Abstract
Background:
Failed anterior cruciate ligament (ACL) reconstruction (ACLR) can lead to reduced quality of life because of recurrent episodes of instability, restrictions in level of activity, and development of osteoarthritis. A profound knowledge of the causes of a failed surgery can ultimately help improve graft survival rates.
Purpose:
To investigate the patient-related risks of inferior outcomes leading to revision surgery after ACLR.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
From a prospective cohort of primary ACLRs performed at a single center, patients who required later revision surgery were matched with a control group of uneventful primary ACLRs. Patient characteristics, data from the preoperative examinations, KT-1000 arthrometer laxity testing, Tegner activity scale, International Knee Documentation Committee subjective score, Knee injury and Osteoarthritis Outcome Score, and perioperative data from the initial surgery were included.
Results:
A total of 100 revision cases and 100 matched controls, with a median follow-up time of 11 years, were included in the study. Those who had undergone revision surgery were younger at the time of reconstruction and had a shorter time from injury to surgery than their matched controls (P = .006). The control group—of uneventful ACLRs—had a higher incidence of meniscal repair at reconstruction (P = .024). Also, the revision group more frequently experienced later failure of the previous meniscal repair (P = .004). Surgeon experience was not found to affect the risk of revision ACL surgery. Those who had undergone ACL revision surgery had more frequently received a hamstring tendon graft size of <8 mm (P = .018) compared with the controls.
Conclusion:
The current study demonstrated that failed meniscal repair and a hamstring tendon graft size of <8 mm were associated with primary ACLR failure. Also, younger age at the time of surgery and shorter time from injury to surgery were found to affect the risk of undergoing revision ACL surgery.
Keywords: revision surgery, anterior cruciate ligament, meniscal repair
Despite efforts to improve outcomes after anterior cruciate ligament (ACL) reconstruction (ACLR) for many decades, there is still a persistent and significant failure rate.5 ACL registries commonly report an overall revision rate of 3%-10%,1,5,20 but in subgroups of patients, up to 22%-30% experience failure.6,38 Graft rerupture can have detrimental effects on quality of life because of recurrent episodes of instability, restrictions in the level of activity, and potential early development of osteoarthritis.2,7 Also, results after revision surgery are commonly described as inferior to what is seen after the first reconstruction.7
Failure can in part be because of return to high-risk sports, as is commonly seen in the youngest group of patients.37 The greatest risk is found in those returning to pivoting sports.37 Also, the magnitude of injury at the initial ACL tear, defined as concomitant injures to other structures, can affect the outcome after surgery.35 Further, predispositions such as female sex, a high posterior tibial slope, or a joint hyperlaxity add to patient-related risks.13,18,19 Finally, factors related to surgery, such as choice of the graft, size of the graft, and choice of fixational devices, have also been found to be of importance.18,26,30 Graft tunnel positioning, especially whether anatomic tunnel placement was achieved, is another topic that has been highlighted.11,22
As an increasing number of patients are being assessed for revision surgery,5 there is a continuous need for knowledge on why the primary surgery fails. Such knowledge can help surgical decision-making at repeat surgery and lower the risk of overall failure. The current study, therefore, aimed to investigate the potential risks of failure after ACLR in a retrospective case-control study that utilized prospectively collected data. A group of patients in need of revision surgery after their primary reconstruction were compared with a matched control group of patients with an uneventful postoperative course. The null hypothesis was that no difference would be found in pre- and peroperative potential risk factors for failure between the groups.
