Abstract
Background:
Nuclei located in the dorsal midline thalamus, such as the paraventricular nucleus of the thalamus (PVT), are crucial to modulate fear and aversive behaviour. In addition, the PVT shows a dense expression of µ-opioid receptors (MORs) and could mediate the anxiolytic effects of opioids.
Methods:
We analysed the contribution of MORs in the dorsal midline thalamus (i.e. the PVT) to the performance of mice in a classical fear conditioning paradigm. We locally injected a specific agonist (DAMGO), an antagonist (CTAP) of MOR or saline as a control into the dorsal midline thalamus of male mice, prior to fear extinction training. We assessed freezing as a typical measure of fear and extended our analysis by evaluation of aversive, non-aversive and neutral behavioural features using compositional data analysis.
Results:
Pharmacological blockade of MORs through CTAP in the dorsal midline thalamus induced a fear memory extinction deficit, as evidenced by maintained freezing during extinction sessions. Stimulation of MORs by DAMGO resulted in an overall increase in locomotor activity, associated with decreased freezing during recall of extinction. Compositional data analysis confirmed the freezing-related pharmacological effects and revealed specific differences in basic behavioural states. CTAP-treated mice remained in an aversive state, whereas DAMGO-treated mice displayed predominantly neutral behaviour.
Conclusions:
Fear extinction requires the integrity of the µ-opioid system in the dorsal midline thalamus. Pharmacological stimulation of MOR and associated facilitation of fear extinction recall suggest a potential therapeutic avenue for stress-related or anxiety disorders.
Keywords: Fear extinction, dorsal midline thalamus, mu-opioid receptor, opioids
Introduction
Organisms have to discriminate continuously noticeable (i.e. salient) stimuli in their environment and assign them a relevant predictive value (i.e. negative or positive valence). From this associative process animals elaborate an appropriate response that manifests in the behaviour of the animal. Nuclei located in the dorsal midline thalamus (dMT), such as the paraventricular nucleus of the thalamus (PVT), play a critical role in this task. The PVT seems to modulate a variety of aspects, such as arousal, awareness and stress coping (Kirouac, 2015), and PVT neurons have recently been shown to code for stimulus saliency (Zhu et al., 2018). The PVT also regulates emotional and behavioural responses (Kirouac, 2015), likely due to its projections to emotional valence-related structures, such as the nucleus accumbens, the extended amygdala or the prefrontal cortex (Goedecke et al., 2019; Haight and Flagel, 2014). Thus, the dMT is a critical hub for information processing in valence-coding circuits and the coordination of the behavioural response.
The dMT is rich in various opioid receptors (Brunton and Charpak, 1998) and the opioid system modulates aversiveness associated with opioid withdrawal (Zhu et al., 2016). In particular, µ-opioid receptors (MORs) regulate opioid-mediated aversive behaviour during fear memory retrieval, particularly in some brain areas such as the ventrolateral periaqueductal gray (vlPAG) (McNally et al., 2005). Opioids are also involved in valence and emotional circuits, and in fear memory retrieval and extinction processes, with implications for diseases such as anxiety disorders (Colasanti et al., 2011). However, the specific contribution of MORs in the dMT to the modulation of aversive behaviour has not been established so far.
Classical (Pavlovian) fear conditioning is a well-established procedure to evaluate aversive behavioural changes, and freezing is usually monitored as one behavioural output variable. This variable largely reflects passive coping behaviour, which can be complemented in several ways. First, activity scores can be used to capture the active component of the behavioural coping strategy (Fucich et al., 2016). In addition, it is mandatory that a broader spectrum of behavioural responses is analysed (i.e. including rearing, grooming, flight responses, etc.) in an attempt to study environmental or pharmacological influences (Laxmi et al., 2003; Perusini and Fanselow, 2015; Remmes et al., 2016). A more general concern is that behavioural traits often mutually exclude and complement each other, resulting in intrinsic negative correlations, which in turn require the implementation of compositional data analysis (CoDA) (Schilling et al., 2012; Smith et al., 2016).
Here we hypothesized that MORs in the dMT alter fear behaviour in a fear conditioning paradigm. We injected a MOR-selective agonist ([D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin (DAMGO)), an antagonist (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP)) or saline (Control) into the dMT of mice prior to extinction sessions. We analysed behavioural responses such as freezing to a conditioned stimulus (CS+) and to a neutral stimulus (CS−), and complemented it with an active coping behaviour analysis and with a CoDA of aversive, non-aversive and neutral behavioural features. Our experiments suggest that the µ-opioid system in the dMT participates in fear memory extinction processes.
Material and methods
Animals and housing
All procedures were performed according to European regulations on animal experimentation (Directive 2010/63/EU of the European parliament and the council), and under the surveillance of the local authorities (Bezirksregierung, Münster), and approved by the ‘Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen’. Twenty-seven male mice aged between eight and 14 weeks (C57Bl6/J; Charles River, Germany) were maintained under standard housing conditions (12 : 12 h light:dark cycle with lights on at 06:00 h, ~21°C temperature, 50–60% relative humidity), with ad libitum access to water and food.
Drug preparation and dosage
DAMGO (Cat No 1171; Tocris-Biotechne, Germany) was dissolved in 0.9% saline solution, in a stock concentration of 2 mM. For injections, the stock was freshly diluted to 80 µM in saline. CTAP (Cat. No. 1560; Tocris-Biotechne, Germany) was diluted in 0.9% saline to 500 µM. Aliquots were kept frozen and thawed freshly before use. The final amount of substance injected into the dMT of the animals was 40 pmol for DAMGO and 250 pmol for CTAP, both delivered in 500 nL saline solution. DAMGO and CTAP doses were adapted from McNally and Cole (2006) and Meyer et al. (2007), respectively.
