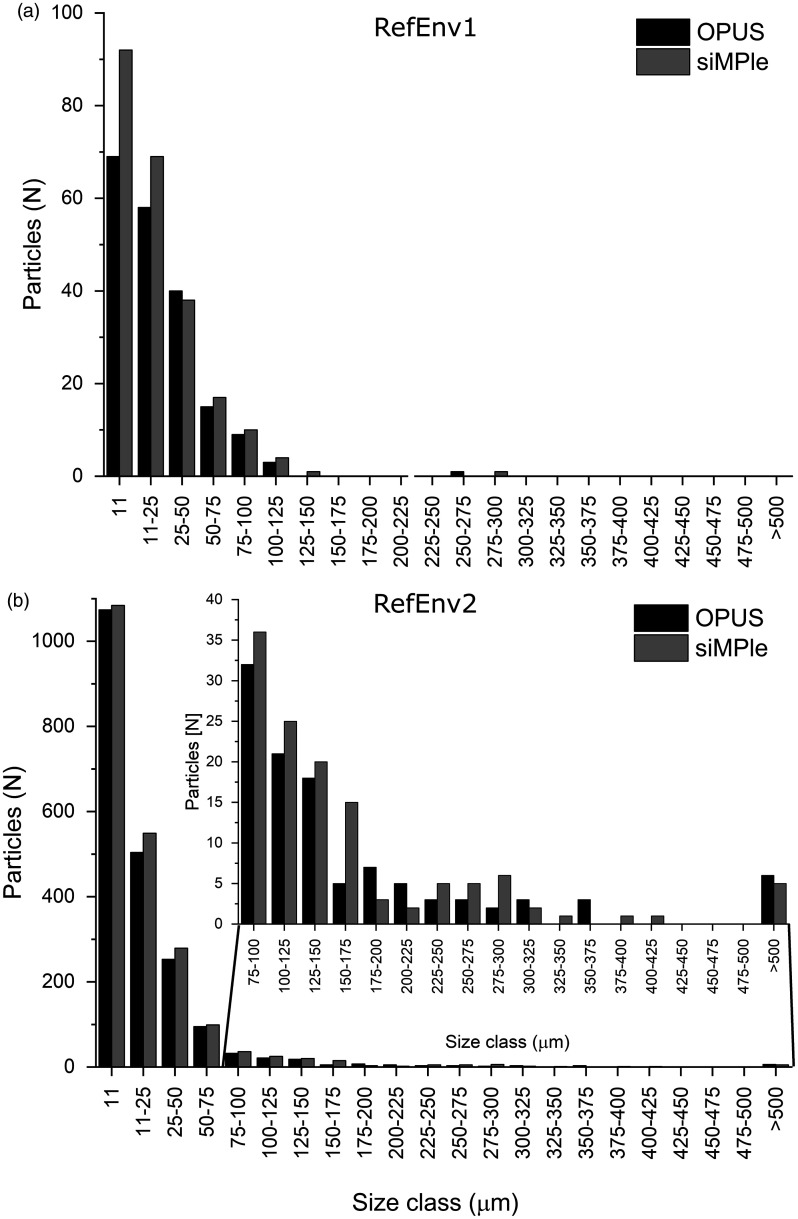
The data sets RefEnv1 and RefEnv2 were also analyzed using siMPle and OPUS. The siMPle analysis required only 2 h for RefEnv1 and 3 h for RefEnv2, which is 12 times faster than the analysis with OPUS using spectral correlation only for raw and first derivative data. With a look at the polymer composition (see Table S1 for details), it was found that siMPle was more sensitive and identified higher numbers of polymers and also larger sized polymer particles (Fig. 4) in comparison to OPUS.
Figure 4.
MP size classes derived from the automated image analysis21 for the reference data sets (a) RefEnv124 and (b) RefEnv224 analyzed using Bruker OPUS and siMPle.
