Abstract
Background:
Fresh osteochondral allograft transplant (OCA) has good outcomes in the knee. However, donor tissue for patellar OCA is limited. Outcomes after nonorthotopic OCA of the patella using more readily available femoral condylar allograft (FCA) tissue have not been previously reported.
Purpose:
To assess short-term magnetic resonance imaging (MRI) and minimum 2-year clinical outcomes of nonorthotopic patellar OCA using an FCA donor.
Study Design:
Case series; Level of evidence, 4.
Methods:
A prospective institutional cartilage registry was reviewed to identify patients treated with patellar OCA using an FCA donor between August 2009 and June 2016. OCA plugs were obtained from the FCA at its trochlear-condylar junction and implanted into the recipient patellar lesion. Early postoperative MRI scans were graded by a blinded musculoskeletal radiologist using the Osteochondral Allograft MRI Scoring System (OCAMRISS). International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC), Knee Outcomes Survey–Activities of Daily Living (KOS-ADL), and pain visual analog scale (VAS) scores were collected preoperatively and at minimum 2 years postoperatively, and outcomes were compared using the paired t test.
Results:
A total of 25 patients were included for clinical outcome analysis and 20 patients for MRI analysis. MRI scans obtained at a mean of 11.4 months (range, 6-22 months) postoperatively showed a mean total OCAMRISS score of 9.0 (range, 7-11); mean bone, cartilage, and ancillary subscores were 2.6, 3.7, and 2.6, respectively. At the latest follow-up (mean, 46.5 months; range, 24-85 months), postoperative improvements were noted in IKDC (from 45.0 to 66.2; P = .0002), KOS-ADL (from 64.3 to 80.4; P = .0012), and VAS (from 5.1 to 3.4; P = .001) scores, with IKDC and KOS-ADL scores above the corresponding previously reported minimal clinically important difference.
Conclusion:
In this study, patellar OCA using nonorthotopic FCA led to significant short-term improvements in pain and patient-reported outcomes. The majority of nonorthotopic patellar grafts demonstrated full osseous incorporation and good restoration of the articular surface on MRI at short-term follow-up.
Keywords: osteochondral allograft, patella, nonorthotopic, MRI
Fresh osteochondral allograft transplant (OCA) is an increasingly popular technique involving the single-stage transfer of mature hyaline cartilage-bone dowels into large chondral defects of the knee. Multiple studies have demonstrated good long-term results, with improvements in both postoperative pain and function2,10,28 and a reported 88% rate of return to sport,14,15 including an 80% rate of return to play in professional and collegiate athletes.3 Anatomically, medial femoral condyle (MFC) and patellar cartilage lesions are the most commonly encountered.4,27 Although most of the existing outcomes literature has focused on condylar OCA, treatment of patellar lesions with OCA has been reported as well. Patellar OCA has been shown to have good outcomes, comparable with other forms of cartilage restoration in the patella,1 with graft survivorship of 78.1% at both 5 and 10 years, maintained improvement in pain and function, and 89% patient satisfaction.9
The unique shape of the patella presents a challenge for osteochondral allograft implantation. Specifically, the bony shape, chondral thickness, and surface function (sliding articulation rather than rolling, which occurs in the condyles) are all distinct challenges to proper matching.6,13 Ideally, transplant of an osteochondral allograft uses a donor graft that matches the morphologic features of the host joint architecture, although a slight mismatch has been shown to be acceptable, with unchanged clinical outcomes.20 Condyle-specific matching, for instance, MFC graft for MFC lesion, was traditionally performed in an effort to minimize incongruity between graft and host. However, this is not always possible because donor tissue availability is limited, with lateral condyle donor grafts being most abundant.24 Recent laboratory and clinical studies have shown that nonorthotopic grafts (such as lateral condyle donor for MFC lesion) lead to minimal surface mismatch and unchanged clinical outcomes compared with orthotopic grafts.8,18,24 For the patella, matching is even more difficult given the large variability in patellar size and shape within the normal population. Traditionally, selection of donor patellar grafts has been based on width of the proximal tibia, as there is no standardized way of quantifying patellar size, facet configuration, or articular contour.6 Matching protocols using radiographic indices have been proposed; however, these protocols are cumbersome and do not fully account for variability in 3-dimensional patellar shape. A further obstacle is the limited supply and rapid use of available patellar allograft donor tissue compared with whole distal femoral or hemicondylar donor tissue. Fresh femoral condylar allografts (FCAs) are more readily available and routinely used for lesions of the condyles and trochlea.
Thus, OCA of patellar lesions using nonorthotopic donor tissue would improve the use of donor graft tissue and increase the likelihood of obtaining a suitable graft in a timely manner. No previous studies have reported results after OCA for patellar lesions using femoral condylar donor graft tissue. Therefore, the purpose of this study was to assess short-term magnetic resonance imaging (MRI) and clinical outcomes of patients treated with nonorthotopic patellar OCA using an FCA donor.
