Abstract
Objective
This study aimed to explore the use of different combinations of alpha-fetoprotein (AFP), Lens culinaris agglutinin-reactive AFP (AFP-L3), and des-gamma-carboxyprothrombin (DCP) for the early diagnosis of hepatocellular carcinoma (HCC) in patients with hepatitis B virus (HBV)-associated liver cirrhosis (LC).
Methods
There were 167 subjects, including 100 with HCC and 67 with LC, who were enrolled into this study. Serum AFP, AFP-L3, and DCP levels were detected by chemiluminescent enzyme immunoassay and analyzed using the receiver operating characteristics (ROC) method.
Results
The sensitivity and specificity of AFP and DCP for differentiating between early HCC and HBV-associated LC were 51.5% and 92.5%, and 60.0% and 84.7%, respectively. Comparative analysis of ROC curves showed no significant difference in the area under the curve (AUC) for AFP and DCP. Moreover, the combination of AFP and DCP showed the largest AUC value with a diagnostic sensitivity and specificity of 67% and 83.1%, respectively.
Conclusion
These results suggest that AFP is the best single biomarker for distinguishing between HBV-associated LC and early HCC induced by HBV. However, the combination of AFP and DCP can enhance the diagnostic value of AFP for differentiating between these diseases.
Keywords: Hepatocellular carcinoma, alpha-fetoprotein, des-gamma-carboxyprothrombin, Lens culinaris agglutinin-reactive alpha-fetoprotein, early diagnosis, liver cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is the main type of primary liver cancer and the third leading cause of cancer-related death worldwide.1,2 The risk factors for HCC include liver cirrhosis (LC) associated with hepatitis virus infection, environmental toxins, metabolic diseases, and alcohol consumption. The major risk factor for HCC in eastern Asia and sub-Saharan Africa is LC associated with chronic hepatitis B virus (HBV) infection.2–4 The estimated prevalence of chronic HBV infection globally varies between 0.1% and 20%.5,6 Up to 40% of patients with chronic HBV infection progress to LC if untreated,7 with a progression rate of 2% to 4% per year.8,9 Despite significant improvements in the diagnosis and treatment of HCC, its overall prognosis remains poor,10 and the 5-year survival rate is <15% in patients with advanced HCC.11 However, few patients are diagnosed with HCC at an early stage when curative treatments such as surgical resection are still possible.12 The results of a meta-analysis suggested a strong link between early tumor detection and improved prognosis of patients with HCC in the LC population,13 strongly suggesting that an early diagnosis of HCC could improve the therapeutic success rate.
Despite the use of HCC-associated biomarkers such as alpha-fetoprotein (AFP), ultrasound, and computed tomography to screen and monitor HCC among patients with LC, HCC is still hard to diagnose at an early stage14,15 because of the difficulty in distinguishing between small HCC masses and LC. More effective methods are, therefore, needed to differentiate between early HCC and LC. Current techniques such as liver biopsy, computed tomography, magnetic resonance imaging, and ultrasonography are only effective for detecting HCC at relatively advanced stages.16 There is, thus, an urgent need to identify reliable serum biomarkers to expand the window of detection to early-stage HCC.
Serum biomarkers such as AFP, des-gamma-carboxyprothrombin (DCP), and Lens culinaris agglutinin-reactive AFP (AFP-L3) have been used complementarily for the early diagnosis of HCC.17 Previous studies found the sensitivity and accuracy of AFP for the diagnosis of HCC were low and varied among etiologies,18–20 although AFP-L3 and DCP could improve the monitoring performance of AFP21,22. However, most of these studies were performed in a mixed group of HCC patients, and studies investigating the value of serum cancer biomarkers for the early detection of HCC in relation to HBV-associated LC are lacking. We, therefore, investigated the value of different combinations of AFP, AFP-L3, and DCP for the early diagnosis of HCC in patients with HBV-associated LC.
