Abstract
Objective
In the present investigation, we evaluated the effects of microRNA-365 (miR-365) on non-small-cell lung cancer (NSCLC) cell metastasis and invasion in patients with bone metastasis of lung cancer.
Methods
Blood samples from patients with NSCLC and healthy controls and the A549 adenocarcinoma cell line were included in this study. Quantitative real-time PCR and microarray were performed on blood samples. The MTT assay, luciferase reporter assay, Transwell assay, ELISA, and western blot were performed to evaluate expression of associated factors.
Results
Expression of miR-365 was reduced in patients with bone metastasis of NSCLC. Downregulation of miR-365 promoted cell growth, metastasis, and invasion of NSCLC. Upregulation of miR-365 reduced cell growth, metastasis, and invasion of NSCLC. Downregulation of miR-365 induced expression of NKX homeobox-1 (NKX2-1), epidermal growth factor receptor (EGFR), phosphoinositide-3-kinase (PI3K), and p-Akt proteins in an in vitro model of NSCLC. Inhibition of NKX2-1 reduced the effects of miR-365 on cell growth, metastasis, and invasion of NSCLC. Activation of EGFR reduced the effects of miR-365 on cell growth, metastasis, and invasion of NSCLC.
Conclusions
The study established that the serum miR-365 suppresses NSCLC cell metastasis and invasion in patients with bone metastasis of lung cancer via EGFR/PI3K through NKX2-1.
Keywords: microRNA-365, non-small-cell lung cancer, bone metastasis, epidermal growth factor receptor, phosphoinositide-3-kinase, NKX homeobox-1
Introduction
At present, lung cancer is the most common malignant tumor worldwide.1 According to estimates by GLOBOCAN 2018, the number of new cases of lung cancer is 1.82 million, accounting for 12.9% of all types of tumors; the number of deaths is 1.59 million, accounting for 19.4% of all types of malignancies globally.2 In China, lung cancer has the highest morbidity and mortality among all malignant tumors.2,3 The number of cases of lung cancer reached 730,000, and the number of death cases reached 610,000 in China in 2018. In addition, the Chinese government has listed lung cancer as a focus of future cancer prevention and treatment.4
Epidermal growth factor receptor (EGFR), a 170-kDa receptor tyrosine kinase (RTK) containing 1186 amino acids located on the short arm of chromosome 7, is the first cell surface receptor considered to be involved in tumorigenesis.5 In addition to binding, with the highest affinity, epidermal growth factor (EGF), EGFR also has ligands, including transforming growth factor (TGF), heparin-binding EGF-like growth factor, and epiregulin (EPR).6 Upon effective binding of EGFR to a ligand, EGFR is activated and homodimerized with its own molecule or heterodimerized with other members within the family, leading to phosphorylation of intracellular tyrosine residues.7
EGFR, which belongs to the erbB family, activates the same downstream signaling pathways, including the Ras-Raf-MAPK signaling pathway and the phosphoinositide-3-kinase (PI3K)/AKT signaling pathway, involving other key effectors such as nuclear factor-κB and mammalian target of rapamycin (mTOR), which could be inhibited by phosphatase and tensin homolog (PTEN).8 Activated EGFR can promote cell cycle progression, proliferation, differentiation, adhesion, survival, invasion, metastasis, and angiogenesis, as well as inhibit cancer cell apoptosis.8
NKX homeobox-1 (NKX2-1) is a transcription factor containing the homologous domain of the NKX2 gene family, which is expressed in human embryonic lung and adult lung to maintain normal lung development and function.9 NKX2-1 has been suggested as a driving factor in lung cancer and a specific biomarker for lung adenocarcinoma.9 Clinical studies have indicated that NKX2-1 can be used as a key indicator for determining the tissue origination of lung adenocarcinoma and pulmonary malignant mesothelioma.10
MicroRNAs (miRNA) are a class of highly conserved endogenous, single-stranded, noncoding RNAs.11 Numerous studies have shown that miRNAs are involved in the pathogenesis and progression of a variety of tumors.11–14 miRNAs have been confirmed to play roles in pancreatic, liver, gastric, and esophageal cancers and other tumors, affecting the invasion and metastasis of tumors.12 In addition, miRNAs can regulate tumor cell growth, inhibit apoptosis, promote tumor angiogenesis, among other functions.12 In the present investigation, we evaluated the effects of miRNA-365 on non-small-cell lung cancer (NSCLC) cell metastasis and invasion in patients with bone metastasis of lung cancer.