Methods
Patients
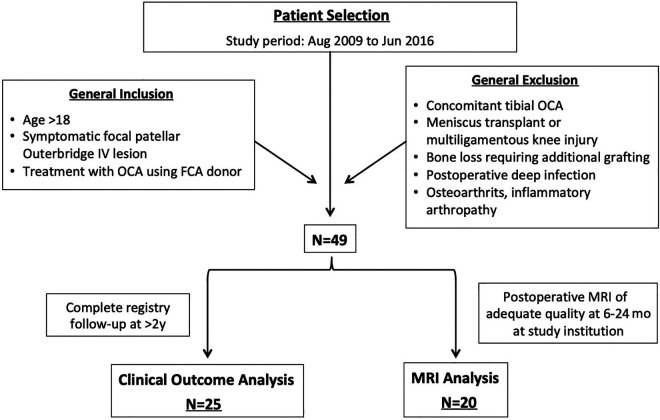
In 1999, a prospective registry dedicated to tracking patient outcomes after articular cartilage restoration procedures was implemented at our institution. An institutional review board approved the registry, and all patients signed an informed consent form before participation. The registry was queried for patients treated between August 2009 and June 2016. Inclusion criteria included (1) symptomatic focal cartilage lesions of the patella that were classified as Outerbridge grade 4 lesions at the time of arthroscopic surgery, (2) no substantial bone loss requiring additional bone grafting, (3) treatment with fresh OCA from FCA donor, and (4) age older than 18 years. Exclusion criteria included (1) tibial OCA (which signifies a more global osteoarthritic change about the knee), (2) meniscal allograft transplant, (3) postoperative deep infection, and (4) contraindications for OCA including advanced osteoarthritis (Kellgren-Lawrence grade 3 or 4) involving the medial or lateral compartments, inflammatory arthritis or autoimmune conditions, and inability to comply with the postoperative rehabilitation protocol. A further inclusion criterion for the clinical outcome cohort was complete registry follow-up at >2 years. Patients with incomplete clinical outcome scores at minimum 2 years were excluded from analysis. For the MRI group, further inclusion criteria were postoperative MRI at the study institution at 6 to 24 months and adequate imaging quality for complete scoring via the Osteochondral Allograft MRI Scoring System (OCAMRISS) as determined by a fellowship-trained musculoskeletal radiologist. A schematic of the study design is presented in Figure 1. Clinical and MRI outcomes were evaluated separately, because not all patients had adequate follow-up of both.
Figure 1.
Flowchart of study design. FCA, femoral condylar allograft; MRI, magnetic resonance imaging; OCA, osteochondral allograft transplant.
Clinical Outcomes
Demographic, preoperative, intraoperative, and postoperative data were collected. Demographic data included age, sex, and body mass index (BMI). Preoperative data included the number and type of previous ipsilateral knee surgical procedures as well as preoperative imaging with measurement of tibial tubercle–trochlear groove distance. Intraoperative data included laterality; examination under anesthesia (range of motion, ligamentous stability); location, size, and depth of the chondral defect; concomitant procedures performed; and postoperative rehabilitation protocol. The following clinical outcome scores were collected preoperatively and at minimum 2 years postoperatively: International Knee Documentation Committee Subjective Knee Evaluation Form (IKDC), Knee Outcomes Survey–Activities of Daily Living (KOS-ADL), and visual analog scale (VAS) for pain.
MRI Outcomes
Postoperative MRI scans were obtained at approximately 6 and/or 12 months as part of the senior author’s (R.J.W.) standard practice to evaluate allograft contour and integration and guide patients’ postoperative rehabilitation. Postoperative MRI scans were included for analysis if they were performed at the study institution (and thus performed using standardized and consistent imaging parameters), were obtained within 24 months postoperatively, and demonstrated adequate imaging quality for OCAMRISS grading upon review by a fellowship-trained musculoskeletal radiologist (A.J.B.). A subset of patients underwent a second postoperative MRI during the study period, outside of the standard follow-up protocol, to evaluate for new or persistent symptoms. The MRI studies that had adequate quality for OCAMRISS grading were included in a subgroup analysis of MRI findings at longer term follow-up in patients with subsequent postoperative MRI scans.
All MRI scans were performed on a 3.0-T scanner (General Electric Healthcare) with a standardized quadrature or 8-channel knee coil (Invivo Inc). MRI pulse sequences included a cartilage-sensitive protocol using a moderate echo time and fast spin-echo technique. All MRI scans were assessed by a fellowship-trained musculoskeletal radiologist (A.J.B.), who was blinded to the patient’s medical history and treatment, using the OCAMRISS (Table 1).16 The OCAMRISS is a reproducible grading system specifically developed to evaluate the radiological characteristics of OCA with the inclusion of features addressing subchondral bone, osseous integration, cartilage congruity and fill, and ancillary features. Each transplanted dowel was graded individually. For knees with >1 transplanted dowel, the scores for each feature were averaged.
Table 1.