Surgical procedures
Animals were anesthetized with pentobarbital (50 mg/kg intraperitoneally) after isoflurane induction (2.5% in O2; CP-Pharma, Germany). One animal, whose cannula was misplaced, was excluded from the analysis. After reaching surgical tolerance, pain was ameliorated with carprofen (Rimadyl: 10 mg/kg subcutaneously (s.c.); Zoetis, Germany) and a local anaesthetic was applied to the incision site on the head (xylocaine 2% s.c., 0.1 mL; AstraZeneca, Germany). The animals were placed in a stereotactic device (Model 692; Kopf, Germany) and a single guiding cannula (26GA 4 mm length; PlasticsOne, VA, USA) was implanted into the PVT (anteroposterior: −0.7 mm; mediolateral: +0.3 mm; dorsoventral: +3.0 mm, angle: +5°). Acrylic cement (Pulpdent; Glasslute, MA, USA) was used to fix the cannula to the skull. The body temperature of the animals was measured using a rectal probe and maintained at 37°C throughout the procedure. Behavioural experiments were performed after 7 days of post-surgical recovery.
Fear conditioning
The mice were subjected to classical (Pavlovian) fear conditioning as previously described, with some modifications (Figure 1; Jüngling et al., 2008). On day 1 (adaptation phase), the animals were placed in the conditioning arena (24.5 cm× 19.5 cm chamber; FearConditioning Sytem; TSE Systems; Germany) and two trains (inter-train interval (ITI), 6 h) of six neutral tones were delivered (CS−; sine tone 2.5 kHz, 85 dB, 10 s). On day 2 (acquisition phase), two trains (ITI 6 h) of four conditioning tones were delivered (CS+; sine tone 10 kHz, 85 dB, 10 s), each of which co-terminated with a 0.4 mA scrambled electric foot-shock (unconditioned stimulus (US)). During ITIs animals were kept in their home cage.
Figure 1.
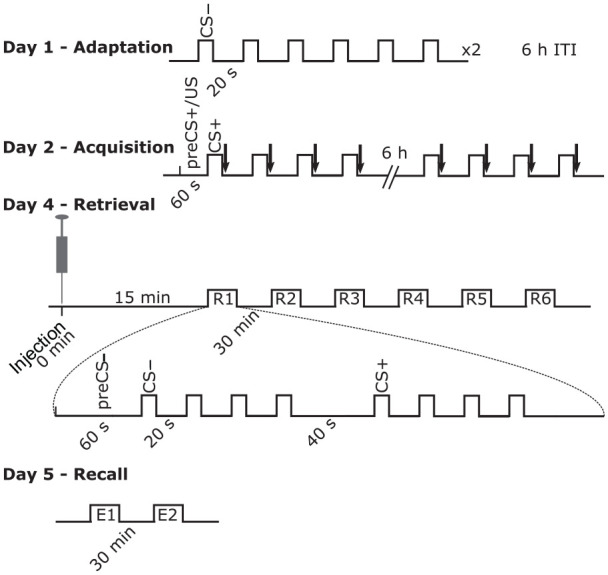
Timeline of the experiments. Mice were subjected to a classical (Pavlovian) fear conditioning paradigm. Conditioned stimulus − (CS−) consisted of a tone of 2.5 kHz (80 dB intensity; 10 s) and conditioned stimulus + (CS+) consisted of a tone of 10 kHz (85 dB intensity; 10 s). The unconditioned stimulus (US; black arrows) was a foot shock of 1 s duration (0.4 mA) co-terminating with the CS+ on day 2. µ-opioid receptor antagonist (CTAP), agonist (DAMGO) or saline (Control) was administered in a single dose on day 4, 15 min before the first R1 session started. Each R (or E) session consisted of four CS− and four CS+ presentations regularly spaced as described.
CTAP: D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; DAMGO: [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin; ITI: inter-train interval
On day 4 (retrieval phase), the animals received a 500 nL injection of either DAMGO, CTAP or saline (Control) into the dMT. The animals were randomly assigned to one of the three groups. The injections were performed under isoflurane anaesthesia (1.5% in O2) and the substances were administered at a flow rate of 0.1 µL/min (CR700-20; Hamilton Company, NV, USA) through a cannula (33GA 5 mm length; PlasticsOne, VA, USA). Fifteen minutes after administration, animals were exposed to six sessions (R1 to R6; ITI 30 min) of tone presentations in a new context (31 cm × 15 cm chamber). Each session consisted of four CS− tones to evaluate potential fear generalization effects followed by four CS+ tone presentations. On day 5 (recall phase), another two sessions of tone presentation were delivered (E1 and E2; ITI 30 min) in the same context as on day 4. All the behavioural sessions were video recorded and analysed offline.
After the behavioural experiments, animals were sedated with isoflurane (2.5% in O2; CP-Pharma, Germany) and killed by decapitation and 500 nL of methylene blue (4 mg/mL) were administered using the same cannula system as on day 4. The brains were immediately extracted, frozen and cut into 50 µm thick coronal slices on a cryostat (CM3050S; Leica, Germany). Images were taken using a transmitted light microscope (Zeiss Axioscope; Zeiss, Germany). The Allen Mouse Reference Atlas served as reference to assess cannula placements (Figure 2(a); Allen Institute for Brain Science, 2011). In addition, histological images were compared with the Paxinos atlas (Paxinos and Franklin, 2001) to assess the anteroposterior distance of the tip to bregma, based on the relative size of adjacent brain structures in the atlas (i.e. ventricles and fimbria).
Figure 2.