Materials and methods
Patients with NSCLC
This protocol was approved by The First Clinical Hospital, Shanxi Medical University. Patients with NSCLC (n = 31) and healthy volunteers (n = 31) were enrolled, blood samples collected, and serum prepared by centrifugation at 1000 ×g for 10 minutes at 4°C. Blood samples in the NSCLC group were from patients with recurrent adenocarcinoma within 2 years or patients without recurrence within 3 years. The enrolled patients met the following criteria. (1) All samples were surgically resected before chemotherapy or radiotherapy and snap frozen. (2) Tumors were all non-small-cell lung adenocarcinoma. (3) All samples were from patients with recurrent adenocarcinoma within 2 years or patients without recurrence within 3 years. (4) All tumor samples were >80% tumor. (5) Normal lung tissue adjacent to each patient was evaluated and collected 2 to 4 cm around the adenocarcinoma. (6) Hematoxylin and eosin (HE)-stained sections were evaluated by a pathologist certified by the Ethics committee of the First Clinical Hospital, Shanxi Medical University to determine that there were no precancerous lesions.
Extraction of RNA and qRT-PCR studies
Total RNA was extracted from blood samples using TRIzol (Invitrogen/Thermo Fisher Scientific Inc., Waltham, MA, USA). cDNA was prepared using a SuperScript First-Strand Synthesis system (Invitrogen/Thermo Fisher Scientific Inc.). The reverse transcription primer of miR-365 was 5′-GGGAAAATGAGGGACTTTTGGG GGCAGATG-3′. The quantitative real-time (qRT)-PCR study was conducted using a StepOne Real-Time PCR system (Applied Biosystems/Thermo Fisher Scientific Inc.) using FastStart SYBR Green qPCR Master Mix (Roche Diagnostics GmbH, Mannheim, Germany), with denaturation at 95°C for 10 s; annealing at 60°C for 30 s; extension at 70°C for 10 s for 40 cycles. The relative mRNA expression was measured through the 2−ΔΔCq method. The qRT-PCR primers were as follows miRNA-365: 5′-CGTAATGCCCCTAAAAAT-3′ and 5′-GTGCAGGGTCCGAGGT-3′; U6: 5′-CTCGCTTCGGCAGCACA-3′ and 5′-AACGCTTCACGAATTTGCGT-3′. U6 was used for normalization of gene expression.
Microarray
Total RNA was purified with the RNeasy kit (Qiagen, Hilden, Germany). cDNA and cRNA were generated and hybridized to HT-12 v4 BeadChips (Illumina Inc., San Diego, CA, USA). Cubic spline-normalized (without background normalization) data were analyzed by the NIA Array Analysis tool (http://lgsun.grc.nia.nih.gov/ANOVA).
Cell lines and culture conditions for in vitro studies
The A549 adenocarcinoma cell line was obtained from American Type Culture Collection (Manassas, VA, USA) and cultured using RPMI-1640 with 10% fetal bovine serum at 37°C. MiRNA-365 (Shanghai Genechem Co. Ltd.), anti-miRNA-365, and negative mimics (Shanghai Genechem Co. Ltd.) were transfected into cells using Lipofectamine 2000 reagent (Thermo Fisher Scientific Inc.).
Luciferase reporter assay
The binding sites for miR-365a-5p in NKX2-1 and the NKX2-1 3′-untranslated region (UTR) fragment were projected using TargetScan (http://www.targetscan.org/) and then amplified by PCR using the reverse primers. The amplified fragment was inserted into the luciferase gene psiCHECK2 vector (Promega, Madison, WI, USA) and named NKX2-1 3′-UTR. Forty-eight hours after transduction, cells were transfected using Lipofectamine 2000 reagent (Thermo Fisher Scientific). The luciferase assay was performed using the Luciferase Reporter Gene Detection Kit (Sigma-Aldrich Co., St Louis, MO, USA).