Osteochondral Allograft MRI Scoring Systema
| MRI Feature | Score |
|---|---|
| Bone features | |
| 1. Subchondral bone plate congruity of graft and host-graft junction | 0: Intact and flush 1: Disrupted or not flush by >1 subchondral thickness |
| 2. Subchondral bone marrow signal intensity vs epiphyseal bone | 0: Normal 1: Abnormal (edema pattern or hypointensity on all sequences) |
| 3. Osseous integration at junction | 0: Crossing trabeculae 1: Discernible cleft |
| 4. Presence of cystic change of graft-host junction | 0: Absent 1: Present |
| Cartilage features | |
| 5. Cartilage signal | 0: Normal 1: Altered intensity but no fluid 2: Fluid signal presence |
| 6. Cartilage fill | 0: 76%-100% 1: 51%-75% or >100% 2: ≤50% |
| 7. Cartilage edge integration at junction | 0: No discernible boundary 1: Discernible boundary 2: Discernible fissure >1 mm |
| 8. Cartilage surface congruity at junction | 0: Flush 1: ≤50% offset of host cartilage 2: >50% offset |
| 9. Calcified cartilage integrity | 0: Intact, thin, and smooth 1: Altered (disrupted, thickened, or blurred) |
| Ancillary features | |
| 10. Opposing cartilage | 0: Normal 1: Abnormal |
| 11. Meniscal tears | 0: Absent 1: Present |
| 12. Synovitis | 0: Absent 1: Present |
| 13. Fat pad scarring | 0: Absent 1: Present |
aMRI, magnetic resonance imaging.
Surgical Technique
After knee arthroscopy, the operative extremity was exsanguinated, and a proximal thigh tourniquet was inflated to 250 mm Hg. A medial or lateral parapatellar incision was made based on the location of the lesion. Hemostasis was achieved, and the fat pad was partially resected to free the patellar tendon from adhesions. The patella was then everted, and the lesion was sized and reamed to create a circular host defect. The depth of the defect was measured, and excess bone was removed. On the back table, a core reamer was used to harvest a donor osteochondral plug allograft from an FCA donor at the trochlear-condylar junction (Figure 2). Fine cuts were made so that the depth of the donor plug matched the host defect. Depth of the osteochondral dowels ranged from 6 to 8 mm, and the graft was gently impacted into place to achieve press-fit fixation. Graft stability was then assessed, the knee was cycled, and in all cases, articular cartilage surface architecture was reestablished without significant step-off. The tourniquet was then released, hemostasis was achieved, and a standard layered wound closure was performed.
Figure 2.
Representative intraoperative photographs of patellar osteochondral allograft transplant (OCA) using femoral condylar allograft (FCA). (A) Harvest site from trochlear-condylar junction of FCA donor tissue (yellow dashed circle). (B) In situ full-thickness patellar osteochondral lesion. (C) Completed OCA graft implantation using press-fit dowel technique.
Postoperatively, patients remained toe-touch weightbearing in a hinged knee brace that was locked in extension during ambulation, with range of motion as tolerated. Patients were permitted to begin active-assisted range of motion exercises, quadriceps sets, straight-leg raises, and patellar mobilization. Full range of motion was permitted immediately and encouraged with the use of a continuous passive motion device. Brace wear was required for a minimum of 2 weeks, with the total duration of bracing dependent on the restoration of quadriceps control and strength. A supervised physical therapy program was undertaken postoperatively in all cases. The duration of the postoperative physical therapy program was dependent on the restoration of normal gait, return of quadriceps function, and performance of sport-specific skills. Return to higher level activities and athletics was initiated on an individual patient basis, typically starting with a running program at 6 months. Sport-specific training and unrestricted activities were progressed thereafter, depending upon return of lower extremity strength.
Statistical Analysis
Power calculation was performed for comparison of paired differences, for a power of 80% and α = .05, to determine the sample size needed to detect clinically meaningful pre- to postoperative change in clinical outcome scores. The previously described minimal clinically important difference (MCID) for IKDC score (17) and KOS-ADL score (10) in patients undergoing OCA of the knee were used as the expected means,23 and previously reported standard deviations from similar studies using this registry were used.26 The sample sizes required for an adequately powered study to detect the MCID for IKDC and KOS-ADL were 14 and 19, respectively. For VAS, there is no well-defined MCID in patients undergoing OCA of the knee. There is a paucity of quality data on VAS outcomes in this particular population, and as such, it is difficult to present credible MCID values.7 Sample size calculation for VAS, using mean and standard deviations reported in a recent cohort study of OCA,21 yielded a required sample size of 17.
For clinical outcome scores, descriptive statistics for pre- and postoperative scores were calculated using Microsoft Excel, and comparative statistics were computed using the paired t test with α = .05. For OCAMRISS scores, descriptive statistics for total, bone, cartilage, and ancillary scores were computed. A post hoc analysis of the association between OCAMRISS score and change in clinical outcome scores was performed using the Pearson correlation coefficient.
