Abstract
Background:
Real-world data on chemotherapy-naïve patients with metastatic castration-resistant prostate cancer (mCRPC) treated with abiraterone plus prednisone are limited, largely deriving from small retrospective studies.
Methods:
ABitude is an Italian, observational, prospective, multicenter study of mCRPC patients receiving abiraterone plus prednisone in clinical practice. Chemotherapy-naïve mCRPC patients were consecutively enrolled at abiraterone start (February 2016 to June 2017) and are being followed for 3 years, with evaluation approximately every 6 months. Several clinical and patients reported outcomes were examined.
Results:
In this second interim analysis, among 481 enrolled patients, 453 were evaluable for analyses. At baseline, the median age was 77 years and ~69% of patients had comorbidities (mainly cardiovascular diseases). Metastases were located mainly at bones and lymph nodes; 8.4% of patients had visceral metastases. During a median follow-up of 18 months, 1- and 2-year probability of radiographic progression-free survival were 73.9% and 56.2%, respectively; the corresponding rates for overall survival were 87.3% and 70.4%. In multivariable analyses, the number of bone metastases significantly affected radiographic progression-free survival and overall survival. During abiraterone plus prednisone treatment, 65% of patients had a ⩾50% prostate-specific antigen decline, and quality of life remained appreciably high. Among symptomatic patients according to the Brief Pain Inventory) (32%), scores significantly declined after 6 months of treatment. Overall, eight patients (1.7%) had serious adverse reactions to abiraterone.
Conclusions:
Abiraterone plus prednisone is effective and safe for chemotherapy-naïve mCRPC patients in clinical practice.
Keywords: abiraterone acetate, effectiveness, metastatic castration-resistant prostate cancer, prospective study, real-world study
Introduction
Prostate cancer is the second most frequent cancer and the fifth leading cause of cancer death in men.1 Most cases are diagnosed in early stages, with localized disease, while some patients (around 4–5 %) had metastatic prostate cancer at first diagnosis.2 Despite initial treatment, some of the patients with localized disease developed progressively metastatic disease.3 Androgen deprivation therapy (ADT) is the mainstay of treatment for patients with metastatic disease; however, these patients inevitably progress to metastatic castration-resistant prostate cancer (mCRPC), with an elevated burden of mortality.4
Until 2010, docetaxel-based chemotherapy was the only therapeutic option improving overall survival (OS) in mCRPC.5,6 Over the last few years, however, the development of new agents with varying mechanisms of actions has dramatically changed the therapeutic landscape of mCRPC.7,8 These included androgen receptor (AR)-directed agents, immunotherapy (i.e. Sipuleucel-T), novel cytotoxic drugs, bone-targeted radiopharmaceuticals, and genetically targeted therapies.
Abiraterone acetate is a prodrug of abiraterone, which is a selective inhibitor of cytochrome P (CYP) 17, a key enzyme in extragonadal and testicular androgen synthesis.9 Abiraterone acetate plus prednisone demonstrated survival benefits in mCRPC patients, initially in mCRPC patients docetaxel pre-treated10,11 and post ADT12,13 and subsequently in high-risk de novo metastatic hormone sensitive prostate cancer (mHSPC) patients. In particular, in the placebo-controlled COU-AA-302 pivotal trial in the post-ADT setting, abiraterone acetate plus prednisone significantly improved OS and radiographic progression-free survival (rPFS) compared with placebo plus prednisone in asymptomatic or mildly symptomatic mCRPC patients without prior chemotherapy.12–14 It also delayed clinical decline, time to cytotoxic chemotherapy, time to opiate use for cancer pain, as well as patient-reported pain progression and health-related quality of life (HRQoL) deterioration.15
Translating results from clinical trials with strict eligibility criteria to unselected patients treated in routine practice remains a concern.16 Clinical trials tended to exclude patients with pre-existing medical conditions and poor prognostic features, and this may have important implications for the generalizability of their results. This is particularly relevant for mCRPC, as most patients are elderly and have significant comorbidities, namely cardiovascular disease, hypertension and diabetes mellitus.17 Such vulnerable patients along with those with visceral metastases are under-represented in clinical trials of mCRPC. Real-life data from well-designed prospective studies provide valuable information on the effectiveness and safety of a drug across the full spectrum of patients who are treated in routine practice. However, to date, real-life data on abiraterone in chemotherapy-naïve mCRPC patients are limited18–30 and derived largely from relatively small retrospective studies. To bridge this crucial gap in knowledge in 2016, ABItude, a multicentric real-life observational prospective study, was initiated. With over 450 enrolled patients, it represents one of the largest prospective “real-world” investigations on the clinical effectiveness of abiraterone in the chemotherapy-naïve setting. ABItude is an ongoing study, and in the first planned interim analysis (~9 months median follow-up) abiraterone was active and safe in an elderly population with a high level of comorbidities.31 The 1-year rPFS was ~74% and adverse reactions occurred in 10% of patients, the vast majority being nonserious. In addition, abiraterone delayed functional decline and improved HRQoL and pain palliation. Here, we present results from the second interim analysis after 18 months of median follow-up.
Methods
ABItude is a prospective, observational, multicentric study conducted in Italy aiming at describing the effectiveness and safety of abiraterone acetate plus prednisone when used in clinical practice for the treatment mCRPC in chemotherapy-naïve patients. From February 2016 to June 2017, all eligible patients with mCRPC naïve to chemotherapy were consecutively enrolled in 49 Italian participating centers (urological, radiotherapy and oncological unit) at the time of initiating abiraterone acetate plus prednisone therapy according to clinical practice. Patients eligible were men aged ⩾18 years with histologically or cytologically confirmed metastatic adenocarcinoma of the prostate, asymptomatic or mildly symptomatic according to clinical judgement, naïve to chemotherapy, surgically or medically castrated, who failed ADT and in whom chemotherapy was not clinically indicated, starting treatment with abiraterone acetate within 30 days after the baseline visit according to clinical practice. Patients already treated with chemotherapy for prostate cancer or participating in experimental clinical trials were excluded.
After enrollment, follow-up visits are planned throughout the 36-month observation period according to clinical practice, that is, around every 6 months unless clinically indicated.