Cell viability assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 5 mg/mL, Thermo Fisher Scientific Inc.) was added to cells and incubated for 4 hours at 37°C. After 4 hours, the old medium was removed and dimethyl sulfoxide (DMSO) was added and incubated for 20 minutes at 37°C. The optical density (OD) values were determined by an enzyme immunoassay analyzer (Thermo Fisher Scientific Inc.) at 490 nm.
Transwell assay
To measure invasion ability, transfected cells were added to the upper chamber, and the lower chamber was filled with basic medium containing 10% FBS. Following incubation for 48 hours, the cells attached to the bottom were fixed with 4% paraformaldehyde and stained with crystal violet (Amresco LLC, Solon, OH, USA) for 20 minutes.
Western blotting
The cells were subjected to lysis using radioimmunoprecipitation assay (RIPA) buffer after incubation for 48 hours, and protein was quantified using a bicinchoninic assay (BCA) assay kit (Pierce Biotechnology/Thermo Fisher Scientific Inc.). Total proteins were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride (PVDF) membranes and blotted. The membranes were incubated with NKX2-1, EGFR, PI3K, p-AKT, and GAPDH at 4°C for 12 hours and washed with Tris-buffered saline-Tween (TBST) for 15 minutes. The membranes were incubated with rabbit anti-goat IgG for 1 hour at 37°C and washed with TBST for 15 minutes. The blots were visualized using enhanced chemiluminescence and analyzed using Image Lab 3.0 (Bio-Rad Laboratories Inc., Hercules, CA, USA).
LDH activity and caspase-3/9 activity assay
Cells were subjected to lysis using RIPA buffer after incubation for 48 hours, and protein was quantified using a BCA assay kit (Pierce Biotechnology/Thermo Fisher Scientific Inc.). Ten micrograms of protein was used to measure LDH and caspase-3/9 activity using LDH and Caspase-3/9 activity kits (Abcam Technology, Cambridge, UK).
Statistical analysis
All data are reported as means ± SD (n = 3). Student’s t-test or one-way analysis of variance (ANOVA) and Tukey’s post-test were used to establish differences. A value of P < 0.05 was regarded as statistically significant.
Results
Serum miRNA-365 expression in patients with bone metastasis of lung cancer
To study the mechanism of miRNAs in patients with bone metastasis of lung cancer, we measured changes in miRNA-365 in patients with bone metastasis of lung cancer using gene chips and qRT-PCR. As shown in Figure 1a and b, serum miRNA-365 expression in patients with bone metastasis of lung cancer was reduced (P < 0.05) compared with the normal group. Overall survival and disease-free survival were higher in patients with high expression of miRNA-365 (P < 0.05) than in those with low expression of miRNA-365 (Figure 1c and d).
Figure 1.
Serum microRNA-365 expression in patients with bone metastasis of lung cancer. (a) Gene chip and (b) quantitative PCR for expression of microRNA-365 in the serum of patients with NSCLC; (c) overall and (d) disease-free survival associated with low or high expression of miRNA-365. **P < 0.01 compared with healthy volunteer group.
Normal, healthy volunteer group; NSCLC, non-small-cell lung cancer.