Materials and methods
Specimen collection
Patients diagnosed with LC or HCC at Qingdao Sixth People’s Hospital from July 2014 to November 2017 were enrolled in this study. Serum specimens were collected, centrifuged at 1000 ×g for 30 minutes, aliquoted into separate vials, and stored at −80°C. The diagnosis of HCC was made based on the imaging or histopathology findings, and patients with HCC were classified in accordance with the Barcelona Clinic Liver Cancer (BCLC) staging system. Patients with BCLC stage 0 with a single nodule <2 cm in diameter and stage A patients with a single nodule 2 to 5 cm in diameter or two to three lesions each <3 cm in diameter were classified in the early HCC group. Patients with intrahepatic cholangiocarcinoma or mixed HCC were excluded from this study. Patients with LC were diagnosed by liver biopsy and also underwent magnetic resonance imaging or computed tomography screening to exclude the possibility of HCC. Further follow-up for at least 12 months was performed to ensure that no LC patients developed HCC. Chronic HBV infection was confirmed in all patients. All enrolled patients with either LC or HCC were treated with nucleoside analogues in accordance with the EASL 2017 Clinical Practice Guidelines on the management of HBV infection. Patients with hepatitis C virus infection, alcoholic liver disease, and biliary cirrhosis were also excluded.
This study was approved by the Qingdao Sixth People’s Hospital Research Ethics Committee. All patients provided written informed consent for the collection of the information, and their clinical samples were stored and used for research in accordance with the Declaration of Helsinki (1964) and subsequent amendments. The methods used conformed to approved guidelines and regulations.
Analysis of serum AFP, AFP-L3, and DCP
Serum levels of AFP and DCP were detected using a fully automated chemiluminescent enzyme immunoassay (CLEIA) system (LUMIPULSE® G1200, Fujirebio Inc., Tokyo, Japan). AFP-L3 was enriched from serum specimens using a Hotgen Biotech glycosyl capture spin column with Lens culinaris agglutinin (Beijing Hotgen Biological Technology Co., Ltd, Beijing, China) and levels of enriched AFP-L3 were then detected by CLEIA. All procedures were performed in accordance with the manufacturer’s instructions.
Statistical analysis
Data analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Software version 6 (GraphPad Software, San Diego, CA, USA). Differences between two groups were analyzed using the Mann–Whitney test for non-normally distributed data and the Student’s t-test for normally distributed data. The diagnostic performances of the biomarkers were evaluated and compared using receiver-operating curves (ROC) for each of the biomarkers and their combinations. The sensitivity, specificity, and accuracy for each biomarker alone and combined were also calculated. P<0.05 was considered to be statistically significant.
Results
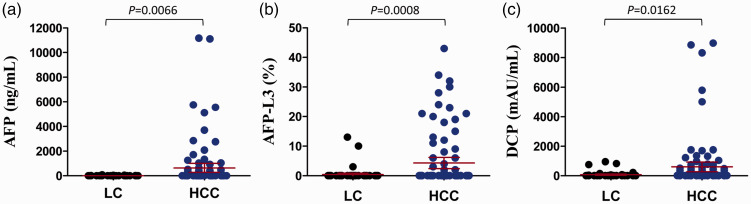
There were 167 patients enrolled into this study, including 100 patients with HCC and 67 with LC. The patients’ clinical characteristics are shown in Table 1. The HCC group included 30 patients with BCLC stage 0 and 70 patients with BCLC stage A. Child–Pugh class A was more common compared with other classes in both groups. AFP and AFP-L3 levels were significantly lower in the LC group compared with the HCC group (Figure 1a and 1b), but there was no significant difference between HCC patients with stage 0 and stage A (data not shown). Serum DCP levels were also significantly lower in the LC compared with the HCC group (Figure 1c).
Table 1.