The study was approved by the ethic committees of the participating centers (ethics committee approval number of the coordinating center: INT-45/15-29/10/2015) and was conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice guidelines.
All patients provided written informed consent to participate in the study.
Clinical information was retrieved by medical records. At baseline, the following data were collected: demographic and anthropometric characteristics, relevant medical history (e.g. hepatic, renal, and cardiovascular function), historical data on prostate cancer (tumor–node–metastasis (TNM) stage, Gleason score at diagnosis, date of diagnosis, and date of castrate resistance), and previous treatment for prostate cancer (including ADT and its duration). Data on vital signs, relevant concomitant therapies, Eastern Cooperative Oncology Group (ECOG) performance status (PS), patient-reported pain and HRQoL were collected at baseline and at each follow-up visit. The pain was evaluated through the Brief Pain Inventory (BPI), a validated instrument comprising several individual items measured on a 0–10 scale, with lower scores representing lower levels of pain intensity or less interference of pain with activities of daily living (e.g. sleep, mood, and activity). Patients HRQoL was measured using the Functional Assessment of Cancer Therapy-Prostate (FACT-P)32 and the EuroQol-5D (EQ-5D)33 questionnaires. The EQ-5D-3L version includes three levels of severity (no problems, some problems, extreme problems) in each dimension (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and includes a visual analog scale (VAS), which records the respondent's self-rated general state of health on a scale from 0 (worst imaginable health status) to 100 (best imaginable health status). An appropriate algorithm was used to summarize data in an overall score ranging from −1 (worse-than-death health status) to 1 (best health status).34,35 Only questionnaires filled in while the patient was under treatment with abiraterone were evaluated in the analyses.
All available measurements of prostate-specific antigen (PSA) values were recorded during the course of the study. Data on abiraterone acetate and prednisone treatments (start and stop dates, dose and frequency of administration, reason for start and reason for choice of treatment, dose changes and reason, treatment interruptions and discontinuations) and on therapies subsequent to abiraterone (if any), analgesic and opioid use, clinical and radiographic progression, clinical benefit according to clinician judgement, adherence to treatment with abiraterone, adverse events (AEs) and adverse drug reactions (ADRs), and survival were recorded during the observation period. Adherence to treatment with abiraterone was evaluated using the Morisky 8-Item Self-Report Measure of Medication-taking Behavior (MMAS-8).36
Details of the statistical analyses are reported as supplementary material.
Results
Study population
From February 2016 to June 2017, a total of 481 patients were recruited in the study; 474 did start abiraterone treatment and were considered in the safety analysis; among them, 453 were evaluable for the analyses (full-analysis set). Analyses on PSA decline were based on a subset of 413 patients with PSA data at baseline and follow-up. Figure S1 (supplementary material) presents the study flowchart showing criteria violations and analysis sets.
At the time of the second planned interim analysis, the median follow-up time was 18.1 months [interquartile range (IQR) 12.1–24.0]. A total of 331 patients (73%) out of 453 were managed in oncology, 64 (14%) in urology and 58 (13%) in radiotherapy centers. In 24% of cases, abiraterone treatment choice was taken by a multidisciplinary team (73 of 305 patients with available information) (data not shown). At the time of first prostate cancer diagnosis, 14.6% of patients presented distant metastases (information was unknown for 9 patients) and 58.9% of patients had Gleason score ⩾8 (information was unknown for 59 patients).
Demographic and clinical patients’ characteristics at baseline are presented in Table 1. At the initiation of abiraterone treatment, the median age was 77 years (range: 51–93), and 265 patients (58.5%) were ⩾75 years old. The large majority of patients (94.8%) had ECOG-PS of 0 or 1. Over 42% of patients had metastases at bones only, 22% at lymph nodes only, and 23% at bones and lymph nodes; 8% of patients had visceral metastases. Among patients with bone metastases, about 22% had 10 or more lesions. The large majority of patients underwent medical castration (94%) as compared to surgical castration (6%). Over two-third of patients had at least one comorbidity at the start of treatment. In particular, 56% of patients had stable and well-compensated cardiovascular disorders (46% had hypertension) and 23.2% had metabolic disorders (11% had diabetes and 11.5% had hypercholesterolemia).
Table 1.
Demographic and clinical patients’ characteristics at baseline.
| Patients (N = 453) | |
|---|---|
| Age (years) | |
| median (q1–q3) | 77 (71–82) |
| 75 years, n (%) | 265 (58.5) |
| PSA (ng/ml), median (IQR) | 15.0 (4.7–41.5) |
| Extent of disease, n (%) | |
| Bone only | 190 (41.9) |
| Bone + lymph nodes only | 103 (22.7) |
| Lymph nodes only | 99 (21.9) |
| Visceral | 38 (8.4) |
| Othera | 23 (5.1) |
| Bone metastases, n (%) | |
| <10 | 225 (78.1) |
| ⩾10 | 63 (21.9) |
| No bone metastases | 133 |
| Missing | 32 |
| Time from first prostate cancer diagnosis to abiraterone (months), median (IQR) | 62.1 (27.0–111.9) |
| Time from ADT start to abiraterone (months), median (IQR)b | 34.7 (13.7–72.4) |
| ECOG-PS, n (%) | |
| 0 | 251 (56.7) |
| 1 | 169 (38.1) |
| ⩾2 | 23 (5.2) |
| Missing | 10 |
| No. of comorbidities, n (%) | |
| 0 | 142 (31.3) |
| 1 | 138 (30.5) |
| ⩾2 | 173 (38.2) |
| Type of comorbidityc, n (%) | |
| Cardiovascular disordersd | 260 (57.4) |
| Metabolic disorderse | 105 (23.2) |
| CSN disorders | 26 (5.7) |
| Renal disorders | 16 (3.5) |
| Hepatic disorders | 9 (2) |
| Hormonal disorders | 3 (0.7) |
| Other disorders | 91 (20.1) |
CSN, central nervous system; ECOG-PS, Eastern Cooperative Oncology Group performance status
IQR, interquartile range; PSA, prostate-specific antigen.
Including prostatic bed (n = 12) and prostate (n = 4).
One missing value.
A patient could have more than one relevant medical condition/disease.