MiRNA-365 regulated NSCLC cell metastasis and invasion
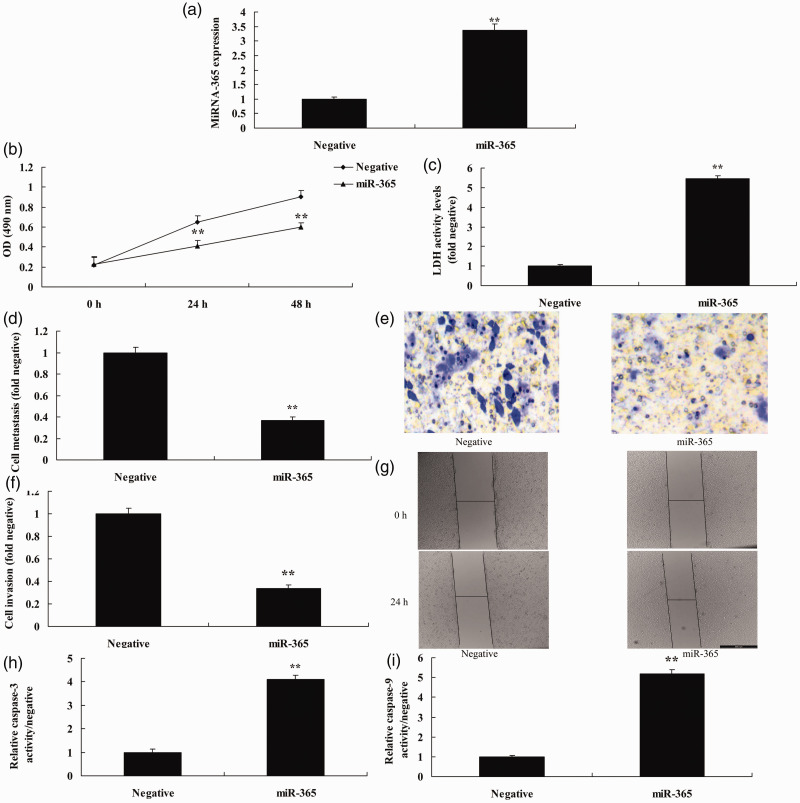
To confirm the effects of miRNA-365 on NSCLC cell metastasis and invasion, we assessed miRNA-365 expression using miRNA-365 or anti-miRNA-365 mimics. Anti-miRNA-365 mimics reduced expression of miRNA-365 in vitro following miRNA-365 overexpression (P < 0.05) compared with the negative group (Figure 2a). Downregulation of miRNA-365 promoted cell growth, metastasis, and invasion, and reduced LDH, caspase-3 and caspase-9 activity levels (all P < 0.05) in the in vitro model compared with the negative group (Figure 2b–i). Next, we observed an increase in miRNA-365 expression in vitro following miRNA-365 overexpression (P < 0.05) compared with the negative group (Figure 3a). Overexpression of miRNA-365 reduced cell growth, metastasis, and invasion, and increased LDH, caspase-3, and caspase-9 activity levels in vitro (all P < 0.05) compared with the negative group (Figure 3b–i).
Figure 2.
Downregulation of miRNA-365 promoted cell growth and migration of NSCLC. (a) miRNA-365 expression, (b) cell growth, (c) LDH activity, (d, e) migration rate, (f, g) cell invasion, and (h, i) caspase-3 and caspase-9 activity levels. **P < 0.01 compared with negative group.
Negative, negative mimic group; anti-365, downregulation of miR-365 group. NSCLC, non-small-cell lung cancer.
Figure 3.
Upregulation of miRNA-365 reduced cell growth and migration of NSCLC. (a) miRNA-365 expression, (b) cell growth, (c) LDH activity, (d, e) migration rate, (f, g) cell invasion, and (h, i) caspase-3 and caspase-9 activity levels. **P < 0.01 compared with negative group.
Negative, negative mimics group; miR-365, miR-365 overexpression group. NSCLC, non-small-cell lung cancer.
MiRNA-365 regulated EGFR/PI3K through NKX2-1
We confirmed the mechanism of miRNA-365 on NSCLC cell metastasis and invasion. The gene chip results showed that miRNA-365 regulated gene expressions, and TargetScan prediction determined that the NKX2-1 wild-type (WT) 3′-UTR and miR-365 had a conservative matching area (Figure 4a–c). Luciferase activity levels were reduced in vitro following transfection with NKX2-1 WT 3′-UTR miRNA-365 (P < 0.05) compared with the negative group (Figure 4d). Overexpression of miRNA-365 reduced NKX2-1 protein expression and suppressed expression of EGFR, PI3K, and p-AKT in the in vitro model (P < 0.05) compared with the negative group (Figure 5a–e). Downregulation of miRNA-365 promoted NKX2-1 protein expression, and induced expression of EGFR, PI3K, and p-AKT in vitro (P < 0.05) compared with the negative group (Figure 5f–j).