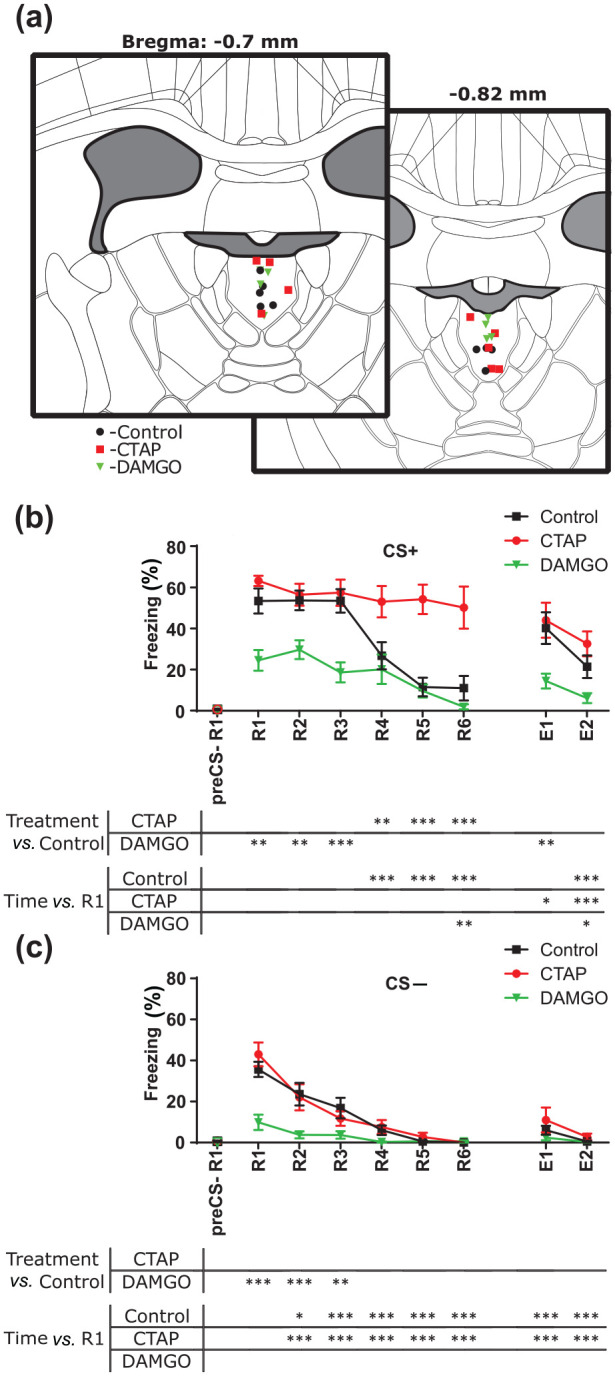
µ-opioid receptors in the dorsal midline thalamus modulate freezing behaviour of mice in a Pavlovian fear conditioning paradigm. (a) Schematic representation of the cannula tip positions. Image credit: Allen Institute. Mean ± SEM of time spent freezing on the conditioned stimulus + (CS+) (b) and conditioned stimulus − (CS−) (c) on day 4 and day 5 for each animal. Asterisks represent significance with respect to time or treatment. Repeated measures two-way analysis of variance and Dunnett post-hoc test.
*p < 0.05
**p < 0.01
***p < 0.001
CTAP: D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; DAMGO: [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin; E: recall; R: retrieval
Behavioural analysis
Mouse behaviour was video-recorded and videos were analysed using the Solomon coder software (v.17.03.22). Freezing was used as readout for passive fear-related behaviour and was defined as ‘body immobility and the absence of vibrissae movement associated with sniffing’ (Bouton and Bolles, 1980), excluding physiologic respiration, with a minimum duration of 1 s. In the retrieval sessions on days 4 and 5, behavioural reactions were evaluated during 1 min before the first tone presentation (preCS−) and during the tone presentations (CS− and CS+). In addition to freezing, a more elaborate behavioural profile was scored, which included the estimation of six other behavioural parameters: rattling was defined as brief and fast tail movements; flight as fast active escape movements; grooming as all movements involving self-care licking; rearing as the detachment of the forepaws from the floor; exploring as the movement of the animal aiming at the surroundings, including only head movements; and quiet as the lack of movement or sleep, without any reaction to the tone. These behavioural categories were adapted from previous work (Remmes et al., 2016).
We further evaluated the average velocity of the animals during preCS−, CS+ and CS− using Ethovision software (v.14.0; Noldus Information Technology, USA). This parameter was used to evaluate active coping strategies (Pliota et al., 2018).
Statistical procedures
All statistical procedures were implemented in either GraphPad Prism software (v.6.07; GraphPad Software, CA, USA) or R software (v.3.5.1) implemented in RStudio (v.1.0.456).
To evaluate the passive coping strategies, the percentage of time freezing was used. During acquisition, freezing during single CS/US presentations was evaluated. During retrieval (R, day 4) and recall sessions (E, day 5), the four CS+ and the four CS− presentations were averaged for each R and E session. Data were evaluated using a two-way repeated measures analysis of variance. If only the main effect of time was detected, treatments were pooled and differences over time were evaluated with a Dunnett’s post-hoc test. When significant interaction was present, two different Dunnett’s post-hoc tests were performed: a time course analysis, to evaluate changes with respect to values in R1 in each treatment, and a treatment comparison, to evaluate differences with respect to Control at a given time. When many p-values are reported at the same time, the most conservative value is given (i.e. the smallest for non-significant values and the highest for significant values). PreCS− behavioural measurements were used to verify standard baseline conditions in the animals, but excluded from the global statistical analysis.
To evaluate the active coping strategies, mean velocity during the tone presentations was used. During the acquisition average velocities for each tone were scored. The average velocity 1 min before the first CS+/US event (preCS+/US) was scored to define the basal locomotor state, but excluded from the statistical analysis. During retrieval and recall sessions, in preCS− time, the average velocity during four CS− and four CS+ presentations was measured and averaged for each R and E session. Analogous statistical testing as for the freezing behaviour was performed.
In order to extract an overall behavioural profile for each animal, its freezing behaviour and six additional behavioural expressions were evaluated in a CoDA. The seven behavioural features were grouped into three categories: the non-aversive category included rearing, grooming and quiet periods; the neutral category included exploring; and the aversive category combined freezing, flight and rattling. The compositional nature of the data is reflected in the way that all seven behavioural features add up to a constant value for each animal and session. To deal with zero values, they were replaced with the minimal amount of scorable freezing in the Solomon Coder (0.2 seconds), and an isometric log–ratio (ilr) transformation was applied to the three behavioural categories (Egozcue et al., 2003; Supplementary material, Material and methods online) with the compositions R package (v.1.40-2). ilr transformation can be understood as a centred log–ratio (clr) transformation followed by an isometric rotation (Supplementary Material and methods, Figure S4(c) to (e)). In a spatial representation, clr transformation shifts the origin of the coordinate system into the centre of the plane with a logarithmic transformation (Supplementary Figure S4(d)). Following isometric rotation constrains the representation of the data to a 2D plane, keeping the distances between the data points (Supplementary Figure S4(e)). Thereafter multivariate analysis of variance with time session as a within factor, and Hotelling’s comparison corrected by Bonferroni method were performed.