Both analyses found a strong trend toward smaller MP sizes. Especially striking was the higher identification rate for cellulose (plant fiber) during the analysis with siMPle (see RefEnv2, Table S1). Sample RefEnv2 also showed the largest differences in the size distribution and showed a better particle assignment compared to the data derived via OPUS (Fig. S3).
Here, it was found that larger particles were identified more accurately with siMPle in comparison to OPUS, which missed areas of larger particles yielding in a separation into two particles. Furthermore, the analysis using siMPle improved closing holes, which is important for morphological analysis of the particles, see for example, the large PP particle on the rightmost edge of the filter. In summary, these results show that data determination with siMPle is better suited for the analysis of imaging data due to transparent data handling and easy data validation.
To test the ability for a harmonized analysis of MP, the performance of siMPle was assessed for the FT-IR imaging data from instruments from the four mentioned manufacturers. In this case, the computation of a full spectral analysis based on Pearson correlation for the untreated data, the first derivative and the second derivative were performed.22 The calculation time was determined on the same computer systems applied for the automated analysis via the OPUS software of the Bruker data set. This allows a comparison of calculation times with existing studies using OPUS.26,28 The determined calculation times are summarized in Table 1 together with further information on the data sets, such as pixel size on the filter area, the size of the analyzed area and the number of spectra recorded.
Table I.
Calculation times of the different data sets measured on systems of four different manufacturers using siMPle.
| Data set | Size .spe | Spectra | Pixel size | Calculation time | Calculation performance spectra | Filter area measured |
|---|---|---|---|---|---|---|
| GB | N | µm | s | s | mm | |
| Agilent | 9.01 | 3 211 264 | 5.5 | 29 979 | 107 | 10 × 10 |
| Bruker | 4.14 | 1 806 336 | 11.05 | 16 464 | 110 | 14.9 × 14.9 |
| PerkinElmer | 0.66 | 215 296 | 25 | 2877 | 75 | 11.6 × 11.6 |
| ThermoFisher Scientific | 0.25 | 221 184 | 20 | 1129 | 195 | 11.5 × 7.2 |
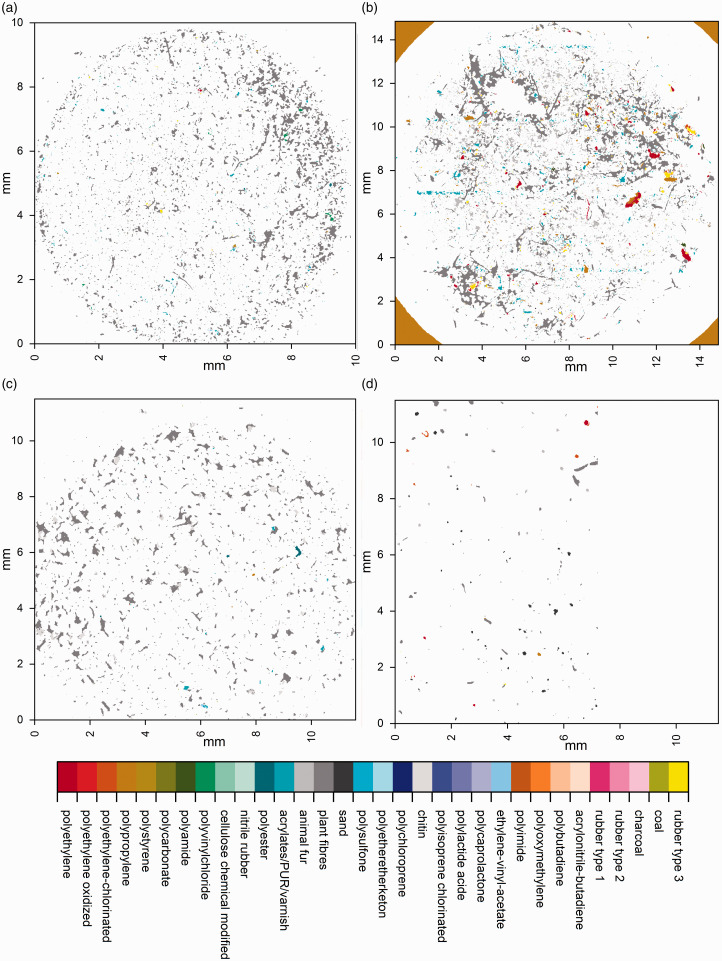
As mentioned previously, the calculation time on the Bruker data sets was reduced considerably when applying siMPle (5 h, in comparison to 48 h using OPUS) which also included the second derivative (it was omitted during OPUS analysis). When the calculation time was normalized to the number of analyzed spectra, the spectra from Thermo Fisher Scientific were correlated twice as fast compared to the other data sets. The reason is the spectral resolution of 16 cm−1 instead of 8 cm−1. Still, one has to keep in mind that the Bruker system and the Thermo Fisher Scientific system need an additional transformation step within their respective software which increases the overall handling and calculation times independent from siMPle. In Fig. 5, the false color images of the analyzed samples are shown.