Figure 4.
MiRNA-365 regulates NKX2-1 signaling in NSCLC. (a) Gene chip, (Bb representative classification of repressed genes by KEGG analysis, (c) NKX2-1 3′-UTR and miR-365 had a conservative matching area, (d) luciferase activity levels, (e, f) network analysis diagram. **P < 0.01 compared with negative group.
NKX2-1, NKX homeobox-1; NSCLC, non-small-cell lung cancer; Negative, negative mimics group; miR-365, miR-365 overexpression group; WT, wild-type; MUT, mutant.
Figure 5.
MiRNA-365 regulates EGFR/PI3K through NKX2-1. Expression of (a) NKX2-1, (b) EGFR, (c) PI3K, and (d) p-AKT proteins by statistical analysis and (e) western blotting assays by overexpression of miR-365; expression of (f) NKX2-1, (g) EGFR, (h) PI3K, and (i) p-AKT proteins by statistical analysis and (j) western blotting assays by downregulation of miR-365. **P < 0.01 compared with negative group.
NKX2-1, NKX homeobox-1; EGFR, epidermal growth factor receptor; PI3K, phosphoinositide-3-kinase; p-AKT, phosphorylated protein kinase B; Negative, negative mimics group; miR-365, miR-365 overexpression group; anti-365, miR-365 downregulation group.
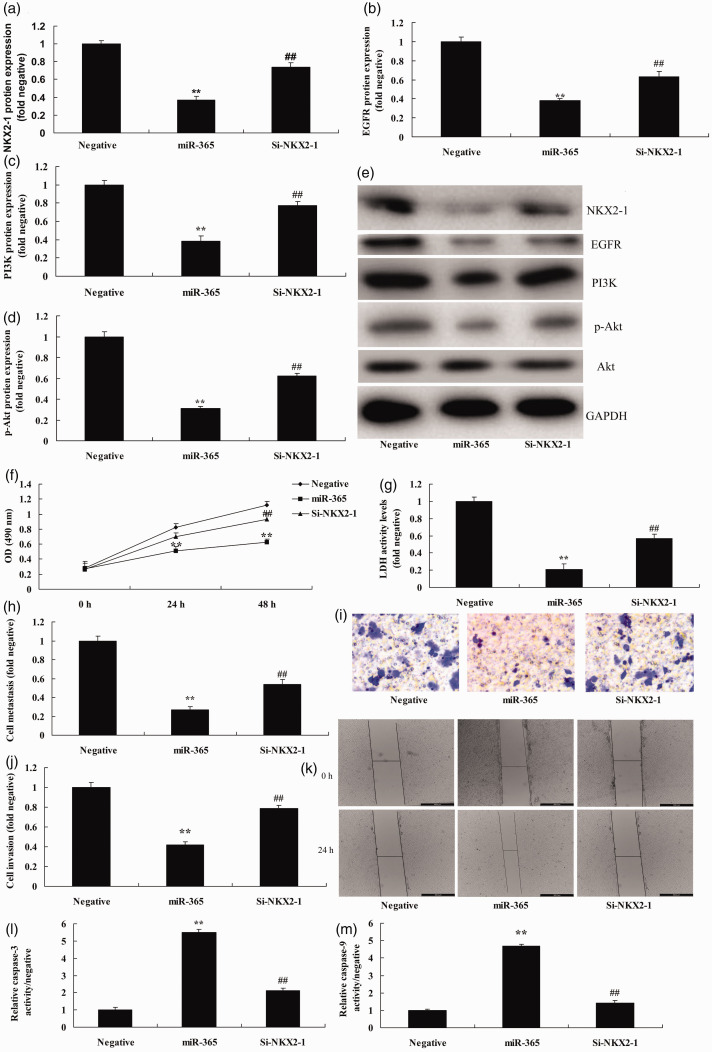
Inhibition of NKX2-1 reduced the effects of miRNA-365 on NSCLC cell metastasis and invasion
We explored the function of NKX2-1 on the effects of miRNA-365 on NSCLC cell metastasis and invasion; short interfering (si)-NKX2-1 reduced the expression of protein, and induced expression of EGFR, PI3K, and p-Akt protein in vitro following overexpression of miRNA-365 (P < 0.05) compared with the miRNA-365 group (Figure 6a–e). The inhibition of NKX2-1 reduced the effects of miRNA-365 on the inhibition of cell growth and promotion of LDH, caspase-3, and caspase-9 activity levels in the in vitro model following overexpression of miRNA-365 (P < 0.05) compared with miRNA-365 group (Figure 6f–m).