Hypertension: 48.6%; history of myocardial infarction: 5.5%; arrhythmia: 4.4%; cardiomyopathy: 3.5%; angina pectoris: 0.7%; atherosclerosis: 0.4%; other: 8.8%.
Hypercholesterolemia 11.5%; diabetes 10.8%; hyperglycemia: 0.7%; obesity: 0.7%; other: 5.1%.
Treatment exposure and clinical outcomes
Median duration of abiraterone treatment was 14 months (IQR 7.2–20.5). During follow-up, 285 patients permanently discontinued abiraterone, mainly because of disease progression (184 patients, 65% who discontinued abiraterone). Other reasons for treatment discontinuation included death (n = 27, 9.5%), personal choice (n = 17, 6%), adverse reaction (n = 14, 4.9%) and AEs (not drug-related) (n = 11, 3.9%).
Figure 1 shows rPFS and OS for our cohort of chemotherapy-naïve mCRPC patients. During a median follow-up of 18 months, 307 patients (67.8%) did not experience any radiographical progression or death during the treatment with abiraterone. Median rPFS on abiraterone treatment was not reached and the 1- and 2-year probability of rPFS were 73.9% and 56.2%, respectively. Over the same observation period, 102 patients (22.5%) died. Median OS was not reached and the 1- and 2-year survival rates were 87.3% and 70.4%, respectively.
Figure 1.
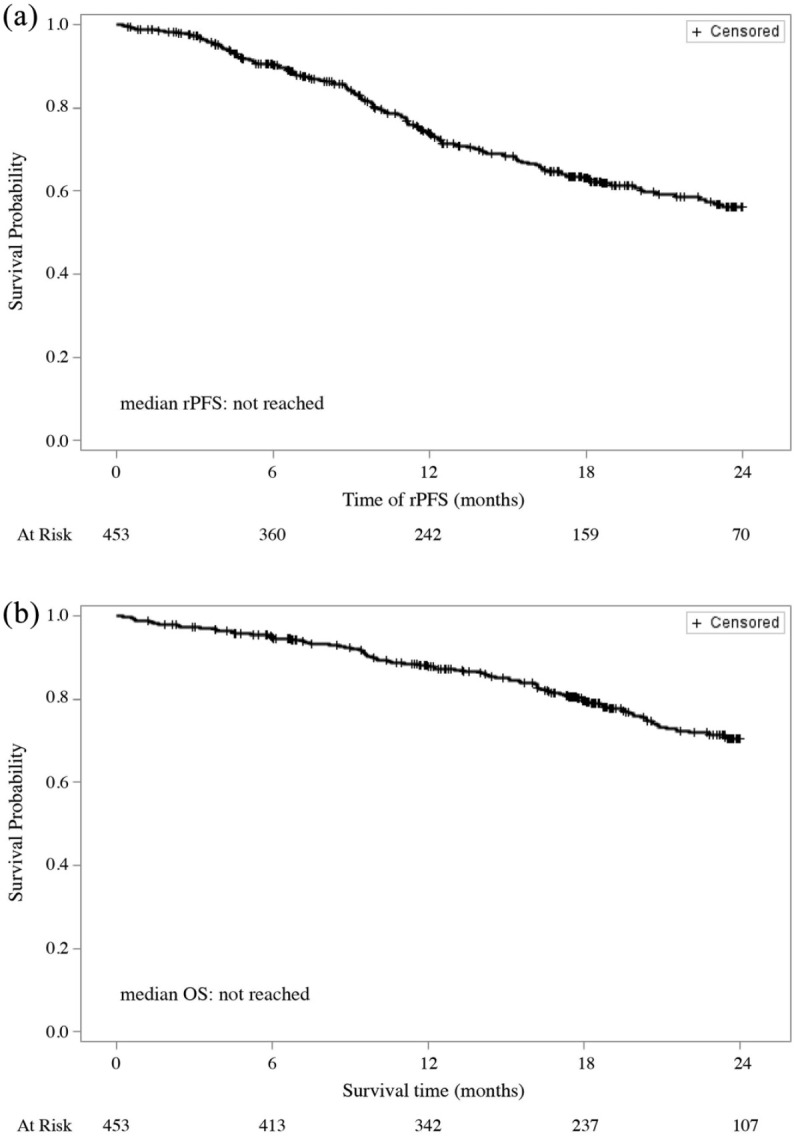
(a) Radiographical progression-free survival (rPFS) and (b) overall survival (OS) during abiraterone treatment.
Table 2 shows results for the association of selected demographic and clinical characteristics with rPFS and OS. Results from univariate and multivariate analyses were consistent. In multivariate analyses, the number of bone metastases was a significant prognostic factor for rPFS [hazard ratio, (HR) 1.76, 95% confidence interval (CI): 1.03–3.03, p = 0.04, for ⩾10 bone metastases versus 0] and OS (HR 1.98, 95% CI: 1.10–3.58, p = 0.023, for 1–9 versus 0 and HR 2.31, 95% CI: 1.12–4.76, p = 0.023, for ⩾10 versus 0); age, presence of comorbidities and visceral disease did not significantly affect rPFS and OS. In further analyses on the type of comorbidities, presence of cardiovascular (adjusted HRs of rPFS: 1.03, 95% CI: 0.71–1.49, p = 0.883; adjusted HRs of OS: 1.10, 95% CI: 0.68–1.77, p = 0.705) and metabolic conditions (adjusted HRs of rPFS: 1.12, 95% CI: 0.69–1.81, p = 0.651; adjusted HRs of OS: 1.49, 95% CI: 0.84–2.66, p = 0.174) were not significantly associated with rPFS and OS (data not shown).
Table 2.