Figure 5.
Overview images of the measured filters using the automated analysis pipeline for the (a) Agilent, (b) Bruker, (c) PerkinElmer, and (d) Thermo Fisher Scientific samples. Sample (d) was measured in a rectangular shape and the area of on the right side was left blank to avoid irritations.
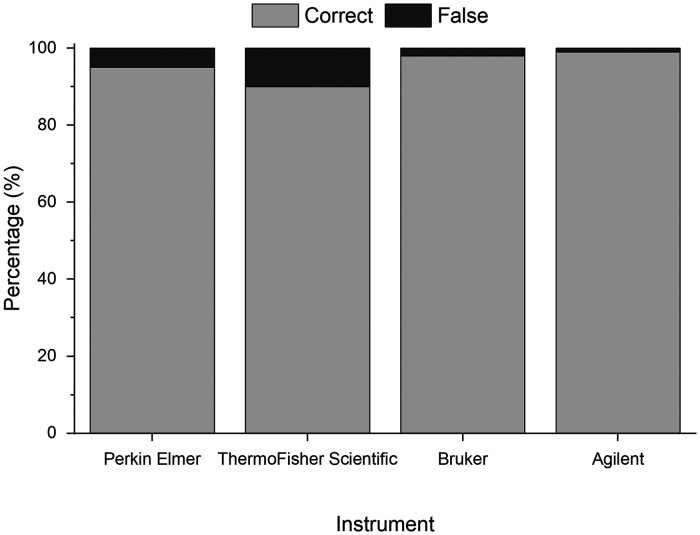
Qualitatively, it can be observed from the images that the samples are similar in nature, containing a large proportion of natural materials (Fig. 6, gray colors), among which a number of artificial polymers are successfully identified, irrespective of the manufacturer of the instrument or the various sampling and extraction methods employed prior to analysis (see ESM2.xslx, Supplemental Material, for details). Due to the varying nature of the WWTP sampled from, and the variety of sampling and extraction methods utilized between samples, commentary on any differences in enumeration of MP between the samples is beyond the scope of this study. However, application of the software on these real-world example data sets demonstrates promising consistency in the proportion of particles identified which are of synthetic origin (3–25%) and of the major polymer types which are identified across the samples (see ESM2.xslx). Compared to existing commercial software solutions, the harmonized analysis via siMPle saves working time and computational costs. Current computers can run several instances of the software unattended, allowing the data analysis of up to 16 samples per day compared to OPUS (two days) for 1.8 million spectra per file. Still, it is possible also to use low-cost office computers which normally can calculate up to three samples per day containing 1.8 million spectra. As a minimum requirement, a processor speed of 3 GHz is advised with 8 GB of RAM. To assess the performance of siMPle on these data sets, a QA/QC analysis on the overall result was performed (Fig. 6).
Figure 6.