Methods
Patient Selection
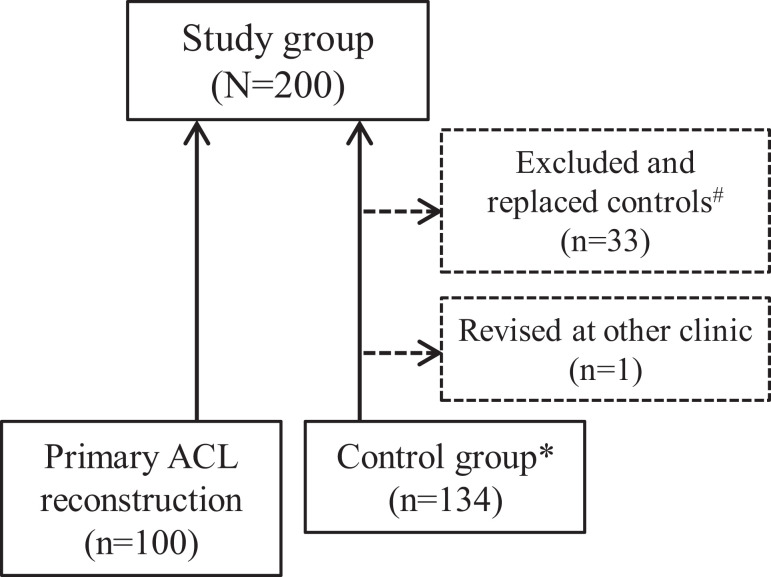
Patients who had undergone ACLR and had later undergone revision surgery from 1999 to 2015 at our clinic were eligible for the study and thus defined the sample size. Patients who had undergone concomitant ligament surgery such as medial collateral ligament, lateral collateral ligament, posterior cruciate ligament, or posterior cruciate ligament reconstruction were excluded. A matched control group of patients who had undergone ACLR without the need for revision immediately following in time to each of the cases that needed revision (and therefore made out the case group) was also included7 (Figure 1). The ratio of case to control was set as 1:1. Such matching was performed to adjust for changing surgical techniques in the period of inclusion. A minimum of 2-year follow-up was required for patients to be included in the study. All participants included in the control group were contacted to ensure they had not undergone revision surgery at another clinic. If so (n = 1), they were excluded in favor of the next consecutive patient that had been reconstructed after the index case (later undergoing revision). The study was approved by a regional ethical review board.
Figure 1.
Patient selection for the revision and control groups. *Selected as the next consecutive primary anterior cruciate ligament (ACL) reconstruction leading to revision. #Excluded as controls owing to concomitant ligament surgery, loss to follow-up, or patient deceased.
Data Collection
All data were collected from a prospective institutional quality assessment database. The following preoperative data were extracted: age, side of injury, sex, height, weight, activity at injury, time of injury, time of surgery, and time of any revision surgery. Further, perioperative data such as graft type and size, the surgeon’s level of experience (defined by the number of previous ACL surgeries performed: 0-25, under supervision; 26-100, moderate experience; and >100, experienced), and any concomitant lesion of meniscal or articular cartilage were included. Tegner activity score, International Knee Documentation Committee (IKDC) 2000 subjective score, and Knee injury and Osteoarthritis Outcome Score (KOOS) were extracted from preoperative assessment. Also, preoperative Lachman test, pivot-shift test,23 and KT-1000 arthrometer (MEDmetric) measurements were included. For the latter, the maximum manual difference between injured and normal knee (side-to-side difference) was calculated and used for analyses.
Surgical Procedure
The primary reconstruction in both the revision group and the control group was performed from April 1990 to August 2014. Therefore, a certain variety in tunnel placement strategies (transtibial and anteromedial portal techniques), choice of graft source (hamstrings and patellar tendon autograft only), and graft fixation methods were seen. Surgical technique was, however, not individualized based on patient characteristics but rather a reflection of what was seen as the gold standard at all times throughout the period. Meniscal repair was done with one (or combinations) of the following techniques: inside-out, outside-in, or all-inside suture devices—depending on the type, size, and position of the tear. The rehabilitation protocol allowed partial weightbearing for 2-4 weeks and free range of motion. In cases of concomitant meniscal repair, patients were restricted to partial weightbearing for 6 weeks and range of motion from full extension to 90° of flexion. A standardized follow-up regimen included postoperative visits to both physical therapist and surgeon at the clinic. All patients were offered functional testing at 9-12 months after surgery, including hop tests and isometric strength testing. Further rehabilitation and potential return to sports were advised according to the results of these tests.
