Abstract
Introduction
Anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis is an autoimmune-mediated disease that is common in young female patients with ovarian teratomas. With appropriate immunotherapy, most patients achieve a good prognosis. Nevertheless, some patients may be refractory to first- and second-line immunotherapy, thus alternative treatments are required for these patients.
Case presentation: We present a case of anti-NMDA receptor encephalitis with ovarian teratoma. After the prompt removal of the teratoma and intense immunotherapy was administered, including an intravenous methylprednisolone pulse, intravenous immunoglobin, plasmapheresis, immunoadsorption, intravenous cyclophosphamide, and rituximab, the patient’s neurologic status did not improve. Bilateral salpingo-oophorectomy was then conducted, and intrathecal injection of methotrexate (MTX) and dexamethasone (DXM) was performed. The patient’s neurological symptoms improved dramatically, and she achieved a good prognosis after 23 months.
Conclusions
Intrathecal injection of MTX and DXM may be beneficial for treatment of refractory cases of anti-NMDA receptor encephalitis. Additional research is required to elucidate the mechanisms of intrathecal treatment with this therapy.
Keywords: Anti-NMDA receptor encephalitis, methotrexate, immunotherapy, ovarian teratoma, intrathecal injection, auto-immune disease
Introduction
Anti-N-methyl-D-aspartate (anti-NMDA) receptor encephalitis has recently been reported as a paraneoplastic limbic encephalitis that mainly affects young female patients with ovary teratomas.1 Clinical therapy, including first-line immunotherapy (steroids, intravenous immunoglobulin, and plasmapheresis), second-line immunotherapy (rituximab and cyclophosphamide), and tumor removal are recommended for the treatment of this condition. With appropriate therapy, most patients achieve a good prognosis (modified Rankin Scale, mRS score < 2) within 24 months of follow-up.2 Nevertheless, approximately 19% of patients may have a poor prognosis following first- and second-line treatment.
Ovarian teratomas are reported to be associated with anti-NMDA receptor encephalitis in 58% of cases.3 The teratomas contain neuronal cells that induce immunologic sensitization against NMDA receptors, and even small teratomas containing nervous tissue may result in severe complications secondary to anti-NMDA receptor encephalitis.4 The longest clinical duration of coma in a patient with anti-NMDA receptor encephalitis has not been reported. Herein, we present a case of anti-NMDA receptor encephalitis with right ovarian teratoma. The patient was refractory to the first- and second-line therapy. Bilateral salpingo-oophorectomy was conducted, and intrathecal injection of methotrexate (MTX) and dexamethasone (DXM) was performed. The patient finally recovered consciousness at 17 months and had a good prognosis 23 months after disease onset. This case will assist physicians in clinical practice in treating refractory anti-NMDA receptor encephalitis patients.
Case presentation
A 27-year-old woman was transferred to our department following three seizure episodes and loss of consciousness that occurred on 4 November 2016 and lasted for 1 day.
She was admitted to a local psychiatric department because of acute psychosis that lasted for 3 days, during which she spoke few words and was restless and unwilling to eat. One week before her symptoms appeared, she had cold-like symptoms with a runny nose and low-grade fever between 37°C and 38°C. Her medical history was not remarkable.
On admission, a physical examination revealed that her vital signs were stable (body weight 52.0 kg, height 163 cm), and a neurological examination based on the Glasgow coma scale (GCS) showed that she had a score of 6 (Eye opening 1, Verbal response 1, Motor response 4). Her laboratory and electroencephalogram (EEG) results were not remarkable. Cranial magnetic resonance imaging with contrast showed mild signal changes in the bilateral hippocampus and left temporal cortex and local meningeal congestion. Anti-NMDA receptor antibodies were detected in the serum and cerebrospinal fluid (CSF) (1:1000 and 1:100, respectively).
Abdominal ultrasound screening showed a weak liquid echo of the right ovary, and teratoma was highly suspected. Tumor removal was initiated after the diagnosis was made on 5 November 2016, and the pathology report confirmed the diagnosis of a teratoma containing nerve tissues.
The patient was comatose with persistent facial involuntary movement including lip peristalsis and uncontrolled eye blinking, which were treated with a large dose of anesthetic agents.