Results
Activation of the µ-opioid system in the dMT modulates passive behaviour
As previous work has demonstrated that the dMT participates in fear memory retrieval (Padilla-Coreano et al., 2012), we evaluated whether MORs in the dMT alter freezing behaviour in our auditory fear conditioning paradigm. To do so, we divided mice into three treatment groups (DAMGO, CTAP, Control; Figures 1 and 2). To assess passive behavioural coping strategies associated with fear expression of the animals, we analysed freezing behaviour during successive presentations of neutral (CS−) and aversive (CS+) conditioned stimuli. The randomly allocated DAMGO, CTAP and Control mice learned successfully during the conditioning sessions on day 2 (main effect of time: F(7, 168) = 7.2845; p < 0.001; CS+/US1 vs. CS+/US2 to CS+/US8: padj < 0.001; Supplementary Figure S1(a)). The three groups did not differ in fear acquisition (main effect of treatment: F(2, 24) = 0.8389; p = 0.445; interaction: F(14, 168) = 0.6768; p = 0.7948; Supplementary Figure S1(a)). During fear memory retrieval and extinction, Control mice showed successful fear memory extinction for the CS+ from R4 on (interaction: F(14, 168) = 3.412; p < 0.001; R1 vs. R4 to R6: padj < 0.001; R1 vs. E2: padj < 0.001; Figure 2(b)). In contrast, freezing behaviour decreased after R6 in DAMGO-treated mice (R6 vs. R1: padj = 0.007; R1 vs. E2: padj = 0.042) and after E1 in CTAP-treated mice (R1 vs. E1: padj = 0.031; R1 vs. E2: padj < 0.001). When we compared treatments within sessions, freezing to the CS+ remained elevated in CTAP-treated mice as compared with Control in sessions R4 to R6 (R4: Control vs. CTAP: padj = 0.004; R5: padj < 0.0001; R6: padj < 0.0001). DAMGO-treated animals showed significantly less freezing from R1 to R3 (Control vs. DAMGO; R1: padj = 0.001; R2: padj = 0.009; R3: padj < 0.001), as well as in E1 (Control vs. DAMGO: E1: padj = 0.005).
For the CS−, Control and CTAP-treated animals showed significantly less freezing from R2 on with respect to R1 (interaction: F(14, 168) = 5.127; p < 0.001; padjs < 0.014; Figure 2(c)). In contrast, in DAMGO-treated mice displayed low freezing values from R1 on (padjs > 0.081). Treatment comparison within session suggests that DAMGO treatment significantly decreased freezing in R1, R2 and R3 (Control vs. DAMGO: R1: padj < 0.001; R2: padj < 0.001; R3: padj = 0.008). These results showed that DAMGO induced overall low freezing values, whereas CTAP-treated mice showed high freezing levels during all sessions on day 4 (R1 to R6).
Effects of CTAP and DAMGO in the dMT on active behaviour
DAMGO-treated animals showed strongly reduced freezing during retrieval and extinction of conditioned fear. However, such a decrement in freezing does not necessarily reflect an attenuated fear response, since animals might simply show more activity or express more active coping strategies (i.e. fast evasive moving as flight response) under a potentially equivalent fear state.
Before testing for a potential influence of CTAP or DAMGO on locomotor activity during CS presentations, we first evaluated the basal locomotor activity of the animals and analysed their average velocity during the acquisition and retrieval before tone presentations (preCS−). No differences were found in the locomotor activity during the preCS+/US time (F(2, 24) = 2.868; p = 0.076). During acquisition, velocity decreased compared with CS+/US1 presentation (main effect of time: F(7, 168) = 3.346; p = 0.002; Supplementary Figure S1(b)) in CS+/US2 (padj < 0.001), CS+/US4 (padj = 0.023) and CS+/US6 (padj = 0.038) without differences between treatments (main effect of treatment: F(2, 24) = 1.178; p = 0.325; interaction: F(14, 168) = 0.436; p = 0.961). In retrieval and recall, Control and CTAP-treated mice did not change the average speed with respect to R1 during the preCS− (interaction: F(14, 168) = 7.783; p < 0.001; R1 vs. R2 to E2: padjs > 0.0810; Figure 3(a)). In contrast, DAMGO increased preCS− activity in R2 (R1 vs. R2: padj = 0.002) and decreased activity from R4 on (R1 vs. R4 to E2: padjs < 0.001). When comparing treatments within sessions, DAMGO-treated animals significantly increased their average speed in the R1 to R3 sessions during the preCS− (Control vs. DAMGO; R1 and R2: padj < 0.001; R3: padj = 0.006). These results suggest that a DAMGO injection in the dMT before R1 induced high locomotor activity in mice.
Figure 3.
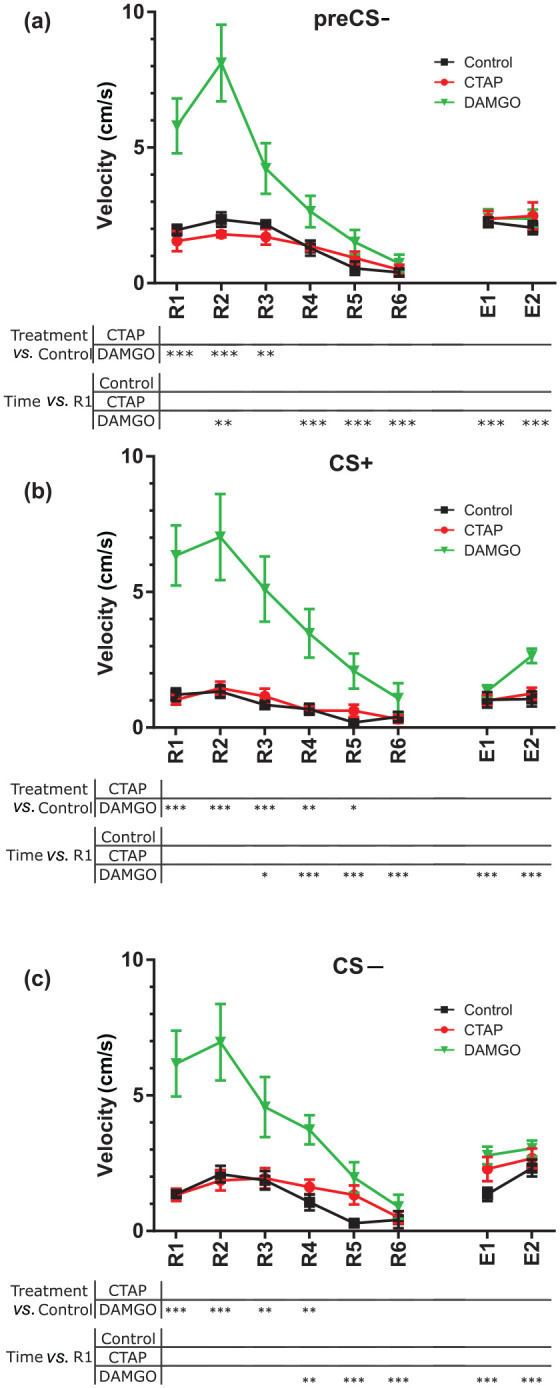
Effects of µ-opioid receptors in the dorsal midline thalamus on the active coping strategy during fear memory retrieval and recall. (a) Mean ± SEM of the average speed of each animal during the 60 s before the first tone was delivered at each R and E session. (b, c) Mean ± SEM of the active coping performance during the tone presentations in the conditioned stimulus + (CS+) (b) and conditioned stimulus − (CS−) (c) presentations. Repeated measures two-way analysis of variance and Dunnett post-hoc test.