Statistical Analysis
All statistical analyses were performed using the SPSS 23.0 software (IBM Corp). As measures of central location and spread of data, mean and standard deviation or median and range were calculated. Normality of continuous variables was investigated using QQ plots and Shapiro-Wilk test. If normality was found, independent-samples t tests were used; if not, the nonparametric Mann-Whitney U test was applied. Chi-square test was used for testing distributions of categorical variables such as sex and injured side. An a priori P value of .05 was used to denote statistical significance. A group size calculation was performed, aiming to detect a minimally clinical important difference for IKDC subjective score of 9 points.24 With a statistical significance of .05, a β value of 0.1 (power of 0.9), and a standard deviation of IKDC of 15 (based on earlier data), a group size of 58 was found to be sufficient.
Results
A total of 100 patients who had undergone revision surgery and 100 control patients were included in the study. There was no difference in median follow-up time (11 years) between the revision group and the control group. The median time from surgery to revision was 2.1 years (range, 14 days-21 years). One case was revised after 14 days because of an infection. Characteristic data for the 2 groups are presented in Table 1. The revision group was significantly younger at the time of surgery (P = .006) and had a significantly shorter time from injury to surgery (P = .041), as compared with the control group. Sex, injured side, body mass index (BMI), and preinjury Tegner score did not differ between the groups. Further, type of activity at the time of injury was not significantly different between the 2 groups, with approximately 50% of injuries being related to soccer, 15% from team handball, and 15% from alpine skiing; and 20% of injuries were related to work, traffic accidents, or other activities.
Table 1.
Characteristic Data in Revision and Control Groupsa
| Revision (n = 100) | Control (n = 100) | P | |
|---|---|---|---|
| Age at surgery, y | 24.2 | 28.4 | .006b |
| Female patients | 56 | 44 | nsc |
| Body mass index | 24.4 ± 4.0 | 24.9 ± 4.3 | nsb |
| Time from injury to surgery, mo, mean ± SD | 11.9 ± 19.0 | 22.7 ± 43.4 | .041b |
| Follow-up time, y, median (range) | 11.0 (2.2-26.4) | 10.9 (2.1-25.5) | nsb |
| Injured side (left) | 46 | 46 | nsc |
| Tegner preinjury, mean ± SD | 7.58 ± 1.63 | 7.44 ± 1.60 | nsb |
ans, not significant.
bNonparametric Mann-Whitney U test.
cChi-square test.
The preoperative clinical examination displayed no differences in mean KT-1000 arthrometer side-to-side difference, distribution of pivot-shift, or Lachman grading. Also, KOOS and IKDC subjective score were not found to differ significantly between those who had undergone revision and controls (Table 2).
Table 2.
Findings From Preoperative Clinical Examinations and Patient-Reported Outcome Measuresa
| Revision (n = 100) | Control (n = 100) | P | |
|---|---|---|---|
| KT-1000 arthrometer side-to-side difference, mm | 6.5 ± 3.0 | 6.8 ± 2.6 | nsb |
| ALRI | nsc | ||
| 0 | 2 | 0 | |
| 1 | 3 | 5 | |
| 2 | 59 | 61 | |
| Classification not possible because of muscular guarding | 14 | 16 | |
| Lachman grade | nsc | ||
| 0 | 0 | 0 | |
| 1 | 13 | 8 | |
| 2 | 71 | 75 | |
| 3 | 10 | 8 | |
| IKDC | 57 ± 15 | 55 ± 15 | nsb |
| KOOS, Sports and Recreation | 44 ± 27 | 44 ± 26 | nsb |
| KOOS, Knee-Related Quality of Life | 32 ± 18 | 31 ± 16 | nsb |
aIKDC, International Knee Documentation Committee; ALRI, anterolateral rotational instability; KOOS, Knee injury and Osteoarthritis Outcome Score; ns, not significant.
bIndependent-samples t test.
cChi-square.
There was no significant difference between the groups in the distribution of meniscal injuries, articular cartilage injuries, treatment of cartilage injuries, or meniscal resections concomitant to the primary reconstruction. The control group patients were more frequently treated with meniscal repair compared with the revision group (P = .024) (Table 3). Of those who had undergone a meniscal repair, 6 of 8 in the revision group had a later meniscal resection, while only 6 of 21 in the control group had undergone later resection (P = .038).
Table 3.