Results
A total of 49 patients were treated with patellar OCA using FCA during the study period, 25 of whom met inclusion criteria for analysis of 2-year clinical outcomes, and 20 of whom met criteria for analysis of postoperative MRI. Analysis of clinical and MRI outcomes was performed separately for these 2 cohorts because not all patients had adequate follow-up in both categories. Of the initial 49 patients, 24 patients had incomplete or absent clinical outcomes follow-up beyond the 2-year time point and were thus excluded from statistical analysis of clinical outcomes. Similarly, 29 of the initial 49 patients did not have adequate MRI scans within the early follow-up period and were therefore excluded from imaging analysis. Demographic data are presented in Table 2.
Table 2.
Demographic Dataa
| P | |||||
|---|---|---|---|---|---|
| Factor | Total (N = 49) | Clinical (n = 25) | MRI (n = 20) | Clinical | MRI |
| Age, y | 38.8 ± 10.9 | 42.0 ± 11.6 | 35.8 ± 9.6 | .25 | .29 |
| Body mass index | 25.1 ± 4.5 | 24.8 ± 5.2 | 25.8 ± 4.9 | .80 | .57 |
| Female, % | 57 | 72 | 58 | .31 | ≥.999 |
| TT-TG, mm | 13.4 ± 6.7 | 13.4 ± 4.6 | 13.7 ± 6.3 | ≥.999 | .86 |
| Lesion area, mm2 | 336.0 ± 146.6 | 305.5 ± 158.9 | 339.0 ± 138.7 | .41 | .94 |
| Lesion type, n (%) | .87 | .87 | |||
| Focal degenerative | 41 (84) | 22 (88) | 16 (80) | ||
| Osteochondritis dissecans | 6 (12) | 2 (8) | 3 (15) | ||
| Acute traumatic | 2 (4) | 1 (4) | 1 (5) | ||
| No. of patellar FCA plugs, n | .70 | .86 | |||
| 1 plug | 46 | 24 | 19 | ||
| 2 plugs | 3 | 1 | 1 | ||
| Concomitant trochlear OCA (bipolar), n | 10 | 5 | 4 | .97 | .97 |
| Osteotomy, n | |||||
| Prior | 5 TTO | 2 TTO | 1 TTO | .76 | .82 |
| Concomitant | 5 TTO, 1 DFO | 2 TTO | 1 TTO, 1 DFO | ≥.999 | .54 |
| Allograft donor type, n (%) | .92 | .69 | |||
| Lateral hemicondyle | 33 | 18 (72) | 13 (65) | ||
| Medial hemicondyle | 7 | 4 (16) | 4 (20) | ||
| Whole distal femur | 4 | 3 (12) | 3 (15) | ||
| Unspecified condyle | 5 | 0 | 0 | ||
aData are reported as mean ± SD unless otherwise indicated. P values represent comparison between “Total” and specified cohort (Clinical or MRI). DFO, distal femoral osteotomy; FCA, femoral condylar allograft; MRI, magnetic resonance imaging; OCA, osteochondral allograft transplant; TTO, tibial tubercle osteotomy; TT-TG, tibial tubercle–trochlear groove distance.
Clinical Outcomes
A total of 25 patients had minimum 2-year follow-up in clinical outcome scores at a mean of 42.2 ± 16.3 months. Significant postoperative increases were seen in all 3 scores (Table 3): IKDC, KOS-ADL, and VAS. The mean IKDC increased by 21.2 (95% CI, 11.2-31.2), from 45.0 to 66.2 (P = .0002). The mean KOS-ADL increased by 16.1 (95% CI, 7.2-25.1), from 64.3 to 80.4 (P = .0012). The mean VAS improved by 1.70 (95% CI, 0.77-2.62), from 5.09 to 3.39 (P = .001). Improvements for IKDC and KOS-ADL were above the previously reported MCID values. Subanalysis of the cohort that received bipolar OCA (5 patients) revealed that improvements in clinical outcome scores were not statistically different from the unipolar cohort (Table 4). It must be noted, however, that our study was not powered for this post hoc comparison.
Table 3.
Changes in Clinical Outcome Scores After Patellar Osteochondral Allograft Transplant With Femoral Condylar Allografta
| Score | Preoperative | Postoperative | Change | 95% CI | P |
|---|---|---|---|---|---|
| IKDC | 45.0 | 66.2 | 21.2 | 11.2 to 31.2 | .0002 |
| KOS-ADL | 64.3 | 80.4 | 16.1 | 7.2 to 25.1 | .0012 |
| VAS | 5.09 | 3.39 | –1.70 | –0.77 to –2.62 | .001 |
aIKDC, International Knee Documentation Committee Subjective Knee Evaluation Form; KOS-ADL, Knee Outcomes Survey Activities of Daily Living; VAS, visual analog scale for pain.
Table 4.