*p < 0.05
**p < 0.01
**p < 0.001
CTAP: D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; DAMGO: [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin; E: recall; R: retrieval
Once the basal levels of activity were assessed, we evaluated changes in active coping strategies (Pliota et al., 2018). Neither Control nor CTAP-treated mice changed their active coping strategy with respect to R1 during CS+ presentations (interaction: F(14, 168) = 7.202; p < 0.001; padjs > 0.291; Figure 3(b)) or with respect to Control values (padjs > 0.808). Compared with Control, DAMGO increased the velocity of mice from R1 to R5 sessions (Control vs. DAMGO: R1, R2 and R3: padj < 0.001; R4: padj = 0.001; R5: padj = 0.036). In contrast, DAMGO-treated mice decreased their average speed with respect to R1 from R3 on (R1 vs. R3: padj = 0.014; R1 vs. R4 to E2: padj < 0.001).
During the CS− presentations, similar results were obtained. The activity of animals in the CTAP and Control groups did not change over time (interaction: F(14, 168) = 5.641; p < 0.001; padjs > 0.178; Figure 3(c)) and was indistinguishable from each other within sessions (padjs > 0.300). However, compared with Control, DAMGO increased the activity, from R1 to R4 (Control vs. DAMGO: R1 and R2: padj < 0.001; R3 and R4: padj = 0.001), whereas they showed decreased activity with respect to R1 from R4 on (R1 vs. R4: padj = 0.001; R1 vs. R5 to E2: padj < 0.001).
Overall, these results suggest that CTAP did not change the active coping strategy of mice. Interestingly, DAMGO induced hyperlocomotion that interferes with the passive and active coping behaviour during fear retrieval and extinction learning. However, the DAMGO-induced hyperlocomotion was not detected from R4 on and velocities were indistinguishable between the treatment groups in E1 and E2 sessions. Thus, active and passive coping strategies for the DAMGO-treated mice can be evaluated in E1 and E2, indicating that DAMGO decreased freezing in these late sessions.
The µ-opioid system in the dMT affects the behavioural profile in mice
We extended the analysis to include a behavioural profile based on seven features chosen a priori (Supplementary Figures S2, S3), to assess possible effects on coping strategies. These behavioural traits were classified in three categories (aversive, neutral and non-aversive). The behaviour of each animal was characterized by the time spent in each of the three categories, and an ilr transformation was applied (Supplementary Material and methods; Figure S4). During the preCS−, behaviour did not significantly differ between CTAP-treated, DAMGO-treated and Control mice (F(4, 48) = 1.7238; p = 0.1602). During the CS+, Control and DAMGO-treated animals changed their behaviour (interaction: F(28, 336) = 2.394; p < 0.001; Figure 4(a)). Compared with R1, the Control mice became significantly more non-aversive in R5 (padj < 0.001), R6 (padj = 0.023) and E2 (padj < 0.001) and DAMGO-treated animals in R6 (padj = 0.002) and E2 (padj < 0.001). In contrast, the CTAP-treated mice failed to adapt the behaviour across the sessions (padjs > 0.084) and remained in the aversive state until the end of the experimental paradigm. Comparing treatments within sessions, DAMGO changed behaviour in R3 (padj = 0.021) and R5 (padj = 0.003) towards the neutral category, whereas behaviour in CTAP remained aversive in R5 with respect to Control mice (padj = 0.001).
Figure 4.