Concomitant Meniscal and Cartilage Injuries at Time of Surgerya
| Revision (n = 100) | Control (n = 100) | P | |
|---|---|---|---|
| Meniscal tear | 46 | 59 | nsb |
| Meniscal resection | 23 | 22 | nsb |
| Meniscal repair | 8 | 21 | .024b |
| Cartilage injury | 13 | 14 | nsb |
| Later failed meniscal repair | 6/8 | 6/21 | .038b |
ans, not significant.
bChi-square test.
The mean graft size was not found to differ between the 2 groups (8.5 mm in the revision group vs 8.7 mm in controls) (Table 4). A graft size of <8 mm was, however, more frequently used in the revision group than in the control group (P = .018). The distribution of graft type (hamstring or patellar tendon autografts) did not differ between groups. No allografts were used. When examining the effect of surgeon experience, no difference in risk of revision was seen when comparing surgeons under supervision, surgeons with moderate experience, and experienced surgeons.
Table 4.
Perioperative Findings: Graft Size, Graft Type, Length of Surgery, and Surgeon Experiencea
| Revision (n = 100) | Control (n = 100) | P | |
|---|---|---|---|
| Mean graft size, mm | 8.5 ± 0.9 | 8.7 ± 0.8 | nsb |
| Graft size <8 mm | 9 | 1 | .018c |
| Patellar/hamstring tendon autograft | 18/82 | 21/79 | nsc |
| Length of surgery, min | 107 ± 35 | 107 ± 31 | nsb |
| Surgeon experience | nsc | ||
| 0-25 | 9 | 7 | |
| 25-100 | 24 | 26 | |
| 100+ | 67 | 67 |
ans, not statistically significant.
bNonparametric Mann-Whitney U test.
cChi-square test.
Discussion
The most important finding in the current study is that patients in need of revision ACLR (because of a failed primary ACLR) had a lower survival rate of meniscal repairs when compared with a control group of patients who had not undergone revision surgery. Further, those who were in need of revision ACLR had more frequently received a hamstring tendon autograft of <8 mm diameter. The latter finding adds to the reports from other studies that smaller graft size is an independent risk for failure after ACLR. Differences were also seen between the revision group and the control group regarding time from injury to surgery and age at the time of surgery. Level of surgeon experience was, however, not found to affect the risk of needing later revision ACL surgery.
Proper repair of the meniscus is thought to restore its native function, which includes its role as a secondary stabilizer of the knee along with the reconstructed ACL.16,17 This was evident in an experimental biomechanical study by Stephen et al,33 who examined the effect of a posteromedial meniscocapsular lesion on tibiofemoral joint laxity in the ACL-deficient knee. They found normalization of sagittal and rotational stability of the knee only after the meniscal tear was repaired along with the ACLR. Clinical studies have also displayed the synergistic effect of meniscal repair and ACLR. A recent multivariate analysis of a cohort of US military personnel by Pullen et al30 found concomitant meniscal repair to protect against later revision ACL surgery. Trojani et al34 also noted the importance of meniscal repair. In their study, better functional results and better knee stability were seen after ACLR where repair rather than resection had been performed.