Comparison of Clinical Outcome Scores in Bipolar vs Unipolar Patellar Osteochondral Allograft Transplant Using Femoral Condylar Allografta
| Bipolar (n = 5) | Unipolar (n = 20) | P | |
|---|---|---|---|
| IKDC | |||
| Preoperative mean | 47.8 | 44.2 | |
| Postoperative mean | 68.9 | 65.5 | |
| Change (95% CI) | 21.1 (6.1 to 36.2) | 21.3 (9.7 to 32.8) | .99 |
| KOS-ADL | |||
| Preoperative mean | 65 | 64.2 | |
| Postoperative mean | 79.1 | 80.8 | |
| Change (95% CI) | 14.1 (–5.0 to 33.1) | 16.6 (7.0 to 26.3) | .82 |
| VAS pain | |||
| Preoperative mean | 6.4 | 4.7 | |
| Postoperative mean | 4.0 | 3.2 | |
| Change (95% CI) | –2.4 (–4.9 to 0.12) | –1.5 (–2.4 to –0.6) | .42 |
aIKDC, International Knee Documentation Committee Subjective Knee Evaluation Form; KOS-ADL, Knee Outcomes Survey Activities of Daily Living; VAS, visual analog scale for pain.
MRI Outcomes
A total of 20 patients had postoperative MRI scans performed at the study institution that were of acceptable quality for imaging analysis after surgery. The first postoperative MRI was obtained at a mean of 11.4 months (range, 6-22 months) after surgery. Mean total OCAMRISS score was 9.1 (range, 7-11). Mean bone, cartilage, and ancillary subscores were 2.6, 3.9, and 2.6, respectively. Within the cartilage subscore, 85% (17/20) of grafts demonstrated 76%-100% cartilage fill, whereas 15% (3/20) demonstrated 51%-75% or >100% fill. Normal cartilage signal intensity was seen in 65% (13/20), with the remaining 35% of grafts showing altered intensity but no grafts showing fluid intensity. Within the bone subscore, 60% of all grafts demonstrated full osseous integration with crossing trabeculae and no discernible cleft, and 70% demonstrated absence of cystic change at graft interfaces (Figure 3). All but 1 graft demonstrated persistent subchondral marrow edema relative to the epiphyseal bone, and all but 1 graft demonstrated incongruity of the subchondral plate. Subgroup comparison of patients receiving bipolar OCA versus unipolar OCA showed no difference in OCAMRISS score (9.3 bipolar vs 9.0 unipolar; P = .66).
Figure 3.

Representative magnetic resonance imaging (MRI) scans after patellar osteochondral allograft transplant using femoral condylar allograft. (A) Twelve-month postoperative sagittal and axial MRI scans demonstrating good osseous integration: crossing trabeculae, intact subchondral plate, absence of cystic change at graft interface, and minimal subchondral edema. Good chondral integration is shown by full cartilage fill without change in signal intensity and no visible cleft or fissures. (B) Six-month postoperative MRI scans in a different patient showing subchondral plate incongruity despite a closely approximated chondral surface due to mismatch in cartilage thickness. Altered signal intensity is evident in the chondral layer with slight fissuring laterally. Yellow arrows depict allograft location.
On subanalysis, 9 patients who had OCAMRISS data had minimum 2-year clinical outcome data. For both IKDC and KOS-ADL, there was a trend toward a moderate negative correlation, with increasing OCAMRISS scores associated with decreasing improvement in clinical scores; however, neither reached statistical significance: r = –0.513 (P = .16) for IKDC and r = –0.550 (P = .13) for KOS-ADL.
In total, 10 patients had a second MRI at later postoperative follow-up (mean, 26.7 months; range, 25-33 months). Mean OCAMRISS was 10.4 (range, 7-12) at 26.7 months compared with 9.0 (range 7-12) at 11.4 months (P = .03). Within these 10 patients, 1 patient had an improved OCAMRISS score at subsequent MRI, 4 patients had unchanged scores, and 5 patients had worsened scores. Among the 5 patients with worsened scores, the mean magnitude increase (worsening) in total score was 2.8 points (from 9.0 to 11.8; P = .002), with a 2.6-point mean increase in cartilage subscore (from 3.2 to 5.8; P = .0005), a 0.2-point increase in bone subscore (from 3.0 to 3.2; P = .6), and no change in ancillary subscore (Table 5). The mean age and BMI for these 5 patients was 32.6 years and 24.5, respectively, both of which were lower than those of the overall study population.
Table 5.
Mean OCAMRISS Subscores in Patients With Worsened Scores on Second Postoperative MRIa
| Months Postoperative | Total Score | Cartilage Subscore | Bone Subscore | Ancillary Subscore | |
|---|---|---|---|---|---|
| First postoperative MRI | 11.4 | 9.0 | 3.2 | 3.0 | 2.8 |
| Second postoperative MRI | 26.7 | 11.8 | 5.8 | 3.2 | 2.8 |
| Difference | 15.3 | 2.8 (P = .002) | 2.6 (P = .0005) | 0.2 (P = .6) | 0 |
aMRI, magnetic resonance imaging; OCAMRISS, Osteochondral Allograft MRI Scoring System.