Assignment rates of correct and misidentified spectra for the different instruments based on manual reanalysis similar to Primpke et al.21
In all cases, correct assignment rates >90% were reached, for three systems (PerkinElmer, Bruker, and Agilent), and these were even >95% (Fig. 6). Those correct assignment rates were exceedingly high, independently from instrument and sample preparation, proving the high potential of siMPle as a harmonized tool for MP analysis. Still, it is suggested and recommended that each study conducts an own QA/QC analysis for each sample series for each polymer type identified as demonstrated, e.g., in Lorenz et al.32 Further questions, such as a comparison between existing analytical pipelines, their harmonization, and a full QA/QC analysis will be addressed in a later detailed study.
To conclude, it is noteworthy that the siMPle software is not limited to MP analysis, and it also allows the analysis of other types of data like the spectral comparison of nano-FT-IR data36 or the analysis of single algae species (Fig. S4).
Using siMPle, single cells can be selected or specific characteristics can be highlighted (Fig. S4). Here, the data show a strong Halo effect (Fig. S4b) mainly caused by interference between the sample and the surface of the CaF2 window, which is not visible using a heatmap (Fig. S4a). In the future, heatmaps based on the integration of specific regions will be introduced to allow even more control over the data. Further, additional functions are currently planned to be introduced, and new possibilities can be determined by contacting the authors to explore its application in a broader scope for future research.
Conclusion
With siMPle, we present a freeware data analysis tool for the harmonized and systematic analysis of spectroscopic data, with application, for example, in the identification of MP in the environment. It allows data determination and interpretation in a transparent and reproducible manner. In addition, it provides a simpler quality QA/QC compared to existing commercial software tools like Bruker OPUS and shows an increased identification rate. Furthermore, it allows the analysis independently from the instrument manufacturer for a single spectrum but also for large fields generated by imaging techniques. In particular, the field of FT-IR imaging benefits greatly, as the data calculation time is reduced from several days to 5 h using this software tool. Compared to other techniques, all spectra are correlated via three different data treatments with the database yielding high-quality results for all investigated instrument systems. This new tool improves the application of FT-IR imaging in monitoring studies for MP, as it is accessible for most types of spectrometers, free of charge and reduces the human bias during manual data analysis.
Supplemental Material
Supplemental material, sj-zip-1-asp-10.1177_0003702820917760 for Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (siMPle) for Spectroscopic Analysis by Sebastian Primpke, Richard K. Cross, Svenja M. Mintenig, Marta Simon, Alvise Vianello, Gunnar Gerdts and Jes Vollertsen in Applied Spectroscopy
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by the German Federal Ministry of Education and Research (Project BASEMAN – Defining the baselines and standards for microplastics analyses in European waters; BMBF grant 03F0734A). S.M. Mintenig received funding from the Dutch Technology Foundation TTW (project 13940). The authors wish to thank UK Water Industry Research (UKWIR) for making available three of the anonymised raw datasets from their research and for supporting UKCEH and Richard Cross in the generation of that data.
ORCID iDs
Sebastian Primpke https://orcid.org/0000-0001-7633-8524
Richard K. Cross https://orcid.org/0000-0001-5409-6552
Supplemental Material
All supplemental material mentioned in the text is available in the online version of the journal.