In a multivariate analysis investigating predictors for ACLR failure, Parkinson et al27 found meniscal deficiency to be the single most important factor. The results from our study align with their finding, showing a protective effect of meniscal repair on graft survival. The choice to perform meniscal repair, whenever viable, seems to be paramount when performing ligament surgery. In accordance with the latter belief, a changing attitude toward meniscal surgery is shown in the data from the Norwegian ACL registry.5 The number of ACLRs with concomitant meniscal repair procedures has risen from 7% to 40%, while resections have decreased from 73% to 48%, in the period from 2005 to 2016. In the current study, it is difficult to establish the causality between meniscal repair failure and increased risk of revision ACLR. On one side, one could argue that an injured meniscus can lead to additional strain on the ACL graft, but it might also be that residual laxity in and of itself increases the risk of a repeat meniscal tear. In addition to the favorable effect of meniscal repair on knee kinematics, one could speculate whether the resulting slower rehabilitation after a meniscal repair is also protective for risk of later revision surgery. This could, in particular, be the case for patients receiving hamstring tendon grafts, as they are believed to need a longer time for graft-to-bone healing.12,25
The relationship between graft diameter and patient outcomes has been the subject of investigation in several studies. Magnussen et al18 reported that hamstring tendon autograft diameter of <8 mm was a predictor for early revision after surgery. Park et al26 demonstrated how graft diameter was dependent on patient BMI, sex, and athletic level. Further, although there was no relation between smaller graft diameters and risk of revision ACL, patients with a graft size of <8 mm displayed inferior clinical outcomes.26 In a study from the Swedish ACL registry, more than 2000 patients who underwent reconstruction with hamstring tendon autografts were analyzed.32 The main finding was an increasing likelihood of undergoing revision surgery for each 0.5-mm decrease in graft diameter from 10 to 7 mm. In contrast to the studies mentioned above is a recent report from the Norwegian ACL registry investigating the effect of BMI and graft size on risk of undergoing revision.10 When graft diameter was related to patient weight and height, no difference in the risk of revision surgery based on hamstring tendon autograft diameter was seen. In the present study, a smaller graft size of <8 mm was seen more frequently in the revision group compared with the control group. This adds to the notion that surgeons should aim to upsize the graft size whenever encountering a small diameter during surgery. A careful consideration should, however, be made toward this upsizing, since data from a publication by Pennock et al28 point toward higher graft failure rates when augmentation with allograft is used to achieve this.
The present study found patients who had undergone revision surgery to be younger at the time of surgery compared with the patients who had not undergone revision surgery.19,29,36 In a prospective cohort by Kamien et al,13 age below 25 years was found to be an independent risk factor for ACLR failure. Studies by Magnussen et al18 and Shelbourne et al31 have displayed the same findings. Further, reports4,6,19 from the Swedish, Danish, and Kaiser Permanente registries also emphasize age as an independent risk factor for revision. With younger age comes a greater desire to return to high-risk activities that expose patients to repeat injury of their knee. As discussed by Marx et al,21 participation in high-risk activity is likely the confounding factor making younger-aged patients more prone to undergo ACL revision surgery.
Several studies have reported on early versus delayed reconstruction after ACL rupture. The level of knee instability, concomitant injuries, and patient expectations are among the factors that influence timing of surgery.9,14 As highlighted by Krutsch et al,15 there might be a risk of secondary injuries related to delayed surgery. In their study, an increase in irreparable meniscal lesions was seen in those who underwent delayed surgery. A previous study8 reported that patients returning to lower level sports can be managed well with proper nonoperative treatment and that “watchful waiting” can therefore be an option. Results from the current study indicated a shorter time from injury to surgery in patients who were in need for revision surgery, as compared with the control patients. While this finding could be interpreted as support for “watchful waiting,” we believe that time from injury to surgery is also a derived factor, reflecting a population more eager to return to sports—exposing them to further risk of new injuries. It has to be acknowledged that this mean time from injury to surgery of 22.7 months could influence meniscus healing potential and thereby the results of the study.
There are several inherent limitations in the current study. First, defining failure only by the need for revision surgery will likely underestimate the number of failed ACLRs. Crawford et al3 found that the overall rate of failure increased from 6% to 12% when patient-reported outcome measures and clinical evaluation of laxity were also accounted for. Not all patients who fail choose to undergo repeat surgery. Further, level of osteoarthritis and return to sports are unknown factors that could help define whether the primary surgery was successful or not. Strengths of the current study include the relatively large prospective cohort of ACLRs undergoing later revision surgery and a homogenous group of controls operated on by the same group of surgeons at a single center. Although data were collected through a long period of time, we believe that the design of the study, applying a matched control group, counterbalances the differences caused by the changing surgical techniques over the time span of the study. We acknowledge that the retrospective design is less robust toward confounding factors than if a prospective design had been applied. The study design supports the generalizability of the results, since in the included patients were an unselected group of patients who experienced failure after ACLR.
Conclusion
The current study, investigating the risks for failure after ACLR, demonstrated an association between failed meniscal surgery and the need for repeat ACL surgery. Second, the study found that hamstring tendon autografts of <8 mm diameter were more frequent in those who underwent revision ACLR. Finally, younger age at the time of surgery and shorter time from injury to surgery—both believed to reflect the risk of returning to high-risk activities—were also found to affect the risk of undergoing revision ACL surgery. With an increasing number of patients in need of repeat ACL surgery, knowledge of causes for failure is increasingly important. There is also a need for a better definition of what constitutes failure; thus, further studies should focus on this topic.