After the diagnosis of anti-NMDA receptor encephalitis was made, first-line therapy including an intravenous methylprednisolone pulse (IVMP), intravenous immunoglobin (IVIG), plasmapheresis, and immunoadsorption was initiated (Table 1). However, the patient was refractory to all treatments. Her neurological status did not improve, and the anti-NMDA receptor antibody titers of both the serum and CSF were persistently high (Table 1). Considering the poor reaction to treatment, second-line therapy including rituximab (100 mg Qw for 4 weeks) and intravenous cyclophosphamide (0.4 g, once; 0.6 g, once) was initiated. The patient did not react to this treatment.
Table 1.
Clinical treatment and serum and CSF antibody titers of the patient.
| Date | Immunotherapy | Serum anti-NMDA receptortiter | CSF anti-NMDA receptor titer | Infections |
|---|---|---|---|---|
| 5 Nov 2016 | Plasma exchange for 5 days | 1:1000 | 1:100 | |
| 6 Nov 2016 | IVMP with 0.5 g/day for 5 days and tapered to oral MP 40 mg | 1:1000 | 1:100 | |
| 10 Nov 2016 | IVIG with 20 g for 5 days | 1:1000 | 1:100 | |
| 21 Nov 2016 | IVIG with 20 g for 5 days | 1:1000 | 1:100 | |
| 1 Dec 2016 | Plasma exchange for 5 days | 1:1000 | 1:100 | |
| 15 Dec 2016 | Oral MP 40 mg | 1:1000 | 1:100 | Septicemia with Staphylococcus caprae |
| 22 Dec 2016 | IV CTX 0.4 g | 1:1000 | 1:100 | |
| 29 Dec 2016 | IV CTX 0.6 g | 1:1000 | 1:100 | |
| 11 Jan 2017 | IVIG with 20 g for 5 days | 1:1000 | 1:100 | |
| 13 Jan 2017 | IVMP with 0.5 g/day for 5 days and tapered to oral MP 40 mg | 1:1000 | 1:100 | |
| 17 Feb 2017 | Immunoadsorption for 5 days | 1:1000 | 1:100 | |
| 7 Mar 2017 | Rituximab 100 mg Qw for 4 weeks | 1:300 | 1:100 | Septicemia with klebsiella pneumoniae |
| 28 Apr 2017 | Plasma exchange for 5 days | 1:300 | 1:100 | |
| 14 May 2017 | Plasma exchange for 5 days | 1:300 | 1:100 | |
| 12 Dec 2017 | Mycophenolate mofetil 0.75 g bid | 1:300 | 1:100 | |
| 26 Dec 2017 | IVIG with 20 g for 5 days | 1:300 | 1:100 | |
| 26 Dec 2017 | IVMP with 0.5 g/day for 3 days and tapered to oral MP 40 mg | 1:300 | 1:100 | |
| 16 Jan 2018 | Intrathecal therapy with DXM and MTX for 5 times (once per week) | 1:300 | 1:32 |
CTX: cyclophosphamide; DXM: dexamethasone; IVMP: intravenous methylprednisolone pulse; IVIG: intravenous immunoglobin; MP: methylprednisolone; MTX: methotrexate.
Because the patient manifested a high antibody titer, we considered the possibility of non-visible teratomas of the ovaries. After obtaining informed consent from the family members, bilateral salpingo-oophorectomy was conducted on 26 October 2017, and the pathology report revealed inflammation, but no teratoma was observed.
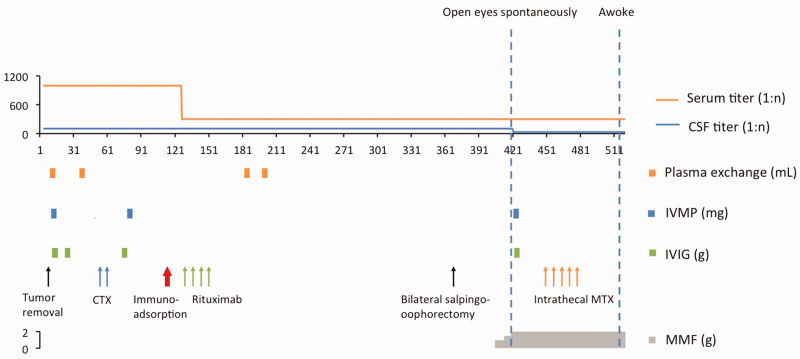
Immunosuppressant therapy including mycophenolate mofetil (MMF, 0.75 g bid) was initiated after failure of the first- and second-line therapies. Intrathecal MTX (10 mg) and DXM (10 mg) were given once a week for 5 weeks, and the antibody titer in the CSF gradually decreased (Table 1, Figure 1); during follow-up, the antibody titer decreased to 1:10 on 20 April 2018. After the therapy was completed, the patient awoke after 17 months with a GCS score of 9T. She could open and close her eyes, extend her tongue, and turn her head as instructed. After completing 6 months of physical therapy, she achieved a good prognosis with an mRS score of 1. During the follow-up, an X-ray examination of the patient’s shoulders showed diffuse muscular ossification.