Clinical characteristics of study patients
| LC | HCC | |
|---|---|---|
| Number | 67 | 100 |
| Sex (male/female) | 43/24 | 77/23 |
| Age, years | 45.77 ± 7.50 | 53.72 ± 10.53 |
| TP, g/L | 67.28 ± 8.31 | 69.20 ± 6.57 |
| AST, U/L | 42.46 ± 50.17 | 34.49 ± 19.93 |
| ALT, U/L | 28.49 ± 21.49 | 36.62 ± 27.11 |
| ALP, U/L | 88.28 ± 44.55 | 88.23 ± 43.86 |
| GGT, U/L | 51.50 ± 60.52 | 72.17 ± 94.06 |
| TBIL, µmol/L | 22.21 ± 15.71 | 19.95 ± 16.82 |
| TBV, µmol/L | 34.19 ± 77.07 | 14.39 ± 18.66 |
| PLT, 109/L | 146.91 ± 107.17 | 141.97 ± 64.57 |
| HBV DNA <2,000 IU/mL | 67 | 100 |
| HBeAg | ||
| Positive | 48 | 68 |
| Negative | 19 | 32 |
| Child–Pugh class | ||
| A | 55 | 86 |
| B | 11 | 14 |
| C | 0 | 1 |
| BCLC stage | ||
| Pre-cancer | 67 | 0 |
| 0 | 0 | 30 |
| A | 0 | 70 |
| Tumor number | ||
| Single | 0 | 88 |
| Multiple | 0 | 12 |
| Tumor size | ||
| ≤2 cm | 0 | 30 |
| >2 cm | 0 | 70 |
| AFP, ng/mL | 5.86 (0.9–72) | 635.57 (1.1–5558) |
| AFP-L3, % | 0.38 (0–13) | 4.31 (0–43) |
| DCP, mAU/mL | 71.77 (2.5–955) | 601.17 (9–8980) |
Values are presented as the mean ± standard deviation, number, or median (range).
HCC, hepatocellular carcinoma; LC, liver cirrhosis; TP, total protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyltransferase; TBIL, total bilirubin; TBV, total blood volume; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; BCLC, Barcelona Liver Cancer; PLT, platelet count; AFP, alpha-fetoprotein; AFP-L3, Lens culinaris agglutinin-reactive AFP; DCP, des-gamma-carboxyprothrombin
Figure 1.
Comparison of serum levels of AFP (a), AFP-L3 (b), and DCP (c) between patients with liver cirrhosis and hepatocellular carcinoma. LC, liver cirrhosis; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein, AFP-L3, Lens culinaris agglutinin-reactive AFP; DCP, des-gamma-carboxyprothrombin
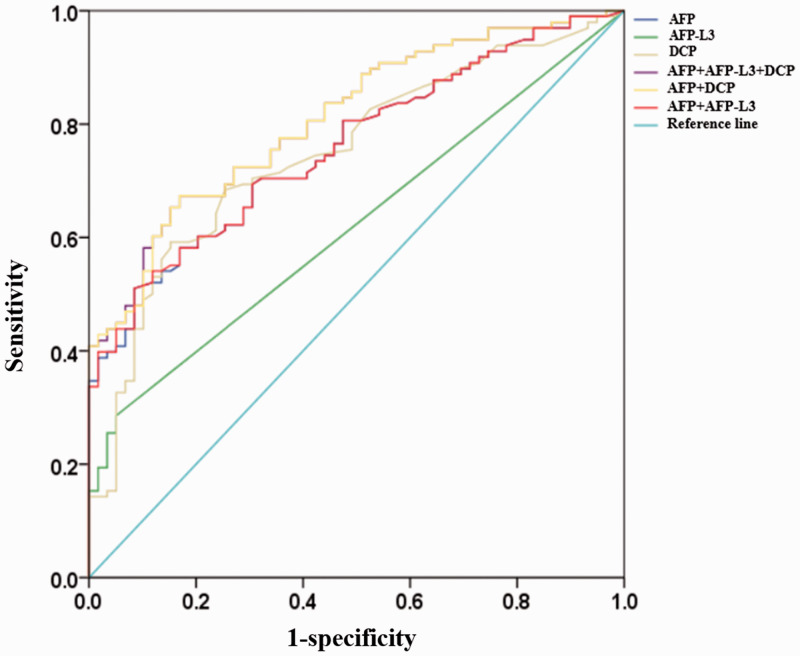
ROCs were plotted to compare the diagnostic values of AFP, AFP-L3, and DCP for distinguishing between HCC and LC (Figure 2). The optimal cut-off value for AFP was 10 ng/mL and the area under the curve (AUC) was 0.76 (95% confidence interval [CI] 0.69, 0.83) with a sensitivity of 51.5% and a specificity of 92.5%. The optimal cut-off value for DCP was 38 mAU/mL and the AUC was 0.75 (95% CI 0.67, 0.83) with a sensitivity of 60% and a specificity of 84.7% (Table 2). Comparative analysis of the ROC curves showed no significant difference in AUC values for AFP and DCP. The cut-off value for AFP-L3 was 10%, with an AUC of 0.62 (95% CI 0.53, 0.71). The combination of AFP and DCP showed a sensitivity and specificity of 67% and 83.1%, respectively, for the diagnosis of HCC, with a larger AUC compared with any of the biomarkers alone (0.81, 95% CI 0.74, 0.88) (Table 2 and Figure 2). The combination of AFP with DCP significantly increased the sensitivity from 51.5% to 67% but decreased the specificity from 92.5% to 83.1% compared with AFP alone (Table 2). Additionally, the AUC value for the combination of AFP, AFP-L3, and DCP was similar to that of the combination of AFP and DCP (Table 2).