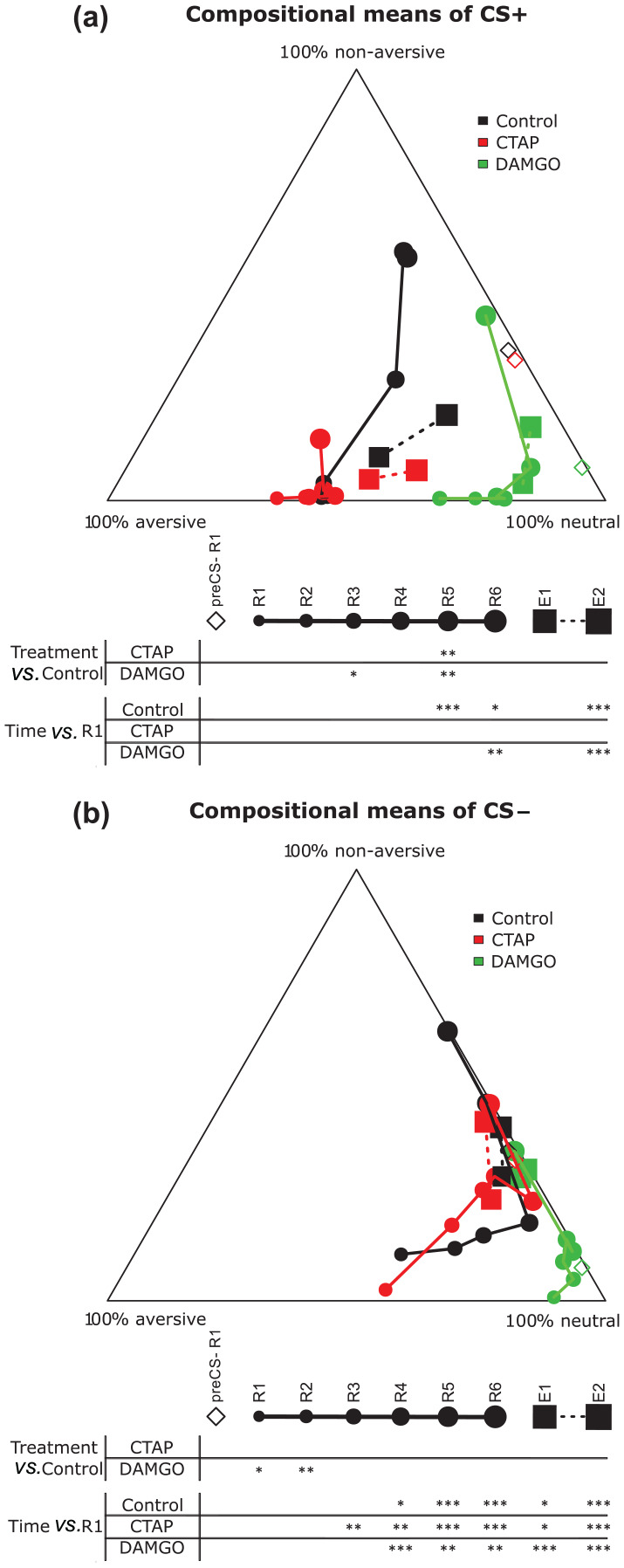
Compositional analysis of the seven feature based behavioural profiles for the CTAP, DAMGO and Control groups, during fear memory retrieval and recall. Compositional plots of the mean behaviour exhibited by each group across extinction sessions in conditioned stimulus + (CS+) (a) and conditioned stimulus − (CS−) (b). The proximity to each corner (aversive, neutral and non-aversive) indicates the percentage of time spent showing the corresponding behaviour. Asterisks represent significance respect time or treatment. Repeated measures two-way multivariate analysis of variance, with Hotelling’s test (corrected by Bonferroni method) for pairwise comparison.
*p < 0.05
**p < 0.01
***p < 0.001
CTAP: D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2; DAMGO: [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin; E: recall; R: retrieval
During CS− presentations, all treatments induced behavioural changes (interaction: F(28, 336) = 1.897; p = 0.005; Figure 4(b)). CTAP-treated mice changed from neutral to non-aversive behaviours (padjs < 0.025), whereas for DAMGO-treated and Control animals, changes became significant from R4 on (padjs < 0.034), first switching from mildly aversive to neutral and later to non-aversive behaviours. Comparing treatments within sessions, DAMGO induced a shift to neutral behaviour in R1 (padj = 0.049) and R2 (padj = 0.024). These results suggest that CTAP kept the behavioural reaction to the CS+ tone invariable, not allowing behavioural changes along the multiple tone presentations.
Discussion
In the present work, we analysed the role of the µ-opioid system in the dMT in fear memory retrieval and extinction. Pharmacological blockage of MORs in the dMT impaired fear extinction and kept the behaviour in an aversive passive state. In contrast, local MOR activation by a single dose of DAMGO induced a transient increase in locomotion during fear extinction, and, more importantly, decreased freezing during recall of extinction, which was tested 24 h later when direct DAMGO effects were excluded.
The blockage of the µ-opioid system by CTAP in the dMT reveals its involvement in fear extinction processes. Previous work has shown that the dMT is implicated in fear memory retrieval (Do-Monte et al., 2015; Li et al., 2014; Padilla-Coreano et al., 2012; Penzo et al., 2015), likely due to its glutamatergic projections to fear-relevant structures such as the medial prefrontal cortex and the amygdala (Haight and Flagel, 2014). Projections from the PVT to the basolateral amygdala (BLA) are, in fact, modulated by MORs in the PVT (Goedecke et al., 2019). Given that MOR activation induces a membrane hyperpolarization in neurons of the dMT (Brunton and Charpak, 1998; Goedecke et al., 2019), CTAP may facilitate neuronal activity in dMT to BLA projections. As PVT inactivation causes a fear memory retrieval deficit (Padilla-Coreano et al., 2012), CTAP should increase fear memory retrieval during R1, but instead, we detected a prominent fear extinction deficit. In line with this, local blockage of the µ-opioid system in other brain structures, such as the vlPAG (McNally et al., 2005), or systemic inhibition of the opioid system by naloxone (Kim and Richardson, 2009) affects fear extinction rather than expression. We conclude that the MOR system in the dMT participates in the fear extinction task.
In auditory Pavlovian fear conditioning, freezing is the most widely studied behavioural response in rodents. Of note, freezing is a passive expression of fear, and there are indicators of rather active coping strategies such as locomotor activity (Pliota et al., 2018). Active coping strategies during stressful events might prevent passive maladaptive responses in fear extinction paradigms (Fucich et al., 2016), which should be considered in behavioural analyses of fear. However, DAMGO application in the dMT resulted in an increase in locomotor activity, already present under baseline conditions before tone presentation, which can be assumed to interfere with active or passive behavioural response components. The persistence of this hyperlocomotion can be evaluated with the preCS− activity and seems to be relevant at least until the R4 session, and may affect the retrieval sessions during the retrieval day. Therefore, changes in behaviour induced by DAMGO before that time point cannot be interpreted appropriately. Opioids have been previously described to induce hyperlocomotion in rodents (Vanderschuren et al., 1997), and these effects have been linked to the activation of the mesolimbic reward pathway. As DAMGO decreases the neural activity of the PVT in vitro (Goedecke et al., 2019), hyperlocomotion might be associated with a low activity of the dMT. However, there is no hyperlocomotion effect reported after PVT lesion (Li et al., 2014) or pharmacological application of muscimol, a GABA-A receptor agonist (Padilla-Coreano et al., 2012). Some other authors even reported a hypolocomotional effect after the injection of the GABA-A receptor and GABA-B receptor agonists muscimol and baclofen into the PVT (Barson and Leibowitz, 2015). Therefore, DAMGO-induced hyperlocomotion cannot be attributed to a simple decrease of neural activity in the PVT. Rather, a specific neuronal subpopulation expressing MOR may lead these effects.