Discussion
In this study, patients who underwent OCA of the patella using FCA had statistically significant improvement in IKDC, KOS-ADL, and VAS at short-term follow-up. Both IKDC and KOS-ADL improvements were above the previously reported MCID values. There is no well-established MCID for VAS in this study population, and previously reported MCID values for VAS in knee pathologies range from 1.0 to 3.0.5,12,19 Therefore, although patients in the study cohort were found to have clinically significant improvements in IKDC and KOS-ADL, the clinical significance of the statistically significant VAS improvement is unclear. On postoperative MRI, the majority of patients showed full osseous integration with good cartilage fill and surface congruity but persistent incongruity at the subchondral plate. These findings suggest that OCA using nonorthotopic FCA donor for patellar lesions produces good short-term clinical and radiographic results while eliminating the potential for surgical delays associated with obtaining a patellar allograft. Longer term follow-up studies are required, however, to elucidate the sustainability of these results. To our knowledge, this is the first study in the literature to assess both MRI and clinical outcomes after nonorthotopic OCA in the patella.
Although no previous studies report outcomes after patellar OCA using FCA donor tissue, the outcomes from this study are comparable with those reported in studies of patellar osteochondral defects treated with orthotopic allografts. Gracitelli et al9 investigated minimum 2-year clinical outcomes after patellar OCA using orthotopic patellar grafts and reported improvements in IKDC score from 36.5 to 66.5 and KOS-ADL from 64.6 to 80.5. That study, however, included patients younger than 18 years, and the mean lesion size was 10.1 cm2. In contrast, the current study included only patients older than 18 years, and the mean lesion size was approximately 3 cm2. Jamali et al11 investigated 20 knees treated with patellar OCA and showed a significant increase in clinical scores, from 11.7 to 16.3, when using the Merle d’Aubigné-Postel 18-point scale. Of note, Jamali et al reported 5 treatment failures (requiring revision allograft, arthrodesis, or arthroplasty), and their study included treatment of bipolar lesions (12 bipolar, 8 patella only). A long-term follow-up study by Torga Spak and Teitge22 showed that clinical scores significantly improved in 14 patients treated with patellar OCA for early degenerative change, at a mean of 10 years of follow-up (minimum 2.5 years) after OCA of the patellofemoral joint. On radiographic analysis of their cohort, knees with intact allografts showed no progression in degenerative disease. In a cohort consisting of only bipolar OCA, Mirzayan et al17 showed that significant improvements in numerous clinical outcome scores could be achieved, with no revisions or conversion to arthroplasty at a mean of 33 months (minimum 1 year). Those authors reported radiographic outcome using the OCAMRISS score, with a mean score of 2.23 for the patella, which is lower than the mean score in the present study. One possible explanation is that the majority of patients included in the Mirzayan et al study were primarily treated for patellar instability, whereas the patients in the present study were treated primarily for degenerative change; this could lead to differences in both the type of chondral injury and biological healing response. In a recent systematic review, Andrade et al1 showed that when compared with osteochondral autograft transfer, autologous chondrocyte implantation, particulated juvenile allograft cartilage, and autologous matrix-induced chondrogenesis, OCA of the patella led to similar improvements in clinical scores above the MCID, with no definitive differences between groups.
Despite the limited literature on patellar OCA, treatment of condylar defects using OCA is known to have good outcomes. The mean total OCAMRISS score, as well as the bone, cartilage, and ancillary subscores reported in this study, are comparable with previously reported scores at 1 year after OCA for condylar defects.25 Similarly, the osseous integration rate of 60% without a discernible cleft on MRI is comparable with previous reports of condylar OCA at 1 year, reported to have 56% full osseous integration without a discernible cleft.25 It is known that patients function very well after orthotopic OCA for condylar defects,2,10,28 and an 88% rate of return to sport has been reported.14,15 Although condylar and patellar defects are distinct pathologies and thus results from condylar OCA cannot be extrapolated to patellar OCA, the similar graft integration rates and similar improvements in short-term outcomes suggest that condylar donor tissue may be a feasible option for treatment of patellar defects.
In the current study, 10 patients had a second postoperative MRI scan at a mean of 26.7 months, for comparison with the first postoperative MRI at mean 11.4 months. Total scores were slightly different: 9.0 at first MRI and 10.4 at second, suggesting a slight decline in radiographic appearance overall. Of this group, 5 patients experienced decline in OCAMRISS score, whereas 1 patient improved and 4 patients remained unchanged. Among the 5 patients with worsened scores, analysis of the subscores showed that most of the decline came from a decline in the cartilage subscore, while the bone and ancillary subscores remained essentially unchanged. Although the study was not powered for such an analysis, this finding suggests that over longer term follow-up, while bony incorporation persists, chondral incorporation may be shorter-lived. However, given the small sample size, bias and confounding may be at play, and further studies are required to answer this particular question. The clinical effects of this difference in cartilage subscore are difficult to ascertain in the current study.
It is known that the patellar cartilage is thicker than that of the femoral condyles, and previous studies have shown that mismatch of host and graft height leads to significantly increased contact pressures.6,13 However, in the case of nonorthotopic grafting, given different thicknesses of the cartilage layer, even if equal overall height is achieved, it is possible that a mismatch in subchondral plate congruity may lead to aberrant loading on the deep layer of the cartilage, which may portend poorer outcome. Further investigation is warranted to characterize the radiographic morphologic characteristics over time and elucidate the longevity of nonorthoptic patellar OCA using FCA donor in comparison with other cartilage regeneration techniques.