Figure 6.
The inhibition of NKX2-1 reduced the effects of microRNA-365 on non-small-cell lung cancer cell metastasis and invasion. Expression of (a) NKX2-1, (b) EGFR, (c) PI3K, and (d) p-AKT proteins by statistical analysis and (e) western blotting assays, (f) cell growth, (g) LDH activity levels, (h, i) migration rate, (j, k) cell invasion, and (l, m) caspase-3 and caspase-9 activity levels. **P < 0.01 compared with negative group; ##P < 0.01 compared with miR-365 overexpression group.
NKX2-1, NKX homeobox-1; EGFR, epidermal growth factor receptor; PI3K, phosphoinositide-3-kinase; p-AKT, phosphorylated protein kinase B; Negative, negative mimics group; miR-365, miR-365 overexpression group; si-NKX2-1, miR-365 and short interfering (si)-NKX2-1 overexpression group.
Discussion
Lung cancer is one of the most difficult malignancies to treat and a leading cause of death worldwide.3 NSCLC accounts for 85% of all lung cancer types.5 The invasion and metastasis of lung cancer are the most important factors affecting its prognosis.5 Therefore, it is important to explore potential targets affecting the invasion and metastasis of lung cancer.15 miRNAs are small, noncoding RNAs that can inhibit translation or degrade targeted mRNA.15 miRNAs can also regulate the stability and translation of targeted mRNA.16 Studies have shown that miRNAs participate in diverse biological processes of various tumors by targeting different transcripts, including those involved in cell proliferation, apoptosis, metabolism, and differentiation.15 In this study, we discovered that expression of miRNA-365 in serum of patients with bone metastasis of lung cancer was reduced compared with that of the negative group. Downregulation of miRNA-365 promoted cell growth, metastasis, and invasion, and reduced LDH, caspase-3, and caspase-9 activity levels in vitro. Sun et al.17 established that miRNA-365 suppresses cell growth and invasion in esophageal squamous cell carcinoma.
EGFR is expressed in normal lung epithelium but is also highly expressed in lung cancer. High expression of EGFR is associated with grade and stage of lung cancer.3 The EGFR/PI3K/Akt pathway is an important signaling pathway regulating tumor cell proliferation and apoptosis.18 After EGFR binds to a ligand, it activates signaling pathways through cascade reactions, including PI3K/Akt and Ras/Raf/MEK/ERK.18 A variety of growth factors are involved in signal transduction in the PI3K/Akt pathway, which is an important survival signaling pathway in vivo.19 The PI3K/Akt pathway not only promotes cell division and proliferation and participate in angiogenesis, but it also plays a role in invasion and metastasis, formation, and progression of tumors.19 The PI3K/Akt pathway is regarded as the primary pathway of cancer cell survival.20 Inhibition of the EGFR/PI3K/Akt signaling pathway has been shown to suppress the survival and migration of NSCLC cell lines.20 We confirmed that downregulation of miRNA-365 suppressed protein expression of NKX2-1 and induced expression of EGFR, PI3K, and p-Akt protein in vitro. Zhu et al.21 reported that miRNA-365 inhibits proliferation, migration, and invasion of glioma by targeting phosphatidylinositol 3-kinase regulatory subunit gamma (PIK3R3).