DAMGO shows a short half-life in vivo (~15 min (Szeto et al., 2001)). As a consequence, DAMGO-induced hyperlocomotion is absent on day 5 and the reduced freezing behaviour on that day cannot be masked directly by the hyperlocomotion. Interestingly, other hyperlocomotion-inducing stimulants, such as amphetamine, did not facilitate fear memory extinction consolidation on day 5 (Mueller et al., 2009). Therefore, although we cannot fully exclude that in our hands the hyperlocomotion itself is triggering the fear memory extinction consolidation in day 5, this is likely not the case. Despite this, the hyperlocomotion present on day 4 impedes a proper fear memory retrieval analysis, raising the question of whether DAMGO was able to erase the fear memory or facilitate the extinction. Some evidence points out that opioids might be able to erase fear and pain memory traces (Sandkühler and Lee, 2013). However, this is unlikely in our experimental conditions. Memory erasure procedures typically involve experimental designs affecting memory consolidation some hours (6 h) after the memory acquisition, which is far from the time steps used in this work (~36 h from the last acquisition trial), where the consolidation procedures have presumably finished. We, rather, suggest that the activation of the MOR in the dMT during the CS re-exposure facilitates fear extinction consolidation. This effect is likely associated with the inactivation of the dMT, as the optogenetic inhibition of the PVT to central amygdala pathway has similar effects in fear extinction consolidation (Do-Monte et al., 2015). In fact, projections from the PVT to the central amygdala might mediate the effects on freezing behaviour (Do-Monte et al., 2015; Penzo et al., 2015), which can be influenced by DAMGO treatment (Goedecke et al., 2019). Our results are, therefore, in line with previous findings, and suggest that the activation of MORs in the dMT influences fear extinction consolidation.
Finally, to evaluate changes in the behavioural pattern of the animals along the extinction sessions, we implemented a behavioural analysis that completes the aforementioned freezing with other complementary behavioural traits. As the values tend to sum to a constant value, data require special statistical handling known as CoDA (Aitchison, 1982). With this we were able to avoid spurious correlations in the statistical analysis.
Through the presentation of the successive tones, control animals showed aversive behaviours at the beginning of the day 4, developing towards neutral behaviour, and finally ended being mostly non-aversive. The behaviour of all the groups rapidly changed in the case of the CS− presentation, but for the CS+, CTAP-treated mice remained in an aversive state. DAMGO-treated mice showed predominantly a neutral behaviour, yet they were still able to adapt their behaviour. The dMT is involved in behavioural flexibility in tasks where rodents have to adapt their behaviour to rewarding cues changing over time (Marton et al., 2018). Our results show that specifically MORs in the dMT participate in the elaboration of the behaviour, which, in turn, might influence the fear status in later sessions.
The following points have to be considered when interpreting our data. First, as only males were used, results might not be directly generalized to female subjects. Second, while correct placement of cannulas was documented and confirmed to be in the PVT, drug effects in adjacent regions like the paratenial, the mediodorsal or centromedial nucleus cannot be excluded completely, due to the small nature of this thalamic structure. Therefore, injections are expected to affect mainly PVT, with partial involvement of other adjacent dMT structures. Finally, in our experimental conditions we detected a slight increase in CS− freezing in contrast to previous results in our group (see, for instance, Chauveau et al., 2012). We consider this a consequence of delaying the retrieval one day more with respect to our previous experiments. This delay was necessary to ensure the recruitment of the PVT into the fear memory circuit (Do-Monte et al., 2015; Padilla-Coreano et al., 2012). As it has been proposed that contextual fear memory generalization increases when a delay between acquisition and retrieval is present (Wiltgen and Silva, 2007), we suggest that cued fear generalization is also affected when retrieval sessions are delayed.
Our results suggest that MORs in the dMT are a potential target for pharmacological interventions in fear- and anxiety-related disorders. Indeed, human studies have shown lower incidence of post-traumatic stress disorder (PTSD) in patients that have received morphine after a traumatic event (Bali et al., 2015; Bryant et al., 2009; Holbrook et al., 2010). In animal studies, the intervention with morphine in a PTSD model facilitates fear memory extinction (Szczytkowski-Thomson et al., 2013), indicating that a local access to the dMT is not necessary to make opioid treatments effective. In light of the addictive potential and possible misuse of medication targeting MORs, alternative pharmacological tools are studied that aim to increase endogenous opioid levels. In fact, local application of an enkefalinase inhibitor in the rodent vlPAG facilitated fear extinction (McNally, 2005), as MORs in this brain structure participate in fear extinction processes (McNally et al., 2005). These results show that pharmacologically targeting the opioid system may benefit the treatment of anxiety spectrum disorders.
Using pharmacological interventions in combination with detailed behavioural analysis, we show the involvement of the µ-opioid system in fear extinction. Extinction learning following cued Pavlovian fear conditioning in rodents can be considered a model of exposure therapy in phobic patients, where the subjects are exposed to the phobogenic object in a controlled manner. Interestingly, application of opioid antagonists during exposure therapy has been shown to decrease the tolerance to the exposure agent (Kozak et al., 2007; Merluzzi et al., 1991). Taken together, we suggest that the µ-opioid system in the dMT participates in the anxiolytic effects of the systemic application of µ-opioid agonists in humans (Bali et al., 2015; Bryant et al., 2009; Holbrook et al., 2010) and rodents (Szczytkowski-Thomson et al., 2013).
Supplemental Material
Supplemental material, Supplementary_material for The µ-opioid system in midline thalamic nuclei modulates defence strategies towards a conditioned fear stimulus in male mice by Xabier Bengoetxea, Lena Goedecke, Peter Blaesse, Hans-Christian Pape and Kay Jüngling in Journal of Psychopharmacology
Footnotes
Author contribution: XB performed the experiments, the statistical analysis. LG, PB and HCP provided expert opinion, reviewed and approved the final manuscript. XB wrote the paper, with contributions by KJ and HCP. KJ supervised the study, reviewed and approved the final manuscript.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Interdisciplinary Centre for Clinical Research (IZKF) (Jün3/003/17 to KJ) and German Research Foundation (Fear, Anxiety, Anxiety Disorders; SFB-TRR 58 TP A08 to KJ).
ORCID iD: Xabier Bengoetxea  https://orcid.org/0000-0001-8651-6320
https://orcid.org/0000-0001-8651-6320
Supplemental material: Supplemental material for this article is available online.