Figure 2.
Receiver operating characteristic curve analysis of AFP, AFP-L3, and DCP alone or combined to distinguish between liver cirrhosis and hepatitis B virus-related hepatocellular carcinoma. AFP, alpha-fetoprotein, AFP-L3, Lens culinaris agglutinin-reactive AFP; DCP, des-gamma-carboxyprothrombin
Table 2.
Diagnostic performances of AFP, DCP, and AFP-L3 for hepatitis B virus-associated hepatocellular carcinoma
| AUC (95%CI) | Cut-off | SEN | SPE | PPV | NPV | |
|---|---|---|---|---|---|---|
| AFP | 0.76 (0.69,0.83) | 10 (ng/mL) | 51.5% | 92.5% | 91.1% | 56.4% |
| AFP-L3 | 0.62 (0.53,0.71) | 0.1 | 28.3% | 95.5% | 90.3% | 47.4% |
| DCP | 0.75 (0.67,0.83) | 38 (mAU/mL) | 60.0% | 84.7% | 87.0% | 55.6% |
| AFP + DCP | 0.81 (0.74,0.88) | 0.49* | 67.0% | 83.1% | 87.0% | 59.8% |
| AFP + AFP-L3 | 0.76 (0.69,0.83) | 0.51* | 53.5% | 89.6% | 88.3% | 56.6% |
| AFP + AFP-L3 + DCP | 0.81 (0.75,0.88) | 0.48* | 66.7% | 83.1% | 86.8% | 59.8% |
AFP, alpha-fetoprotein; AFP-L3, Lens culinaris agglutinin-reactive AFP; DCP, des-gamma-carboxy-prothrombin; AUC, area under the curve; CI: confidence interval; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value
*The optimal probability was calculated by applying the logistic regression model that showed the highest value of sensitivity + specificity−1.
Discussion
The incidence of HCC was higher in eastern Asia and sub-Saharan Africa, where the major risk factor is LC associated with HBV infection, compared with the incidences in the USA and Europe.2,23 A previous report found that the relative risk of developing HCC among chronic HBV carriers was 63-times higher compared with that of uninfected individuals.24 The poor prognosis of patients with HCC is mainly because of the delay in diagnosis, and an early diagnosis is the most important factor in improving the long-term survival of patients with HCC. However, early HBV-related HCC is difficult to differentiate from LC that is induced by HBV in clinical practice using imaging techniques alone.
In this study, we evaluated the combined and isolated performances of AFP, AFP-L3, and DCP for distinguishing between early HBV-associated HCC and LC. The combination of AFP and DCP had the largest AUC for distinguishing between these conditions, while further addition of AFP-L3 did not improve the diagnostic performance compared with the combination of AFP and DCP (0.810 and 0.810, respectively). These results suggest that the combination of AFP and DCP might be the best and most cost-effective strategy for monitoring patients for HCC. The combination of AFP and AFP-L3 showed a similar performance to AFP alone in our study (0.761 and 0.760, respectively). However, our results were not consistent with previous reports.25 This apparent discrepancy may be because the current study participants had HBV-associated LC, and patients with chronic HBV, alcohol-related LC, and hepatitis C virus infection had already been excluded.