NKX2-1 controls the expression of surface-active materials, which are associated with the stability of lung tissue and host defense of lung tissue.22 NKX2-1 was previously considered to be only related to the development and function of lung tissue. In a genetic study of 371 cases of lung cancer, frequent mutations of the NKX21 gene were found.23 By using RNA interference and gene knockout, it was confirmed that in lung adenocarcinoma cell lines expressing NKX2-1, NKX2-1 was essential for survival and tumor growth of lung adenocarcinoma.22 NKX2-1 is specifically expressed in the lung and highly expressed in lung adenocarcinoma, suggesting that it might be a lineage survival oncogene for lung cancer.10 Large-scale analysis of lung tumors and lung cancer cell lines showed expansive proliferation of NKX2-1 in lung adenocarcinoma.22
Serum protein expression of NKX2-1 in patients with primary lung cancer is significantly higher than that in healthy controls.10 Quantitative analysis has shown that the protein level of NKX2-1 in lung cancer group is twice as high in patients with lung cancer as that in the healthy control group.23 The abnormally elevated level of serum NKX2-1 protein is significantly higher than that of carcinoembryonic antigen (CEA) in patients with primary lung cancer.24 In addition, the sensitivity, total coincidence rate, and kappa value of NKX2-1 are higher but the specificity of NKX2-1 is lower compared with that of CEA. The positive rate of NKX2-1 expression in adenocarcinoma and small cell lung cancer (SCLC) can be as high as 87.5%, which is similar to NKX2-1 expression in the pathological types of lung cancer (expression of NKX2-1 is high in lung adenocarcinoma and SCLC).24 Our experiments demonstrated that inhibition of NKX2-1 or activation of EGFR reduced the effects of miRNA-365 on NSCLC cell metastasis and invasion. Kang et al.25 showed that miRNA-365 regulates NKX2-1 in lung cancer. Moisés et al.22 reported a negative correlation between NKX2-1 and miR-365 expression in early-stage NSCLC. These results show that NKX2-1 is a target for cell growth in NSCLC.
In conclusion, in the present study, we provide strong evidence that downregulation of miRNA-365 promoted cell growth, metastasis, and invasion, and reduced LDH, caspase-3, and caspase-9 activity levels in an in vitro model of NSCLC via the EGFR/PI3K pathway through NKX2-1. These findings provide further support for current clinical trials aimed at estimating the protective effect of miRNA-365 in patients with bone metastasis of lung cancer.
Footnotes
Author contributions: YYL conceived and designed the study; JMJ, BS, and HLQ participated in data collecting; GL analyzed data; and BZ and KW commented on drafts of the paper. All authors read and approved the final manuscript.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yanyan Liu https://orcid.org/0000-0001-9707-2083
References
- 1.Mao Y, Yang D, He J, Krasna MJ. Epidemiology of lung cancer. Surg Oncol Clin N Am. 2016; 25(3):439–445. [DOI] [PubMed] [Google Scholar]
- 2.Shankar A, Dubey A, Saini D, et al. Environmental and occupational determinants of lung cancer. Transl Lung Cancer Res. 2019; 8(Suppl 1):S31–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019; 10(1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu S, Yu Y, Yang Y. Retrospect and prospect for lung cancer in China: Clinical advances of immune checkpoint inhibitors. Oncologist. 2019; 24(Suppl 1):S21–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sequist LV, Rolfe L, Allen AR. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2015; 373(6):578–579. [DOI] [PubMed] [Google Scholar]
- 6.Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer. 2018; 124(19):3830–3838. [DOI] [PubMed] [Google Scholar]
- 7.Kishi K, Sakai H, Seto T, et al. First-line onartuzumab plus erlotinib treatment for patients with MET-positive and EGFR mutation-positive non-small-cell lung cancer. Cancer Treat Res Commun. 2019; 18:100113. [DOI] [PubMed] [Google Scholar]