Figure 1.
The clinical course of the patient. The serum and CSF antibody titers are indicated. The immunotherapy protocol administered to the patient is illustrated in the figure.
CSF, cerebrospinal fluid; CTX, cyclophosphamide; IVIG, immunoglobin; IVMP, intravenous methylprednisolone pulse; MMF, mycophenolate mofetil.
Discussion
A large cohort study enrolling 577 patients found that in most patients with anti-NMDA receptor encephalitis, responses to immunotherapy and second-line immunotherapy are usually effective when first-line treatments fail. In some patients in this cohort, recovery lasted up to 18 months.2 The patient in our study had a poor response to both the first- and second-line therapy and therefore was considered a refractory case.
The comorbidity of teratoma and anti-NMDA receptor encephalitis has been widely reported. The incidence varies from 6% to 38%, with a lower incidence in younger patients.2,5 Delayed teratoma development and teratomas that are not visible on imaging have previously been reported.1,6 Zainab et al. described a case of a patient who received bilateral oophorectomy even though multiple imaging investigations showed no evidence of teratoma. Ovarian histology confirmed the diagnosis of teratoma with nerve tissues.1 In our case, at the onset of the disease, an ultrasound examination revealed a teratoma of the right ovary, and tumor removal was conducted within 1 week of the diagnosis. The lack of response to treatment suggested the possibility of non-visible teratomas. On the basis of the clinical suspicion of small teratomas, the patient underwent bilateral salpingo-oophorectomy. However, the pathology report did not show any evidence of teratoma.
Intrathecal injection of MTX and DXM is widely used and has been shown to be beneficial for the treatment of neuropsychiatric systemic lupus erythematosus (NPSLE) patients, particularly those patients who were refractory to traditional therapy or those who had contraindications for IVMP and intravenous cyclophosphamide.7–9 A study that evaluated the effect of methylprednisolone combined with MTX and DXM on the level of antibodies against the NR2a/2b NMDA receptor subtype in NPSLE patients reported that after treatment, the positive rates of autoantibodies and anti-NR2 antibodies in both the NPSLE and non-NPSLE group were significantly decreased, while the negative conversion rate was as high as 61.5%.10 In the largest anti-NMDA receptor encephalitis cohort, consisting of 577 patients, clinical improvement was assessed at 4 weeks from initiation of treatment. If no improvement was observed, second-line therapy was initiated, including rituximab, cyclophosphamide, and other immunotherapy agents (azathioprine, MMF, tacrolimus, or MTX).2 In the present case, the patient’s symptoms were severe and refractory to many therapies including IVMP, IVIG, plasmapheresis, rituximab, and cyclophosphamide. Therefore, based on the encouraging effects of intrathecal injection of MTX and DXM in NPSLE patients, we attempted this therapy in our patient. The neurologic status of our patient improved dramatically after intrathecal therapy.
MTX is a potent immunosuppressive agent that cannot penetrate the blood–brain barrier. Intrathecal administration can increase the local concentration of MTX and thus enhance its immunosuppressive effect.8 Nonetheless, intrathecal injection of MTX should be used with caution in patients with anti-NMDA receptor encephalitis. A case report of one patient with MTX neurotoxicity is worthy of consideration. The EEG of a young female patient with leukemia who received a high dosage of intrathecal MTX showed a delta brush pattern,11 which has been reported in approximately 30.3% patients with anti-NMDA receptor encephalitis.12 The NMDA receptor is involved in the pathogenesis of MTX neurotoxicity. MTX interferes with potentially neurotoxic amino acid and neurotransmitter pathways causing accumulation of homocysteine and its metabolites, resulting in a strong excitatory effect on NMDA receptors,13 which seems paradoxical. We hypothesize that MTX affects NMDA receptors, and an appropriate dosage may be beneficial for the treatment of anti-NMDA receptor encephalitis. Further research is warranted to elucidate the underlying mechanisms of the effects of intrathecal MTX administration in patients with anti-NMDA receptor encephalitis.