Univariate and multivariable analysesa for the association of selected baseline demographic and clinical characteristics with rPFS and OS.
| rPFS |
OS |
||||
|---|---|---|---|---|---|
| N (%) | Univariate HR (95% CI) p-value | Adjusted HR (95% CI)b p-value | Univariate HR (95% CI) p-value | Adjusted HR (95% CI)b p-value | |
| Age (years) | |||||
| <75 | 188 (41.5) | 1c | 1c | 1c | 1c |
| ⩾75 | 265 (58.5) | 1.018 (0.735–1.410) | 0.755 (0.525–1.085) | 2.057 (1.343–3.151) | 1.066 (0.657–1.730) |
| p-value = 0.916 | p-value = 0.129 | p-value <0.001 | p-value = 0.795 | ||
| Comorbidities | |||||
| None | 142 (31.3) | 1c | 1c | 1c | 1c |
| ⩾1 | 311 (68.7) | 1.014 (0.715–1.438) | 0.983 (0.684–1.412) | 1.187 (0.768–1.834) | 1.088 (0.685–1.727) |
| p-value = 0.937 | p-value = 0.924 | p-value = 0.439 | p-value = 0.722 | ||
| Visceral disease | |||||
| No | 415 (91.6) | 1c | 1c | 1c | 1c |
| Yes | 38 (8.4) | 1.008 (0.558–1.822) | 0.942 (0.488–1.818) | 1.441 (0.771–2.695) | 1.283 (0.612–2.692) |
| p-value = 0.978 | p-value = 0.859 | p-value = 0.252 | p-value = 0.510 | ||
| Bone metastases | |||||
| 0 | 133 (31.6) | 1c | 1c | 1c | 1c |
| <10 | 225 (53.4) | 1.287 (0.867–1.910) | 1.252 (0.831–1.886) | 2.195 (1.254–3.840) | 1.983 (1.099–3.575) |
| p-value = 0.211 | p-value = 0.282 | p-value = 0.006 | p-value = 0.023 | ||
| ⩾10 | 63 (15.0) | 2.228 (1.347–3.683) | 1.762 (1.025–3.029) | 3.770 (1.952–7.283) | 2.312 (1.123–4.761) |
| Missing data | 32 | p-value = 0.002 | p-value = 0.040 | p-value <0.001 | p-value = 0.023 |
CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival; OS, overall survival.
Cox proportional hazard models.
Model adjusted for age, PSA at baseline, presence of comorbidities, visceral metastases, bone metastases, and using ECOG-PS as stratification factor.
Reference category
Of the 413 patients evaluated for PSA, 269 (65.1%) achieved a decline in PSA of ⩾50% during abiraterone treatment.
Patient-reported outcomes measures
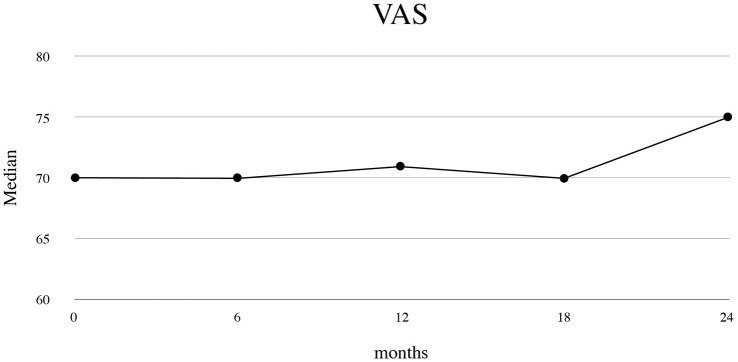
At baseline, the median VAS score was 70.0 (IQR 50.0–80.0) points. VAS score remained quite stable during abiraterone treatment (p-value for comparison with baseline: 0.001 at 6 months, 0.159 at 12, 0.526 at 18 and 0.753 at 24 months of follow-up), with a median of 75 (IQR 60–80) at 24 months of follow-up (Figure 2).
Figure 2.
Patient’s health-related quality of life: visual analog scale (VAS) score during abiraterone treatment.
Data for VAS were available from 431 patients at baseline, 329 at 6-month follow-up, 224 at 12-month follow-up, 139 at 18-month follow-up and 68 at 24-month follow-up. The signed rank test was used to test changes from baseline. p-values were 0.001 for changes at 6 months, 0.159 for changes at 12 months, 0.526 for changes at 18 months, and 0.753 for changes at 24 months of follow-up.
The overall EQ-5D-3L score remained stable over time during abiraterone treatment [median (IQR) equal to 0.9 (0.8–1.0) points both at baseline and at 24 months of follow-up, p-value for comparison = 0.470]. The percentage of patients reporting “no problem” remained appreciably high throughout the period of abiraterone treatment for the mobility, self-care, usual activities and anxiety/discomfort dimensions, and increased from 53.3% (235 of 441 patients) at baseline to 61.4% (43 of 70 patients) at 24 months of follow-up for the pain/discomfort dimension (data not shown).
In the overall population, BPI scores improved while on treatment with abiraterone. Median at baseline and at 24 months of follow-up were 1 (IQR 0–3) and 0 (IQR 0–2.5) for the mean pain intensity, 2 (IQR 0–4) and 0 (IQR 0–3) for the worst pain intensity, and 0.4 (IQR 0–3) and 0.3 (IQR 0–2.1) for interference of pain, respectively.