Footnotes
Final revision submitted May 6, 2020; accepted May 19, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Regional Committee for Medical and Health Research Ethics, Western Norway (REK Helse Vest ID No. 2015/2176).
References
- 1. Ahldén M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J. The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med. 2012;40(10):2230–2235. [DOI] [PubMed] [Google Scholar]
- 2. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crawford SN, Waterman BR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(9):1566–1571. [DOI] [PubMed] [Google Scholar]
- 4. Desai N, Andernord D, Sundemo D, et al. Revision surgery in anterior cruciate ligament reconstruction: a cohort study of 17,682 patients from the Swedish National Knee Ligament Register. Knee Surg Sports Traumatol Arthrosc. 2016;25(5):1542–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Engebretsen L. Yearly Review 2017 - Norwegian ACL Registry. 2017:1–315. https://www.kvalitetsregistre.no/registers/nasjonalt-korsbandregister [Google Scholar]
- 6. Faunø P, Rahr-Wagner L, Lind M. Risk for revision after anterior cruciate ligament reconstruction is higher among adolescents: results from the Danish Registry of Knee Ligament Reconstruction. Orthop J Sports Med. 2014;2(10):2325967114552405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gifstad T, Drogset JO, Viset A, Grøntvedt T, Hortemo GS. Inferior results after revision ACL reconstructions: a comparison with primary ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2011–2018. [DOI] [PubMed] [Google Scholar]
- 8. Grindem H, Eitzen I, Engebretsen L, Snyder-Mackler L, Risberg MA. Nonsurgical or surgical treatment of ACL injuries: knee function, sports participation, and knee reinjury: The Delaware-Oslo ACL Cohort Study. J Bone Joint Surg Am. 2014;96(15):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herbst E, Hoser C, Gföller P, et al. Impact of surgical timing on the outcome of anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):569–577. [DOI] [PubMed] [Google Scholar]
- 10. Inderhaug E, Drogset JO, Lygre SHL, Gifstad T. No effect of graft size or body mass index on risk of revision after ACL reconstruction using hamstrings autograft. Knee Surg Sports Traumatol Arthrosc. 2020;28(3):707–713. [DOI] [PubMed] [Google Scholar]
- 11. Inderhaug E, Raknes S, Østvold T, Solheim E, Strand T. Increased revision rate with posterior tibial tunnel placement after using the 70-degree tibial guide in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):152–158. [DOI] [PubMed] [Google Scholar]
- 12. Irvine JN, Arner JW, Thorhauer E, et al. Is there a difference in graft motion for bone-tendon-bone and hamstring autograft ACL reconstruction at 6 weeks and 1 year? Am J Sports Med. 2016;44(10):2599–2607. [DOI] [PubMed] [Google Scholar]
- 13. Kamien PM, Hydrick JM, Replogle WH, Go LT, Barrett GR. Age, graft size, and Tegner activity level as predictors of failure in anterior cruciate ligament reconstruction with hamstring autograft. Am J Sports Med. 2013;41(8):1808–1812. [DOI] [PubMed] [Google Scholar]
- 14. Kiadaliri AA, Englund M, Lohmander LS, Carlsson KS, Frobell RB. No economic benefit of early knee reconstruction over optional delayed reconstruction for ACL tears: registry enriched randomised controlled trial data. Br J Sports Med. 2016;50(9):558–563. [DOI] [PubMed] [Google Scholar]
- 15. Krutsch W, Zellner J, Baumann F, Pfeifer C, Nerlich M, Angele P. Timing of anterior cruciate ligament reconstruction within the first year after trauma and its influence on treatment of cartilage and meniscus pathology. Knee Surg Sports Traumatol Arthrosc. 2017;25(2):418–425. [DOI] [PubMed] [Google Scholar]
- 16. Levy IM, Torzilli PA, Gould JD, Warren RF. The effect of lateral meniscectomy on motion of the knee. J Bone Joint Surg Am. 1989;71(3):401–406. [PubMed] [Google Scholar]
- 17. Levy IM, Torzilli PA, Warren RF. The effect of medial meniscectomy on anterior-posterior motion of the knee. J Bone Joint Surg Am. 1982;64(6):883–888. [PubMed] [Google Scholar]