Another interesting point in this case is that the patient developed severe and diffuse muscle ossification. This phenomenon is referred to as heterotopic ossification (HO) and is consistent with our experience.14 Severe neurologic symptoms, long-term intensive care, muscular spasticity, and mechanical ventilation likely caused HO to develop in this patient.
Conclusion
Anti-NMDA receptor encephalitis is a newly recognized auto-immune disease. In most patients, a favorable prognosis is achieved with the first- and second-line immune therapy. In some rare cases, in which patients react poorly to strong first- and second-line treatment, intrathecal MTX with DXM may be beneficial.
Acknowledgements
We thank the patient and her family members for their generosity and cooperation.
Footnotes
Authors’ contributions: DW, YW, ZJ, SW and SP were responsible for the study concept and design. YX, KH, YP, HZ and HW provided care to the patient. DW and XZ were responsible for data collection. All authors contributed intellectually. All authors acquired, analyzed, and interpreted the data. The manuscript was prepared by DW and SP. All authors reviewed and made critical revisions to the manuscript.
Ethics approval and consent: Institutional review board/ethics committee approval was obtained from the Institutional Review Board of the Nanfang Hospital, Southern Medical University. Written informed consent was obtained from both the patient and her parents for publication of this case report and any accompanying images.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by the National Key R&D Program of China (2017YFC1307500) and the National Natural Science Foundation of China (No. 81871030). SP was funded by the two funds and was responsible for the concept and design of the study. SP also revised the manuscript.
ORCID iD: Suyue Pan https://orcid.org/0000-0003-2744-1984
References
- 1.Abdul-Rahman ZM, Panegyres PK, Roeck M, et al. Anti-N-methyl-D-aspartate receptor encephalitis with an imaging-invisible ovarian teratoma: a case report. J Med Case Rep 2016; 10: 296. DOI: 10.1186/s13256-016-1067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12: 157–165. DOI: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azizyan A, Albrektson JR, Maya MM, et al. Anti-NMDA encephalitis: an uncommon, autoimmune mediated form of encephalitis. J Radiol Case Rep 2014; 8: 1–6. DOI: 10.3941/jrcr.v8i8.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acien P, Acien M, Ruiz-Macia E, et al. Ovarian teratoma-associated anti-NMDAR encephalitis: a systematic review of reported cases. Orphanet J Rare Dis 2014; 9: 157. DOI: 10.1186/s13023-014-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63–74. DOI: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omata T, Kodama K, Watanabe Y, et al. Ovarian teratoma development after anti-NMDA receptor encephalitis treatment. Brain Dev 2017; 39: 448–451. DOI: 10.1016/j.braindev.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Valesini G, Priori R, Francia A, et al. Central nervous system involvement in systemic lupus erythematosus: a new therapeutic approach with intrathecal dexamethasone and methotrexate. Springer Semin Immunopathol 1994; 16: 313–321. [DOI] [PubMed] [Google Scholar]
- 8.Zhou HQ, Zhang FC, Tian XP, et al. Clinical features and outcome of neuropsychiatric lupus in Chinese: analysis of 240 hospitalized patients. Lupus 2008; 17: 93–99. DOI: 10.1177/0961203307085671. [DOI] [PubMed] [Google Scholar]
- 9.Zhou HQ, Leng XM, Zhang FC. [ Neuropsychiatric manifestations in systemic lupus erythematosus and the treatment of intrathecal methotrexate plus dexamethasone. Zhonghua Yi Xue Za Zhi 2006; 86: 771–774. [PubMed] [Google Scholar]
- 10.Wang J, Zhao Y, Zhang J, et al. Impact analysis of autoantibody level and NR2 antibody level in neuropsychiatric SLE treated by methylprednisolone combined with MTX and DXM intrathecal injection. Cell Biochem Biophys 2014; 70: 1005–1009. DOI: 10.1007/s12013-014-0010-9. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt LS, Kjaer TW, Schmiegelow K, et al. EEG with extreme delta brush in young female with methotrexate neurotoxicity supports NMDA receptor involvement. Eur J Paediatr Neurol 2017; 21: 795–797. DOI: 10.1016/j.ejpn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt SE, Pargeon K, Frechette ES, et al. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012; 79: 1094–1100. DOI: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vezmar S, Becker A, Bode U, et al. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy 2003; 49: 92–104. DOI: 10.1159/000069773. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Wang S, Huang X, et al. Heterotopic ossification following anti-NMDA receptor encephalitis: a case report. BMC Neurol 2016; 16: 232. DOI: 10.1186/s12883-016-0747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]