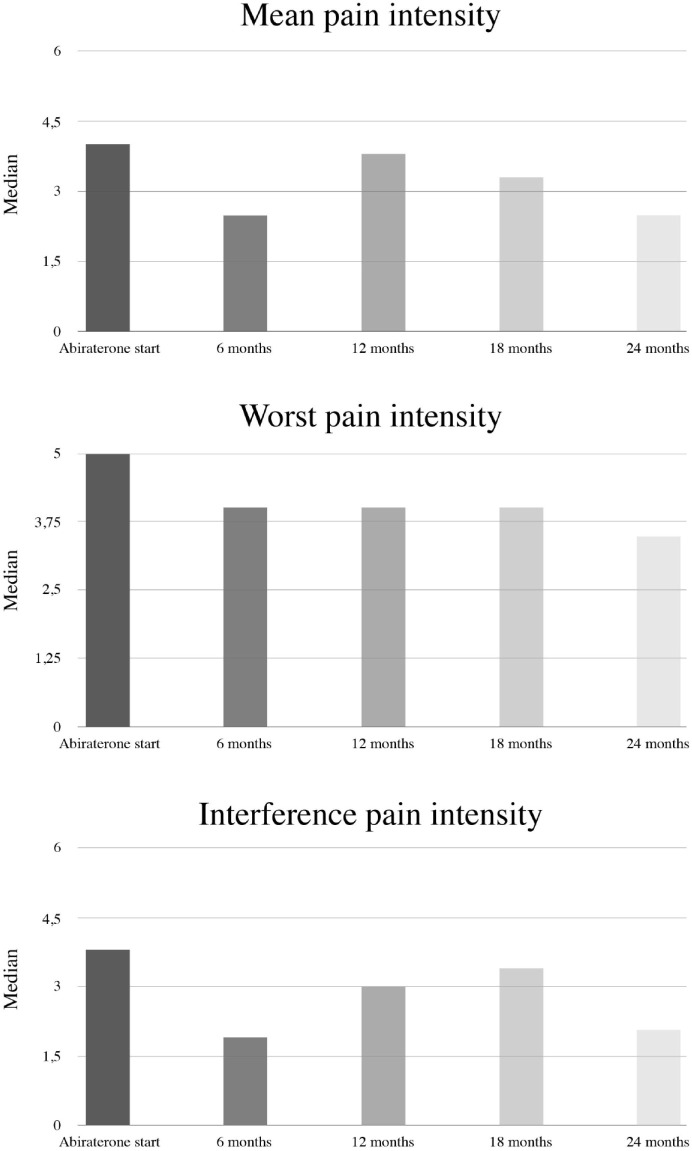
Among symptomatic patients according to BPI (32%), BPI scores improved over time, with significant declines in all the three BPI domains at 6 months of treatment (Figure 3). Median values at baseline were 4.0 (IQR 3.0–5.0) for mean pain intensity, 5.0 (IQR 4.0–7.0) for worst pain intensity and 3.8 (IQR 1.9–6.0) for the interference of pain; corresponding median changes at 24 months of follow-up for the three variables were −1.4 (IQR −2.9, +0.8), −2.0 (IQR −4.0, +1.0), and −0.3 (IQR −2.0, +0.6), respectively. In this subgroup of patients, median rPFS and OS were not reached. The rPFS and OS at 1 year were 66.4% and 77.2%, respectively.
Figure 3.
Pain assessment (Brief Pain Inventory; BPI) during abiraterone treatment in symptomatic patients.
Data for mean pain intensity were available from 119 symptomatic patients at baseline, 71 at 6-month follow-up, 43 at 12-month follow-up, 29 at 18-month follow-up and 12 at 24-month follow-up. Corresponding numbers for worst pain intensity: 125, 74, 44, 30 and 12; interference of pain: 108, 71, 39, 29, 11. The signed rank test was used to test changes from baseline in the three BPI domains. Tests were not performed at 18 and 24 months of follow-up due to the limited number of patients with available information at those time points at the present interim analysis. p-values were <0.001 at 6 months and 0.101 at 12 months for the mean pain intensity domain; <0.001 at 6 months and 0.001 at 12 months for the worst pain intensity domain; and 0.004 at 6 months and 0.008 at 12 months for the interference of pain domain.
*p < 0.05.
Safety
Among the 474 patients analyzed for safety, 231 (48.7) had at least one AE during abiraterone treatment and 84 patients (17.7%) had at least one serious AE (Table S1, supplementary material). Overall, 61 patients (12.9%) had AEs related to abiraterone per clinician’s judgement, but only 1.7% had a serious AE. The details of the AEs are presented in Table 3.
Table 3.
Adverse events occurred in >3% of patients during abiraterone treatment.
| Total number of patients with adverse events (N = 231) |
||||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Asthenia | 29 (12.6%) | 4 (1.7%) | 2 (0.9%) | 35 (15.2%) |
| Edema | 13 (5.6%) | 5 (2.2%) | 16 (6.9%) | |
| Diarrhea | 14 (6.1%) | 1 (0.4%) | 15 (6.5%) | |
| Anemia | 9 (3.9%) | 3 (1.3%) | 3 (1.3%) | 14 (6.1%) |
| Fatigue | 10 (4.3%) | 3 (1.3%) | 1 (0.4%) | 14 (6.1%) |
| Fever | 11 (4.8%) | 2 (0.9%) | 1 (0.4%) | 14 (6.1%) |
| Dyspnea | 9 (3.9%) | 3 (1.3%) | 12 (5.2%) | |
| Nausea | 9 (3.9%) | 3 (1.3%) | 1 (0.4%) | 12 (5.2%) |
| Cough | 10 (4.3%) | 1 (0.4%) | 11 (4.8%) | |
| Constipation | 8 (3.5%) | 2 (0.9%) | 1 (0.4%) | 10 (4.3%) |
| Pain | 9 (3.9%) | 2 (0.9%) | 10 (4.3%) | |
| Death unexpected | 8 (3.5%) | 8 (3.5%) | ||
| Atrial fibrillation | 2 (0.9%) | 5 (2.2%) | 7 (3.0%) | |
| Hot flushes | 6 (2.6%) | 1 (0.4%) | 7 (3.0%) | |
| Pneumonitis | 1 (0.4%) | 4 (1.7%) | 2 (0.9%) | 7 (3.0%) |
| Back pain | 5 (2.2%) | 2 (0.9%) | 7 (3.0%) | |
| Urinary incontinence | 7 (3.0%) | 7 (3.0%) | ||
Discussion
In the present second interim analysis of the ABItude study, chemotherapy-naïve mCRPC patients treated with abiraterone in routine clinical practice showed good clinical outcomes, preserved HRQoL, and achieved good pain control over a median of 18 months of follow-up.
In agreement with previous real-world studies,18,20,22,24–26,28,37, including Italian ones,21,27 our findings confirm, in a real-life setting, the efficacy and safety of abiraterone demonstrated in the registration trial (COU-AA-302), despite the poorer clinical features of the enrolled patients, including the advanced age (60% of our patients were ⩾75 years old) and the high prevalence of comorbidity.