The relationship between appearance on MRI and improvement in clinical outcome scores is still unclear. This study was not initially powered for this post hoc subgroup analysis. In this study, only 9 patients in the study cohort had both minimum 2-year clinical outcomes and adequate postoperative MRI scans for OCAMRISS grading. Among these patients, there was a moderate negative correlation of increasing OCAMRISS score with decreasing clinical scores, although this was not statistically significant. Although the results of this study suggest that there are good short-term clinical and radiographic results after patellar OCA using FCA donor tissue, conclusions cannot be drawn regarding the correlation of radiographic signs and clinical outcome scores.
There are numerous important limitations to our study. Although data were prospectively collected through an institutional registry, analysis was done retrospectively, introducing various confounders. Although the demographic information collected preoperatively did not differ between the overall cohort and separate clinical and MRI groups, other possible confounders may influence the results of the intervention: history of patellar instability, activity level, sports participation, smoking status, or postoperative compliance with rehabilitation protocols. There was a large loss to follow-up in both the clinical and the MRI groups, since only patients with complete outcome data at >2 years or patients with MRI scans from the early postoperative period were included, which introduces participation bias. Although the inclusion and exclusion criteria define a distinct population and outcomes from this study are comparable with those previously reported in similar patients, there is still potential for sampling bias, as the study population is drawn from a single institution. Furthermore, no control or comparison group was included, precluding direct comparison with other described techniques. To capture a larger number of patients with OCA using FCA, we included patients receiving treatment for bipolar lesions. However, our study was not initially powered to draw conclusions based on the post hoc subgroup analyses. Larger sample size and more uniform follow-up are required to draw conclusions regarding changes in MRI characteristics on subsequent imaging follow-up. Specific to patellar osteochondral lesions, although the OCAMRISS score measures the lesion fill, surface congruity, and subchondral congruity, there is no standardized way of measuring the overall topography fit. For instance, while a graft may match the host patella perfectly at the edges without any fissures or step-offs, the surface topography may still be altered; the clinical impact of this phenomenon is unknown and was not measured in this study. Similarly, we did not subanalyze our results based on which region of the patella was affected; although most of the lesions in this study were central, it is possible that patients with steeper patellar anatomy or particular facet lesions tend to have worse outcome after condylar allograft, and these subpopulations would be missed in our analysis.
Conclusion
The results of this retrospective analysis of prospectively collected registry data suggest that use of femoral condylar donor tissue for treatment of patellar chondral lesions produced good short-term clinical and MRI outcomes. Improvements in patient-reported outcome scores and rate of complete osseous integration based on MRI are comparable with previously reported results after OCA for condylar defects. On postoperative MRI follow-up beyond 2 years, a subset of patients had a worsening appearance on MRI scans due to worsening cartilage subscores. The clinical correlation of this finding is unclear. The lack of a comparison group and retrospective nature of this study preclude direct comparison with other techniques. Further well-designed comparative studies are warranted to elucidate differences between the various cartilage restoration techniques for focal patellar defects and further characterize outcomes after the use of condylar donor tissue for patellar defects.
Footnotes
Final revision submitted April 30, 2020; accepted May 18, 2020.
Ethical approval for this study was obtained from the Hospital for Special Surgery (study No. 2013-024-CR7).
One or more of the authors has declared the following potential conflict of interest or source of funding: D.W. has received research support from Arthrex and the Musculoskeletal Transplant Foundation and educational support from Smith & Nephew and Arthrex and has stock or stock options in Cartilage Inc. K.J.J. has received research support from the Musculoskeletal Transplant Foundation, educational support from Arthrex, consulting fees from JRF Ortho and Vericel, and honoraria from the Musculoskeletal Transplant Foundation and Vericel. R.J.W. has received educational support from Arthrex, consulting fees from Arthrex and JRF Ortho, and nonconsulting fees from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Andrade R, Nunes J, Hinckel BB, et al. Cartilage restoration of patellofemoral lesions: a systematic review. Cartilage. Published online December 17, 2019. doi:10.1177/1947603519893076 [DOI] [PMC free article] [PubMed]