- 18. Magnussen RA, Lawrence JTR, West RL, Toth AP, Taylor DC, Garrett WE. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy. 2012;28(4):526–531. [DOI] [PubMed] [Google Scholar]
- 19. Maletis GB, Chen J, Inacio MCS, Funahashi TT. Age-related risk factors for revision anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(2):331–336. [DOI] [PubMed] [Google Scholar]
- 20. Maletis GB, Inacio MCS, Funahashi TT. Analysis of 16,192 anterior cruciate ligament reconstructions from a community-based registry. Am J Sports Med. 2013;41(9):2090–2098. [DOI] [PubMed] [Google Scholar]
- 21. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 22. Morgan JA, Dahm D, Levy B, Stuart MJ; MARS Study Group. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg. 2012;25(5):361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687–695. [PubMed] [Google Scholar]
- 24. Nwachukwu BU, Chang B, Voleti PB, et al. Preoperative Short Form Health Survey score is predictive of return to play and minimal clinically important difference at a minimum 2-year follow-up after anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(12):2784–2790. [DOI] [PubMed] [Google Scholar]
- 25. Park MJ, Lee MC, Seong SC. A comparative study of the healing of tendon autograft and tendon-bone autograft using patellar tendon in rabbits. Int Orthop. 2001;25(1):35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park SY, Oh H, Park S, Lee JH, Lee SH, Yoon KH. Factors predicting hamstring tendon autograft diameters and resulting failure rates after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(5):1111–1118. [DOI] [PubMed] [Google Scholar]
- 27. Parkinson B, Robb C, Thomas M, Thompson P, Spalding T. Factors that predict failure in anatomic single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45(7):1529–1536. [DOI] [PubMed] [Google Scholar]
- 28. Pennock AT, Ho B, Parvanta K, et al. Does allograft augmentation of small-diameter hamstring autograft ACL grafts reduce the incidence of graft retear? Am J Sports Med. 2016;45(2):334–338. [DOI] [PubMed] [Google Scholar]
- 29. Persson A, Fjeldsgaard K, Gjertsen J-E, et al. Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction: a study of 12,643 patients from the Norwegian Cruciate Ligament Registry, 2004-2012. Am J Sports Med. 2014;42(2):285–291. [DOI] [PubMed] [Google Scholar]
- 30. Pullen WM, Bryant B, Gaskill T, Sicignano N, Evans AM, DeMaio M. Predictors of revision surgery after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(12):3140–3145. [DOI] [PubMed] [Google Scholar]
- 31. Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. [DOI] [PubMed] [Google Scholar]
- 32. Snaebjörnsson T, Hamrin Senorski E, Ayeni OR, et al. Graft diameter as a predictor for revision anterior cruciate ligament reconstruction and KOOS and EQ-5D values: a cohort study from the Swedish National Knee Ligament Register based on 2240 patients. Am J Sports Med. 2017;45(9):2092–2097. [DOI] [PubMed] [Google Scholar]
- 33. Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA. Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med. 2016;44(2):400–408. [DOI] [PubMed] [Google Scholar]
- 34. Trojani C, Sbihi A, Djian P, et al. Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):196–201. [DOI] [PubMed] [Google Scholar]
- 35. Ulstein S, Årøen A, Engebretsen L, Forssblad M, Lygre SHL, Røtterud JH. Effect of concomitant cartilage lesions on patient-reported outcomes after anterior cruciate ligament reconstruction: a nationwide cohort study from Norway and Sweden of 8470 patients with 5-year follow-up. Orthop J Sports Med. 2018;6(7):2325967118786219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wasserstein D, Khoshbin A, Dwyer T, et al. Risk factors for recurrent anterior cruciate ligament reconstruction: a population study in Ontario, Canada, with 5-year follow-up. Am J Sports Med. 2013;41(9):2099–2107. [DOI] [PubMed] [Google Scholar]
- 37. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 38. Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]