This present study demonstrated that AFP showed the best performance among the isolated biomarkers for distinguishing between HCC and LC. AFP is, thus, currently considered to be the best biomarker for HCC surveillance.25 In this study, AFP showed the largest AUC when used alone, and there was a slightly lower AUC for DCP (0.76 and 0.75, respectively). DCP is used widely to detect early-stage HCC in clinical studies, but although many previous studies found superior sensitivity of DCP compared with AFP for the early diagnosis of HCC,19,20,26 the specificity of DCP remained lower compared with that of AFP and AFP-L3 in our study. A previous meta-analysis showed that the pooled sensitivity and specificity of DCP for diagnosing HBV-related HCC were 0.71 (95% CI: 0.59, 0.80) and 0.93 (95% CI: 0.87, 0.96), respectively,27 which is consistent with the diagnostic value of DCP in this study. DCP might be a good complementary biomarker for diagnosing early HBV-related HCC. AFP-L3 was considered to be an effective biomarker for distinguishing HCC with relatively low AFP levels.28 AFP-L3 was also found to be more specific than AFP and DCP for diagnosing early HBV-related HCC in the present study; however, its low sensitivity might limit its clinical use. In 2019, Choi and colleagues proposed that DCP, AFP-L3, and AFP could identify early-stage HCC, using optimal cut-off values of 20 mAU/mL, 4%, and 5 ng/mL, respectively, which were far lower than the serum marker cut-off values in the current study.25 However, their research was based on a dynamic large-scale longitudinal observational study in participants with chronic hepatitis B and/or LC. It has also been reported that about 30% to 40% of patients with early-stage HCC display normal serum AFP levels,20,29 and DCP was used to distinguish between HCC and LC in AFP-negative patients.20 AFP-L3 was complementary to AFP for distinguishing between AFP-negative HCC and LC patients.29
Consistent with the current results, Feng et al.30 reported that the combination of AFP and DCP could greatly improve the sensitivity for diagnosing HCC, but with decreased specificity, compared with their individual uses. Sensitivity may be more important than specificity in terms of the surveillance for HCC in patients with LC because a delayed HCC diagnosis might result in its progression from an early to an advanced stage. The widespread use of antiviral drugs has increased the rate of viral suppression along with normalization of alanine aminotransferase, and reduced the rate of false elevation of tumor biomarkers in most patients. It is, therefore, necessary to adapt the cut-off values for biomarkers to a lower level to maintain their sensitivity in the surveillance of HCC, especially in its early stage.
This study had some limitations including the relatively low numbers of patients, retrospective design, and lack of different algorithms to analyze the biomarkers. Further studies are, therefore, needed to address these issues. We also did not assess the role of longitudinal changes in serum biomarkers for detecting early-stage HCC, and this aspect also needs further investigation.
In conclusion, this study demonstrated that AFP remains the best single biomarker for distinguishing between HBV-associated LC and early HCC induced by HBV. However, the combination of AFP and DCP may provide improved diagnostic performance for differentiating between these diseases.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding authors upon reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, 81571973 to HW; 81772165 to BS), the NSFC-NIH Biomedical Collaborative Research Program (81761128001 to HW), the National 13th Five-Year Grand Program on Key Infectious Disease Control (2017ZX10202102-005-003 to BS, 2017ZX10202101-004-001 to TZ, 2018ZX10301-407-005 and 2018ZX10302103-001-003 to TJ), the Beijing Municipal of Science and Technology Major Project (D161100000416003 to HW), and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ORCID iD
Ting Song https://orcid.org/0000-0003-2810-471X
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Yang S, Xu K, et al. Patterns and trends of liver cancer incidence rates in Eastern and Southeastern Asian Countries (1983–2007) and predictions to 2030. Gastroenterology 2018; 154: 1719–1728.e5. [DOI] [PubMed] [Google Scholar]
- 3.Pungpapong S, Kim WR, Poterucha JJ. Natural history of hepatitis B virus infection: an update for clinicians. Mayo Clin Proc 2007; 82: 967–975. [DOI] [PubMed] [Google Scholar]
- 4.Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: The major impact of China. Hepatology 2015; 60: 2099–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Wang FJ. Epidemiology and prevention of hepatitis B virus in China. Mil Med Res 2009; 24: 301–308. [Google Scholar]