References
- 1.Vianello A., Jensen R.L., Liu L., et al. “Simulating Human Exposure to Indoor Airborne Microplastics Using a Breathing Thermal Manikin”. Sci. Rep. 2019. 9(1): 8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rochman C.M., Brookson C., Bikker J., et al. “Rethinking Microplastics as a Diverse Contaminant Suite”. Environ.Toxicol. Chem. 2019. 38(4): 703–711. [DOI] [PubMed] [Google Scholar]
- 3.Lusher A.L., Munno K., Hermabessiere L., et al. “Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization”. Appl. Spectrosc. 2020. 74(9): 1049–1065. [DOI] [PubMed] [Google Scholar]
- 4.Brander S., Renick V., Foley M., et al. “Sampling and Quality Assurance and Quality Control: A Guide for Scientists Investigating the Occurrence of Microplastics Across Matrices”. Appl. Spectrosc. 2020. 74(9): 1099–1125. [DOI] [PubMed] [Google Scholar]
- 5.Zarfl C. “Promising Techniques and Open Challenges for Microplastic Identification and Quantification in Environmental Matrices”. Anal. Bioanal. Chem. 2019. 411(17): 3743–3756. [DOI] [PubMed] [Google Scholar]
- 6.Primpke S., Christiansen S.H., Cowger W., et al. “Critical Assessment of Analytical Methods for the Harmonized and Cost Efficient Analysis of Microplastics”. Appl. Spectrosc. 2020. 74(9): 1012–1047. [DOI] [PubMed] [Google Scholar]
- 7.Schwaferts C., Niessner R., Elsner M., et al. “Methods for the Analysis of Submicrometer- and Nanoplastic Particles in the Environment”. Trends Anal. Chem. 2019. 112: 52–65. [Google Scholar]
- 8.Yu Y., Zhou D., Li Z., et al. “Advancement and Challenges of Microplastic Pollution in the Aquatic Environment: A Review”. Water Air Soil Pollut. 2018. 229(5): 140. [Google Scholar]
- 9.Li J.Y., Liu H.H., Chen J.P. “Microplastics in Freshwater Systems: A Review on Occurrence, Environmental Effects, and Methods for Microplastics Detection”. Water Res. 2018. 137: 362–374. [DOI] [PubMed] [Google Scholar]
- 10.Stock F., Kochleus C., Bansch-Baltruschat B., et al. “Sampling Techniques and Preparation Methods for Microplastic Analyses in the Aquatic Environment: A Review”. TrAC, Trends Anal. Chem. 2019. 113: 84–92. [Google Scholar]
- 11.Ivleva N.P., Wiesheu A.C., Niessner R. “Microplastic in Aquatic Ecosystems”. Angew Chem. Int. Ed. 2017. 56(7): 1720–1739. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann N.B., Huffer T., Thompson R.C., et al. “Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris”. Environ. Sci. Technol. 2019. 53(3): 1039–1047. [DOI] [PubMed] [Google Scholar]
- 13.Löder M.G.J., Imhof H.K., Ladehoff M., et al. “Enzymatic Purification of Microplastics in Environmental Samples”. Environ. Sci. Technol. 2017. 51(24): 14283–14292. [DOI] [PubMed] [Google Scholar]
- 14.Cowger W., Gray A., Christiansen S.H., et al. “Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research”. Appl. Spectrosc. 2020. 74(9): 989–1010. [DOI] [PubMed] [Google Scholar]
- 15.Hermsen E., Mintenig S.M., Besseling E., et al. “Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review”. Environ. Sci. Technol. 2018. 52(18): 10230–10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koelmans A.A., Nor N.H.M., Hermsen E., et al. “Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality”. Water Res. 2019. 155: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Käppler A., Fischer D., Oberbeckmann S., et al. “Analysis of Environmental Microplastics by Vibrational Microspectroscopy: FT-IR, Raman or Both?”. Anal. Bioanal. Chem. 2016. 408(29): 8377–8391. [DOI] [PubMed] [Google Scholar]
- 18.Tagg A.S., Sapp M., Harrison J.P., et al. “Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging”. Anal. Chem. 2015. 87(12): 6032–6040. [DOI] [PubMed] [Google Scholar]
- 19.Löder M.G.J., Kuczera M., Mintenig S., et al. “Focal Plane Array Detector-Based Micro-Fourier-Transform Infrared Imaging for the Analysis of Microplastics in Environmental Samples”. Environ. Chem. 2015. 12(5): 563–581. [Google Scholar]