In the COU-AA-302 trial, only one-third of patients were ⩾75 years old and those with relevant comorbidities were excluded, as well as those with visceral metastases. Conversely, about 8% of our cohort of patients had visceral metastases at treatment initiation. Although a negative prognostic role of visceral disease has been identified, a few evidences confirmed the efficacy of abiraterone in this population. The post-chemo study on mCRPC patients, COU-AA-301,38 and the Latitude study in mHSPC39 showed that patients with visceral disease had a benefit from abiraterone treatment both in rPFS and in OS, similar to the remaining population and irrespective of the organ involved, lung or liver. Furthermore, a recent Italian real-life study found that abiraterone was effective and safe in a small series of patients with visceral metastases, both in the pre-chemotherapy and post-docetaxel settings.40
In our prospective study of chemotherapy-naïve mCRPC patients, age, presence of comorbidities and visceral disease did not significantly affect rPFS and OS in multivariate analyses, thus indicating good response of patients treated with abiraterone with such baseline frail conditions. Presence of at least 10 bone metastases was directly associated to worst clinical outcomes, in agreement with a prognostic model based on COU-AA-302 data.41
In mCRPC patients, the progression of the disease usually leads to worsening symptomatology due to complications of metastases and treatment-related toxicities.42 Delaying symptoms, preserving HRQoL and pain palliation are important therapeutic objectives in the management of mCRPC.43 In clinical trials definition of symptomatic or asymptomatic patients was done through BPI, a validated scale to be filled in by the patient, whereas in clinical practice it is usually defined by clinicians’ judgement. Interestingly, in our real-life study we observed a discrepancy between patient and physician assessment. Indeed, 32% of patients reported symptoms per self-assessment at abiraterone initiation whereas judged asymptomatic or mildly symptomatic by physicians. This is the first time that effectiveness and quality of life is prospectively evaluated in first-line symptomatic mCRPC patients, demonstrating that patients with pain at baseline experienced a significant improvement in pain control in the early months of treatment and achieved good clinical outcomes, similar to the overall population enrolled in our study (66.4% versus 73.9% patients without radiographical progression at 1 year of follow-up respectively). Truly asymptomatic or mildly symptomatic patients maintained very low levels of pain while on treatment with abiraterone. Abiraterone was well tolerated in our cohort of elderly patients with pre-existing cardiac disorders, in line with previous real-life observations.21,44–46 In the largest study published so far by Boegemann et al.,22 2% of patients discontinued due to toxicity, 8% of patients had an ADR and <1% had a serious ADR. In our study similar numbers were reported: discontinuation of therapy because of toxicity occurred in 5% of patients, and 13% of patients had an ADR; only 1.7% reported a serious ADR. The most common ADRs in our study were asthenia/fatigue, atrial fibrillation, nausea, edema, which were, however, reported with low frequency. This pattern of drug-related AEs is consistent with that observed in the pivotal trials in pre- and post-chemotherapy settings.
To our knowledge, this is the largest prospective study assessing the effectiveness and safety of abiraterone acetate plus prednisone in mCRPC patients naïve to chemotherapy in a real-world setting. Our study includes a broad range of patients with a high level of comorbidities from several major oncological, urological and radiotherapy centers across the country, reflecting the mCRPC population encountered in real-world settings. This, together with the inclusion of all consecutive patients meeting the inclusion criteria over a 12-month period, is likely to have minimized selection bias and ensured the generalizability of our results to the mCRPC Italian patient population. Another major strength is the ad hoc and prospective data collection, which limited inaccuracies and incompleteness of the information. This is a second interim analysis of a prospective study. Among the limitations are that we did not include one or more “control” arms to compare the effectiveness of abiraterone to that of other first-line treatments for mCRPC, including chemotherapy and other AR-directed agents. An additional limitation, typical of the real-world setting, concerns the methodology of the assessment of the rPFS. Specifically, according to the observational nature of the study, restaging scans were performed at variable time points according to the discretion of the treating physician and no central radiological review was carried out. However, recently, a higher concordance between real-world and RCT assessments of disease progression has been shown.47
In conclusion, the present large prospective data confirm that treatment with abiraterone plus prednisone is effective and well tolerated in mCRPC patients naïve to chemotherapy, even though in the real-life they are more elderly, vulnerable and have a high burden of disease such as visceral metastases and pain.
Supplemental Material
Supplemental material, Supplementary_material_3 for Effectiveness of abiraterone acetate plus prednisone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer in a large prospective real-world cohort: the ABItude study by Giuseppe Procopio, Vincenzo Emanuele Chiuri, Monica Giordano, Giovanna Mantini, Roberto Maisano, Roberto Bordonaro, Nicola Calvani, Gaetano Facchini, Sabino De Placido, Mario Airoldi, Andrea Sbrana, Donatello Gasparro, Giuseppe Mario Ludovico, Pamela Guglielmini, Emanuele Naglieri, Daniele Fagnani, Massimo Aglietta, Luigi Schips, Patrizia Beccaglia, Alessandro Sciarra, Lorenzo Livi and Daniele Santini in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors acknowledge the contribution of all medical professionals who supported the data collection phase. In particular, they are grateful to: Saverio Cinieri (Brindisi), Luca Galli (Pisa), Rodolfo Passalacqua (Cremona), Lucio Trodella (Roma), Riccardo Santoni (Roma), Giovanni Luca Ceresoli (Bergamo), Stefano Magrini (Brescia), Alessandra Mosca (Novara), Vincenzo Mirone (Napoli), Michele Gallucci (Roma), Mirko Acquati (Monza), Francesco Boccardo (Genova), Giorgio Vittorio Scagliotti (Orbassano), Manlio Mencoboni (Genova), Ugo De Giorgi (Meldola), Virgilio Cicalese (Avellino), Gaetano Lanzetta (Grottaferrata), Donata Sartori (Mirano), Paolo Carlini (Roma), Hector Josè Soto Parra (Catania), Michele Battaglia (Bari), Francesco Uricchio (Napoli), Antonio Bernardo (Pavia), Antonello De Lisa (Cagliari), Giuseppe Carrieri (Foggia), Antonio Ardizzoia (Lecco), Michele Aieta (Rionero in Vulture), Salvatore Pisconti (Taranto), Paolo Marchetti (Roma), and Fabiola Paiar (Pisa).
The authors also thank Carlotta Galeone, who provided medical writing services on behalf of Statinfo srl.