- 8.Pei X, Xiao J, Wei G, et al. Oenothein B inhibits human non-small cell lung cancer A549 cell proliferation by ROS-mediated PI3K/Akt/NF-κB signaling pathway. Chem Biol Interact. 2019; 298:112–120. [DOI] [PubMed] [Google Scholar]
- 9.Clarke N, Biscocho J, Kwei KA, et al. Integrative genomics implicates EGFR as a downstream mediator in NKX2-1 amplified non-small cell lung cancer. PLoS One. 2015; 10(11):e0142061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ida L, Yamaguchi T, Yanagisawa K, et al. Receptor tyrosine kinase-like orphan receptor 1, a target of NKX2-1/TTF-1 lineage-survival oncogene, inhibits apoptosis signal-regulating kinase 1-mediated pro-apoptotic signaling in lung adenocarcinoma. Cancer Sci. 2016; 107(2):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Fang J, Wang Y, Wang H, Sun CC. MiRNA-based therapeutic strategy in lung cancer. Curr Pharm Des. 2018; 23(39):6011–6018. [DOI] [PubMed] [Google Scholar]
- 12.Yao D, Cui H, Zhou S, Guo L. Morin inhibited lung cancer cells viability, growth, and migration by suppressing miR-135b and inducing its target CCNG2. Tumour Biol. 2017; 39(10):1010428317712443. [DOI] [PubMed] [Google Scholar]
- 13.Yue PY, Ha WY, Lau CC, et al. MicroRNA profiling study reveals miR-150 in association with metastasis in nasopharyngeal carcinoma. Sci Rep. 2017; 7(1):12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009; 4:199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verma V, Lautenschlaeger T. MicroRNAs in non-small cell lung cancer invasion and metastasis: from the perspective of the radiation oncologist. Expert Rev Anticancer Ther. 2016; 16(7):767–774. [DOI] [PubMed] [Google Scholar]
- 16.Afonso-Grunz F, Müller S. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci. 2015; 72(16):3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun C, Zhang X, Chen Y, Jia Q, Yang J, Shu Y. MicroRNA-365 suppresses cell growth and invasion in esophageal squamous cell carcinoma by modulating phosphoserine aminotransferase 1. Cancer Manag Res. 2018; 10:4581–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Sai B, Cao P, et al. Iron oxide nanoparticles synergize with erlotinib to suppress refractory non-small cell lung cancer cell proliferation through the inhibition of ErbB/PI3K/AKT and PTEN Activation. J Biomed Nanotechnol. 2017; 13(4):458–468. [DOI] [PubMed] [Google Scholar]
- 19.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008; 8(3):187–198. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Yamamoto H, Sakaguchi M, et al. Combined inhibition of MEK and PI3K pathways overcomes acquired resistance to EGFR-TKIs in non-small cell lung cancer. Cancer Sci. 2018; 109(10):3183–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Zhao H, Rao M, Xu S. MicroRNA-365 inhibits proliferation, migration and invasion of glioma by targeting PIK3R3. Oncol Rep. 2017; 37(4):2185–2192. [DOI] [PubMed] [Google Scholar]
- 22.Moisés J, Navarro A, Santasusagna S, et al. NKX2-1 expression as a prognostic marker in early-stage non-small-cell lung cancer. BMC Pulm Med. 2017; 17(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran L, Mattsson JS, Nodin B, et al. Various antibody clones of napsin A, thyroid transcription factor 1, and p40 and comparisons with cytokeratin 5 and p63 in histopathologic diagnostics of non-small cell lung carcinoma. Appl Immunohistochem Mol Morphol. 2016; 24(9):648–659. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Ruan W, Chen E, et al. Diagnostic value of serum NKX2-1 protein in patients with primary lung cancer. Zhejiang Da Xue Xue Bao Yi Xue Ban 2012; 41(5):535–539. [PubMed] [Google Scholar]
- 25.Kang SM, Lee HJ, Cho JY. MicroRNA-365 regulates NKX2-1, a key mediator of lung cancer. Cancer Lett. 2013; 335(2):487–494. [DOI] [PubMed] [Google Scholar]