- 2. Assenmacher AT, Pareek A, Reardon PJ, Macalena JA, Stuart MJ, Krych AJ. Long-term outcomes after osteochondral allograft: a systematic review at long-term follow-up of 12.3 years. Arthroscopy. 2016;32(10):2160–2168. [DOI] [PubMed] [Google Scholar]
- 3. Balazs GC, Wang D, Burge AJ, Sinatro AL, Wong AC, Williams RJ III. Return to play among elite basketball players after osteochondral allograft transplantation of full-thickness cartilage lesions. Orthop J Sports Med. 2018;6(7):23259 67118786941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. [DOI] [PubMed] [Google Scholar]
- 5. Danoff JR, Goel R, Sutton R, Maltenfort MG, Austin MS. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasty. 2018;33(7)(suppl):S71–S75. [DOI] [PubMed] [Google Scholar]
- 6. Determann JR, Fleischli JE, D’Alessandro DF, Piasecki DP. Patellofemoral osteochondral allografts: can we improve the matching process? J Knee Surg. 2017;30(8):835–841. [DOI] [PubMed] [Google Scholar]
- 7. Devji T, Guyatt GH, Lytvyn L, et al. Application of minimal important differences in degenerative knee disease outcomes: a systematic review and case study to inform BMJ Rapid Recommendations. BMJ Open. 2017;7(5):e015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du PZ, Markolf KL, Levine BD, McAllister DR, Jones KJ. Differences in the radius of curvature between femoral condyles: implications for osteochondral allograft matching. J Bone Joint Surg Am. 2018;100(15):1326–1331. [DOI] [PubMed] [Google Scholar]
- 9. Gracitelli GC, Meric G, Pulido PA, Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med. 2015;43(4):879–884. [DOI] [PubMed] [Google Scholar]
- 10. Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. [DOI] [PubMed] [Google Scholar]
- 11. Jamali AA, Emmerson BC, Chung C, Convery FR, Bugbee WD. Fresh osteochondral allografts: results in the patellofemoral joint. Clin Orthop Relat Res. 2005;437:176–185. [PubMed] [Google Scholar]
- 12. Jones KJ, Kelley BV, Arshi A, McAllister DR, Fabricant PD. Comparative effectiveness of cartilage repair with respect to the minimal clinically important difference. Am J Sports Med. 2019;47(13):3284–3293. [DOI] [PubMed] [Google Scholar]
- 13. Koh JL, Wirsing K, Lautenschlager E, Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32(2):317–320. [DOI] [PubMed] [Google Scholar]
- 14. Krych AJ, Pareek A, King AH, Johnson NR, Stuart MJ, Williams RJ III. Return to sport after the surgical management of articular cartilage lesions in the knee: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(10):3186–3196. [DOI] [PubMed] [Google Scholar]
- 15. Krych AJ, Robertson CM, Williams RJ III. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40(5):1053–1059. [DOI] [PubMed] [Google Scholar]
- 16. Meric G, Gracitelli GC, McCauley JC, et al. Osteochondral Allograft MRI Scoring System (OCAMRISS) in the knee: interobserver agreement and clinical application. Cartilage. 2015;6(3):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mirzayan R, Charles MD, Batech M, Suh BD, DeWitt D. Bipolar osteochondral allograft transplantation of the patella and trochlea. Cartilage. Published online September 3, 2018. doi:10.1177/1947603518796124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mologne TS, Cory E, Hansen BC, et al. Osteochondral allograft transplant to the medial femoral condyle using a medial or lateral femoral condyle allograft: is there a difference in graft sources? Am J Sports Med. 2014;42(9):2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. [DOI] [PubMed] [Google Scholar]
- 20. Nakagawa Y, Suzuki T, Kuroki H, Kobayashi M, Okamoto Y, Nakamura T. The effect of surface incongruity of grafted plugs in osteochondral grafting: a report of five cases. Knee Surg Sports Traumatol Arthrosc. 2007;15(5):591–596. [DOI] [PubMed] [Google Scholar]
- 21. Thomas D, Shaw KA, Waterman BR. Outcomes after fresh osteochondral allograft transplantation for medium to large chondral defects of the knee. Orthop J Sports Med. 2019;7(3):2325967119832299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torga Spak R, Teitge RA. Fresh osteochondral allografts for patellofemoral arthritis: long-term followup. Clin Orthop Relat Res. 2006;444:193–200. [DOI] [PubMed] [Google Scholar]
- 23. Wang D, Chang B, Coxe FR, et al. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. Am J Sports Med. 2019;47(1):71–81. [DOI] [PubMed] [Google Scholar]
- 24. Wang D, Jones KJ, Eliasberg CD, Pais MD, Rodeo SA, Williams RJI. Condyle-specific matching does not improve midterm clinical outcomes of osteochondral allograft transplantation in the knee. J Bone Joint Surg Am. 2017;99(19):1614–1620. [DOI] [PubMed] [Google Scholar]
- 25. Wang D, Lin KM, Burge AJ, Balazs GC, Williams RJ III. Bone marrow aspirate concentrate does not improve osseous integration of osteochondral allografts for the treatment of chondral defects in the knee at 6 and 12 months: a comparative magnetic resonance imaging analysis. Am J Sports Med. 2019;47(2):339–346. [DOI] [PubMed] [Google Scholar]
- 26. Wang T, Belkin NS, Burge AJ, et al. Patellofemoral cartilage lesions treated with particulated juvenile allograft cartilage: a prospective study with minimum 2-year clinical and magnetic resonance imaging outcomes. Arthroscopy. 2018;34(5):1498–1505. [DOI] [PubMed] [Google Scholar]
- 27. Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–182. [DOI] [PubMed] [Google Scholar]
- 28. Williams RJ III, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718–726. [DOI] [PubMed] [Google Scholar]