Footnotes
Conflict of interest statement: G. Procopio has an honoraria/consulting or advisory role for AZ, Bayer, BMS, Ipsen, Janssen, Merk, MSD, Pfizer and Novartis. V. Chiuri is an Advisory Board member and speaker for BMS, Ipsen, Janssen and Pfizer. R. Bordonaro has an honoraria/consulting or advisory role/speaker’s bureau for Bayer, AstraZeneca, Sanofi, Novartis, Amgen, Roche, Pfizer, Janssen Cilag and Bristol Mayer Squibb. S. De Placido is an Advisory Board member and invited speaker for Novartis, Roche, Celgene, AstraZeneca, Amgen, Eisai, Lilly, Pfizer and Gentili. P. Beccaglia is an employee of Janssen.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by Janssen-Cilag SpA.
ORCID iDs: Nicola Calvani  https://orcid.org/0000-0002-9761-7431
https://orcid.org/0000-0002-9761-7431
Andrea Sbrana  https://orcid.org/0000-0003-4460-5282
https://orcid.org/0000-0003-4460-5282
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Giuseppe Procopio, Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, via Venezian 1, Milan, 20133, Italy.
Vincenzo Emanuele Chiuri, Medical Oncology Department, Ospedale Vito Fazzi, Lecce, Puglia, Italy.
Monica Giordano, Medical Oncology Division, ASST-Lariana, Como, Lombardia, Italy.
Giovanna Mantini, Radiochemotherapy Unit, Department of Diagnostics for Imaging, Oncological Radiotherapy and Hematology, Fondazione Policlinico A. Gemelli IRCCS, Rome - University Department of Radiological and Hematological Sciences, Università Cattolica Sacro Cuore, Italy.
Roberto Maisano, Department of Oncology, A.O. Bianchi-Melacrino-Morelli, Reggio Calabria, Calabria, Italy.
Roberto Bordonaro, MD - ARNAS Garibaldi, Catania, Sicilia, Italy.
Nicola Calvani, Medical Oncology Unit, Antonio Perrino Hospital, Brindisi, Puglia, Italy.
Gaetano Facchini, Departmental Unit of Experimental Uro-Andrological Clinical Oncology, Istituto Nazionale Tumori - IRCCS - Fondazione G. Pascale, Naples, Italy.
Sabino De Placido, Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Naples, Campania, Italy.
Mario Airoldi, Oncology Unit 2 - Città della Salute e della Scienza di Torino, Turin, Piemonte, Italy.
Andrea Sbrana, Medical Oncology Unit 2, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy.
Donatello Gasparro, Medical Oncology Unit, Department of General and Specialistic Medicine, University Hospital of Parma, Parma, Italy.
Giuseppe Mario Ludovico, Department of Urology, Ospedale F. Miulli, Acquaviva delle Fonti, Bari, Italy.
Pamela Guglielmini, Oncology Unit, SS Antonio e Biagio e Cesare Arrigo Hospital, Alessandria, Italy.
Emanuele Naglieri, Medical Oncology Unit, IRCCS Giovanni Paolo II, Bari, Italy.
Daniele Fagnani, Medical Oncology Division, ASST Vimercate, Vimercate, Italy.
Massimo Aglietta, Department of Oncology, University of Turin; Candiolo Cancer Institute - FPO- IRCCS, Candiolo, Italy.
Luigi Schips, Department of Medical, Oral and Biotechnological Sciences, G. d’Annunzio University of Chieti, Urology unit, “SS. Annunziata Hospital”, Chieti, Italy.
Patrizia Beccaglia, Janssen SpA, Cologno Monzese, Italy.
Alessandro Sciarra, Department of Urology, Sapienza Rome University Policlinico Umberto I, Rome, Italy.
Lorenzo Livi, Department of Radiation Oncology, Azienda Ospedaliero-Universitaria Careggi, University of Florence, Florence, Italy.
Daniele Santini, Department of Medical Oncology, University Campus Biomedico, Rome, Italy.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Scher HI, Solo K, Valant J, et al. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One 2015; 10: e0139440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 4. Crawford ED, Petrylak D, Sartor O. Navigating the evolving therapeutic landscape in advanced prostate cancer. Urol Oncol 2017; 35S: S1–S13. [DOI] [PubMed] [Google Scholar]
- 5. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 6. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008; 26: 242–245. [DOI] [PubMed] [Google Scholar]
- 7. Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol 2019; 75: 88–99. [DOI] [PubMed] [Google Scholar]
- 8. Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med 2019; 70: 479–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 2008; 26: 4563–4571. [DOI] [PubMed] [Google Scholar]
- 10. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010; 376: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 11. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012; 13: 983–992. [DOI] [PubMed] [Google Scholar]
- 12. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. [DOI] [PubMed] [Google Scholar]
- 14. Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014; 66: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basch E, Autio K, Ryan CJ, et al. Abiraterone acetate plus prednisone versus prednisone alone in chemotherapy-naïve men with metastatic castration-resistant prostate cancer: patient-reported outcome results of a randomised phase 3 trial. Lancet Oncol 2013; 14: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 16. Sargent D. What constitutes reasonable evidence of efficacy and effectiveness to guide oncology treatment decisions? Oncologist 2010; 15(Suppl. 1): 19–23. [DOI] [PubMed] [Google Scholar]
- 17. Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration-resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol 2013; 24: 2972–2977. [DOI] [PubMed] [Google Scholar]
- 18. Rescigno P, Lorente D, Bianchini D, et al. Prostate-specific antigen decline after 4 weeks of treatment with abiraterone acetate and overall survival in patients with metastatic castration-resistant prostate cancer. Eur Urol 2016; 70: 724–731. [DOI] [PubMed] [Google Scholar]
- 19. Shore ND, Saltzstein D, Sieber P, et al. Results of a real-world study of enzalutamide and abiraterone acetate with prednisone tolerability (REAAcT). Clin Genitourin Cancer 2019; 17: 457–463e6. [DOI] [PubMed] [Google Scholar]
- 20. Koninckx M, Marco JL, Perez I, et al. Effectiveness, safety and cost of abiraterone acetate in patients with metastatic castration-resistant prostate cancer: a real-world data analysis. Clin Transl Oncol 2019; 21: 314–323. [DOI] [PubMed] [Google Scholar]