References
- Aitchison J. (1982) The statistical analysis of compositional data. J R Stat Soc B (Methodol) 44: 139–160. [Google Scholar]
- Allen Institute for Brain Science (2011) Allen Mouse Reference Atlas. Available at: http://mouse.brain-map.org/static/atlas (accessed 2 April 2019).
- Bali A, Randhawa PK, Jaggi AS. (2015) Stress and opioids: Role of opioids in modulating stress-related behavior and effect of stress on morphine conditioned place preference. Neurosci Biobehav Rev 51: 138–150. [DOI] [PubMed] [Google Scholar]
- Barson JR, Leibowitz SF. (2015) GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett 609: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. (1980) Conditioned fear assessed by freezing and by the suppression of three different baselines. Anim Learn Behav 8: 429–434. [Google Scholar]
- Brunton J, Charpak S. (1998) μ-Opioid peptides inhibit thalamic neurons. J Neurosci 18: 1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, et al. (2009) A study of the protective function of acute morphine administration on subsequent posttraumatic stress disorder. Biol Psychiatry 65: 438–440. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jüngling K, et al. (2012) Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala. Neuropsychopharmacology 37: 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Rabiner EA, Lingford-Hughes A, et al. (2011) Opioids and anxiety. J Psychopharmacol 25: 1415–1433. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Quiñones-Laracuente K, Quirk GJ. (2015) A temporal shift in the circuits mediating retrieval of fear memory. Nature 519: 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, et al. (2003) Isometric logratio transformations for compositional data analysis. Math Geol 35: 279–300. [Google Scholar]
- Fucich EA, Paredes D, Morilak DA. (2016) Therapeutic effects of extinction learning as a model of exposure therapy in rats. Neuropsychopharmacology 41: 3092–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedecke L, Bengoetxea X, Blaesse P, et al. (2019) µ-Opioid receptor-mediated downregulation of midline thalamic pathways to basal and central amygdala. Sci Rep 9: 17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. (2014) A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci 8: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook TL, Galarneau MR, Dye JL, et al. (2010) Morphine use after combat injury in Iraq and post-traumatic stress disorder. N Engl J Med 362: 110–117. [DOI] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, et al. (2008) Neuropeptide S-mediated control of fear expression and extinction: Role of intercalated GABAergic neurons in the amygdala. Neuron 59: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. (2009) The effect of the μ-opioid receptor antagonist naloxone on extinction of conditioned fear in the developing rat. Learn Mem 16: 161–166. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ. (2015) Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev 56: 315–329. [DOI] [PubMed] [Google Scholar]
- Kozak AT, Spates CR, McChargue DE, et al. (2007) Naltrexone renders one-session exposure therapy less effective: A controlled pilot study. J Anxiety Disord 21: 142–152. [DOI] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape H-C. (2003) Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res 145: 89–98. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong X, Li S, et al. (2014) Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front Behav Neurosci 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. (2005) Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101(S), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci 119: 1672–1677. [DOI] [PubMed] [Google Scholar]
- McNally GP, Cole S. (2006) Opioid receptors in the midbrain periaqueductal gray regulate prediction errors during Pavlovian fear conditioning. Behav Neurosci 120: 313–323. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee B-W, Chiem JY, et al. (2005) The midbrain periaqueductal gray and fear extinction: Opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci 119: 1023–1033. [DOI] [PubMed] [Google Scholar]
- Marton TF, Seifikar H, Luongo FJ, et al. (2018) Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J Neurosci 38: 2569–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi TV, Taylor CB, Boltwood M, et al. (1991) Opioid antagonist impedes exposure. J Consult Clin Psychol 59: 425–430. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Fossum EN, Ingram SL, et al. (2007) Analgesic tolerance to microinjection of the μ-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology 52: 1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Olivera-Figueroa LA, Pine DS, et al. (2009) The effects of yohimbine and amphetamine on fear expression and extinction in rats. Psychopharmacology (Berl) 204: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Coreano N, Do-Monte FH, Quirk GJ. (2012) A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 62: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. (2001) Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. 2nd ed. San Diego: Academic Press. [Google Scholar]
- Penzo MA, Robert V, Tucciarone J, et al. (2015) The paraventricular thalamus controls a central amygdala fear circuit. Nature 519: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Fanselow MS. (2015) Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem 22: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliota P, Böhm V, Grössl F, et al. (2018) Stress peptides sensitize fear circuitry to promote passive coping. Mol Psychiatry. Epub ahead of print 14 June 2018. DOI: 10.1038/s41380-018-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmes J, Bodden C, Richter SH, et al. (2016) Impact of life history on fear memory and extinction. Front Behav Neurosci 10: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J, Lee J. (2013) How to erase memory traces of pain and fear. Trends Neurosci 36: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K, Oberdick J, Schilling RL. (2012) Toward an efficient and integrative analysis of limited-choice behavioral experiments. J Neurosci 32: 12651–12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PF, Renner RM, Haslett SJ. (2016) Compositional data in neuroscience: If you’ve got it, log it! J Neurosci Methods 271: 154–159. [DOI] [PubMed] [Google Scholar]
- Szczytkowski-Thomson JL, Lebonville CL, Lysle DT. (2013) Morphine prevents the development of stress-enhanced fear learning. Pharmacol Biochem Behav 103: 672–677. [DOI] [PubMed] [Google Scholar]
- Szeto HH, Lovelace JL, Fridland G, et al. (2001) In vivo pharmacokinetics of selective mu-opioid peptide agonists. J Pharmacol Exp Ther 298: 57–61. [PubMed] [Google Scholar]
- Vanderschuren LJ, Tjon GH, Nestby P, et al. (1997) Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology 131: 115–122. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. (2007) Memory for context becomes less specific with time. Learn Mem 14: 313–317. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Nachtrab G, Keyes PC, et al. (2018) Dynamic salience processing in paraventricular thalamus gates associative learning. Science 362: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wienecke CFR, Nachtrab G, et al. (2016) A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530: 219–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material for The µ-opioid system in midline thalamic nuclei modulates defence strategies towards a conditioned fear stimulus in male mice by Xabier Bengoetxea, Lena Goedecke, Peter Blaesse, Hans-Christian Pape and Kay Jüngling in Journal of Psychopharmacology