- 20.Harrison J.P., Ojeda J.J., Romero-Gonzalez M.E. “The Applicability of Reflectance Micro-Fourier-Transform Infrared Spectroscopy for the Detection of Synthetic Microplastics in Marine Sediments”. Sci. Total Environ. 2012. 416: 455–463. [DOI] [PubMed] [Google Scholar]
- 21.Primpke S., Lorenz C., Rascher-Friesenhausen R., et al. “An Automated Approach for Microplastics Analysis Using Focal Plane Array (FPA) FT-IR Microscopy and Image Analysis”. Anal. Methods. 2017. 9(9): 1499–1511. [Google Scholar]
- 22.Liu F., Olesen K.B., Borregaard A.R., et al. “Microplastics in Urban and Highway Stormwater Retention Ponds”. Sci. Total Environ. 2019. 671: 992–1000. [Google Scholar]
- 23.Renner G., Nellessen A., Schwiers A., et al. “Data Preprocessing and Evaluation Used in the Microplastics Identification Process: A Critical Review and Practical Guide”. TrAC, Trends Anal. Chem. 2019. 111: 229–238. [Google Scholar]
- 24.Primpke S., Wirth M., Lorenz C., et al. “Reference Database Design for the Automated Analysis of Microplastic Samples Based on Fourier Transform Infrared (FT-IR) Spectroscopy”. Anal. Bioanal. Chem. 2018. 410(21): 5131–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Primpke S., Dias P.A., Gerdts G. “Automated Identification and Quantification of Microfibres and Microplastics”. Anal. Methods. 2019. 11(16): 2138–2147. [Google Scholar]
- 26.Primpke S., Imhof H., Piehl S., et al. “Environmental Chemistry Microplastic in the Environment”. Chem. Unserer Zeit. 2017. 51(6): 402–412. [Google Scholar]
- 27.Kansiz M., Heraud P., Wood B., et al. “Fourier Transform Infrared Microspectroscopy and Chemometrics as a Tool for the Discrimination of Cyanobacterial Strains”. Phytochemistry. 1999. 52(3): 407–417. [Google Scholar]
- 28.Cabernard L., Roscher L., Lorenz C., et al. “Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment”. Environ. Sci. Technol. 2018. 52(22): 13279–13288. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann M., Mützel S., Primpke S., et al. “White and Wonderful? Microplastics Prevail in Snow from the Alps to the Arctic”. Sci. Adv. 2019. 5(8): Eaax1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergmann M., Wirzberger V., Krumpen T., et al. “High Quantities of Microplastic in Arctic Deep-Sea Sediments from the HAUSGARTEN Observatory”. Environ. Sci. Technol. 2017. 51(19): 11000–11010. [DOI] [PubMed] [Google Scholar]
- 31.Haave M., Lorenz C., Primpke S., et al. “Different Stories Told by Small and Large Microplastics in Sediment—First Report of Microplastic Concentrations in an Urban Recipient in Norway”. Mar. Pollut. Bull. 2019. 141: 501–513. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz C., Roscher L., Meyer M.S., et al. “Spatial Distribution of Microplastics in Sediments and Surface Waters of the Southern North Sea”. Environ. Pollut. 2019. 252: 1719–1729. [DOI] [PubMed] [Google Scholar]
- 33.Mani T., Primpke S., Lorenz C., et al. “Microplastic Pollution in Benthic Midstream Sediments of the Rhine River”. Environ. Sci. Technol. 2019. 53(10): 6053–6062. [DOI] [PubMed] [Google Scholar]
- 34.Peeken I., Primpke S., Beyer B., et al. “Arctic Sea Ice is an Important Temporal Sink and Means of Transport for Microplastic”. Nature Commun. 2018. 9(1): 1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olesen K.B., Stephansen D.A., Van Alst N., et al. “Microplastics in a Stormwater Pond”. Water. 2019. 11(7): 10.3390/W11071466. [Google Scholar]
- 36.Meyns M., Primpke S., Gerdts G. “Library Based Identification and Characterisation of Polymers with Nano-FT-IR and IR-sSNOM Imaging”. Anal. Methods. 2019. 11(40): 5195–5202. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-zip-1-asp-10.1177_0003702820917760 for Toward the Systematic Identification of Microplastics in the Environment: Evaluation of a New Independent Software Tool (siMPle) for Spectroscopic Analysis by Sebastian Primpke, Richard K. Cross, Svenja M. Mintenig, Marta Simon, Alvise Vianello, Gunnar Gerdts and Jes Vollertsen in Applied Spectroscopy