- 21. Cavo A, Rubagotti A, Zanardi E, et al. Abiraterone acetate and prednisone in the pre- and post-docetaxel setting for metastatic castration-resistant prostate cancer: a mono-institutional experience focused on cardiovascular events and their impact on clinical outcomes. Ther Adv Med Oncol 2018; 10: 1758834017745819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boegemann M, Khaksar S, Bera G, et al. Abiraterone acetate plus prednisone for the Management of Metastatic Castration-Resistant Prostate Cancer (mCRPC) without prior use of chemotherapy: report from a large, international, real-world retrospective cohort study. BMC Cancer 2019; 19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKay RR, Werner L, Fiorillo M, et al. Predictors of duration of abiraterone acetate in men with castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2016; 19: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poon DM, Chan K, Lee SH, et al. Abiraterone acetate in metastatic castration-resistant prostate cancer - the unanticipated real-world clinical experience. BMC Urol 2016; 16: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thortzen A, Thim S, Roder MA, et al. A single-center experience with abiraterone as treatment for metastatic castration-resistant prostate cancer. Urol Oncol 2016; 34: 291.e1-7. [DOI] [PubMed] [Google Scholar]
- 26. Rocha J, Aprikian AG, Vanhuyse M, et al. Impact of abiraterone acetate with and without prior docetaxel chemotherapy on the survival of patients with metastatic castration-resistant prostate cancer: a population-based study. CMAJ Open 2017; 5: E265–E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cindolo L, Natoli C, De Nunzio C, et al. Safety and efficacy of abiraterone acetate in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer: an Italian multicenter “real-life” study. BMC Cancer 2017; 17: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyake H, Hara T, Terakawa T, et al. Comparative assessment of clinical outcomes between abiraterone acetate and enzalutamide in patients with docetaxel-naïve metastatic castration-resistant prostate cancer: experience in real-world clinical practice in Japan. Clin Genitourin Cancer 2017; 15: 313–319. [DOI] [PubMed] [Google Scholar]
- 29. Chowdhury S, Bjartell A, Lumen N, et al. Real-World Outcomes in First-Line Treatment of Metastatic Castration-Resistant Prostate Cancer: The Prostate Cancer Registry. Targeted Oncology 2020; 15: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manokumar T, Aziz S, Breunis H, et al. A prospective study examining elder-relevant outcomes in older adults with prostate cancer undergoing treatment with chemotherapy or abiraterone. J Geriatr Oncol 2016; 7: 81–89. [DOI] [PubMed] [Google Scholar]
- 31. Sciarra A, Scarcia M, Cindolo L, et al. A prospective real-life study evaluating abiraterone Acetate Plus Prednisone (AAP) for Metastatic Castration-Resistant Prostate Cancer (mCRPC) (Abitude Study). Eur Urol Suppl 2018; 17: 277. [Google Scholar]
- 32. Diels J, Hamberg P, Ford D, et al. Mapping FACT-P to EQ-5D in a large cross-sectional study of metastatic castration-resistant prostate cancer patients. Qual Life Res 2015; 24: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 34. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 35. Scalone L, Cortesi PA, Ciampichini R, et al. Italian population-based values of EQ-5D health states. Value Health 2013; 16: 814–822. [DOI] [PubMed] [Google Scholar]
- 36. Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008; 10: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Marchioni M, Sountoulides P, Bada M, et al. Abiraterone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer: a systematic review of ‘real-life’ studies. Ther Adv Urol 2018; 10: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodman OB, Jr, Flaig TW, Molina A, et al. Exploratory analysis of the visceral disease subgroup in a phase III study of abiraterone acetate in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2014; 17: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019; 20: 686–700. [DOI] [PubMed] [Google Scholar]
- 40. Facchini G, Cavaliere C, D’Aniello C, et al. Abiraterone acetate treatment in patients with castration-resistant prostate cancer with visceral metastases: a real-world experience. Anticancer Drugs 2019; 30: 179–185. [DOI] [PubMed] [Google Scholar]
- 41. Ryan CJ, Kheoh T, Li J, et al. Prognostic index model for progression-free survival in chemotherapy-naïve metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone. Clin Genitourin Cancer. Epub ahead of print 25 July 2017. DOI: 10.1016/j.clgc.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nussbaum N, George DJ, Abernethy AP, et al. Patient experience in the treatment of metastatic castration-resistant prostate cancer: state of the science. Prostate Cancer Prostatic Dis 2016; 19: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prati V, Ruatta F, Aversa C, et al. Cardiovascular safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients: a prospective evaluation. Future Oncol 2018; 14: 443–448. [DOI] [PubMed] [Google Scholar]
- 45. Procopio G, Grassi P, Testa I, et al. Safety of abiraterone acetate in castration-resistant prostate cancer patients with concomitant cardiovascular risk factors. Am J Clin Oncol 2015; 38: 479–482. [DOI] [PubMed] [Google Scholar]
- 46. Verzoni E, Grassi P, Ratta R, et al. Safety of long-term exposure to abiraterone acetate in patients with castration-resistant prostate cancer and concomitant cardiovascular risk factors. Ther Adv Med Oncol 2016; 8: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang Bartlett C, Mardekian J, Cotter MJ, et al. Concordance of real-world versus conventional progression-free survival from a phase 3 trial of endocrine therapy as first-line treatment for metastatic breast cancer. PLoS One 2020; 15: e0227256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material_3 for Effectiveness of abiraterone acetate plus prednisone in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer in a large prospective real-world cohort: the ABItude study by Giuseppe Procopio, Vincenzo Emanuele Chiuri, Monica Giordano, Giovanna Mantini, Roberto Maisano, Roberto Bordonaro, Nicola Calvani, Gaetano Facchini, Sabino De Placido, Mario Airoldi, Andrea Sbrana, Donatello Gasparro, Giuseppe Mario Ludovico, Pamela Guglielmini, Emanuele Naglieri, Daniele Fagnani, Massimo Aglietta, Luigi Schips, Patrizia Beccaglia, Alessandro Sciarra, Lorenzo Livi and Daniele Santini in Therapeutic Advances in Medical Oncology