- 6.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatol 2010; 11: 97–107. [DOI] [PubMed] [Google Scholar]
- 7.Popper H, Shafritz DA, Hoofnagle JH. Relation of the hepatitis B virus carrier state to hepatocellular carcinoma. Hepatology 2010; 7: 764–772. [DOI] [PubMed] [Google Scholar]
- 8.Lampertico P, Agarwal K, Berg T, et al. EASL 2017, Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67: 370–398. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019; 380: 1450–1462. DOI: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 10.Lurje I, Czigany Z, Bednarsch J, et al. Treatment strategies for hepatocellular carcinoma (-) a multidisciplinary approach. Int J Mol Sci 2019; 20: pii: E1465. DOI: E1465 [pii] 10.3390/ijms20061465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilikhan SU, Bilici M, Sahin H, et al. Assessment of the correlation between serum prolidase and alpha-fetoprotein levels in patients with hepatocellular carcinoma. World J Gastroenterol 2015; 21: 6999–7007. DOI: 10.3748/wjg.v21.i22.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BH, Lim YS, Kim EY, et al. Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol 2018; 33: 475–483. DOI: 10.1111/jgh.13848. [DOI] [PubMed] [Google Scholar]
- 13.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. DOI: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuchiya N, Yu S, Endo I, et al. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol 2015; 21: 10573–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song P, Tang Q, Feng X, et al. Biomarkers: evaluation of clinical utility in surveillance and early diagnosis for hepatocellular carcinoma. Scand J Clin Lab Invest Suppl 2016; 76: S70–S76. [DOI] [PubMed] [Google Scholar]
- 16.Benson AB, 3rd, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: Hepatobiliary cancers. J Natl Compr Canc Netw 2009; 7: 350–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peipei S, Jianjun G, Yoshinori I, et al. Biomarkers: Evaluation of screening for and early diagnosis of hepatocellular carcinoma in Japan and China. Liver Cancer 2013; 2: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Zhang Y, Li S, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res 2018; 10: 1947–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol 2008. ;23: 1541– 1548. [DOI] [PubMed] [Google Scholar]
- 20.Ji J, Wang H, Li Y, et al. Diagnostic evaluation of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatitis B virus-related hepatocellular carcinoma in China: A large-scale, multicentre study. PLoS One 2016; 11: e0153227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 2010; 138: 493–502. DOI: 10.1053/j.gastro.2009.10.031 S0016-5085(09)01857-5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol 2016; 14: 875–886.e6. DOI: S1542-3565(16)00044-6 [pii] 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Katayama F, Kato H, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: A review of epidemiology and control measures. J Epidemiol 2011; 21: 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beasley RP, Hwang LY. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis 1984; 4: 113–121. DOI: 10.1055/s-2008-1040651. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Kim GA, Han S, et al. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology 2019; 69: 1983–1994. DOI: 10.1002/hep.30233. [DOI] [PubMed] [Google Scholar]
- 26.Best J, Bilgi H, Heider D, et al. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol 2016; 54: 1296–1305. DOI: 10.1055/s-0042-119529. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Wu G, Li YJ. Evaluation of serum des-gamma-carboxy prothrombin for the diagnosis of hepatitis B virus-related hepatocellular carcinoma: a meta-analysis. Dis Markers 2018; 2018: 8906023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CS, Lee TY, Chou RH, et al. Development of a highly sensitive glycan microarray for quantifying AFP-L3 for early prediction of hepatitis B virus-related hepatocellular carcinoma. PLoS One. 2014; 9: e99959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo P, Wu S, Yu Y, et al. Current status and perspective biomarkers in AFP negative HCC: Towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res 2019. DOI: 10.1007/s12253-019-00585-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Feng X, Song P, Ping B, et al. Des-γ-carboxyprothrombin plasma level in diagnosis of hepatocellular carcinoma in a Chinese population undergoing surgery. Med Sci Monit 2016; 22: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding authors upon reasonable request.