Abstract
Background:
Investigations of the female athlete triad (Triad) in high school athletes have found that 36% had low energy availability, 54% had menstrual abnormalities, and 16% had low bone mineral density (BMD). Limited data are available showing the prevalence of these risk factors in high school distance runners or regarding best practice on screening for the Triad in the adolescent population.
Purpose:
To (1) evaluate the prevalence of Triad risk factors and iron supplementation in high school distance runners and (2) pilot a screening tool for Triad risk score.
Study Design:
Descriptive epidemiology study.
Methods:
The study population included female high school athletes who participated in cross-country/track. Participants completed a survey including questions regarding dietary habits, menstrual history, and bone stress injury (BSI) history. They then underwent evaluation of 25-hydroxyvitamin D, free triiodothyronine (T3), and dual-energy x-ray absorptiometry scan to measure body fat and BMD through use of age-, sex-, and ethnicity-matched Z scores. Triad scores were calculated. Relationships were analyzed using Spearman correlation coefficient.
Results:
There were 38 study participants (mean age, 16.9 years). Average body mass index was 19.8 kg/m2. Disordered eating or eating disorders were reported in 76.3% of runners; in addition, 23.7% reported delayed menarche, 45.9% had a history of amenorrhea or oligomenorrhea, 42.1% had low BMD (Z score < –1.0), and 15.8% reported prior BSI. Low free T3 was significantly associated with higher Triad risk scores (r S = –0.36; P = .028). More than 42% of athletes were supplementing iron.
Conclusion:
The prevalence of Triad risk factors in high school distance runners was high. Free T3 was inversely associated with Triad score, which may serve as an indicator of low energy availability. Nearly half of the athletes were using iron supplementation.
Keywords: female athlete triad, iron deficiency, iron supplementation, disordered eating, bone density, amenorrhea
The female athlete triad (Triad) is a constellation of symptoms that have affected women participating in high-intensity activities of all types since well before the introduction of organized sports. However, it was not until the early 1990s that the American College of Sports Medicine commenced the Task Force on Women’s Issues and first defined the Triad as a syndrome of three distinct but interrelated conditions: disordered eating, amenorrhea, and osteoporosis.13 In 2014, the Female Athlete Triad Coalition Consensus Statement updated this definition to involve any one of the three components: (1) low energy availability with or without disordered eating, (2) menstrual dysfunction, and (3) low bone mineral density (BMD).7 In this context, low energy availability refers to any imbalance of caloric intake to energy expenditure, which could include restricted diet, excessive exercise, or a combination of the two.
The Triad affects a large proportion of young athletes. Beals and Hill,3 evaluating National Collegiate Athletic Association (NCAA) Division II athletes, found that 25% had disordered eating, 26% reported menstrual dysfunction, and 10% had low BMD. Hoch et al8 investigated athlete and nonathlete high school females, of whom a total of 36% of the athletes were classified as having low energy availability, 54% menstrual abnormalities, and 16% low BMD measured by dual-energy x-ray absorptiometry (DXA).
Adolescence is a critical time for bone acquisition, highlighting the importance of understanding risk factors for impaired bone health in this population. A cross-sectional study found that amenorrhoeic, adolescent endurance athletes had lower BMD than both athletes with eumenorrhea and nonathletic controls.6 BMD was correlated with lean mass, body mass index (BMI), insulin-like growth factor 1 (IGF-1), duration of amenorrhea, and bone age.6 A separate 2008 study evaluating 93 adolescent female runners identified the following risk factors for impaired bone health: longer duration of running participation, menstrual irregularities, lower BMI, and less lean tissue mass.2 A more recent study found that prior fracture history was also a risk factor for low bone mass (defined as BMD Z score ≤ –1.0).18 Factors that impede bone density accumulation in this age group are particularly detrimental, as they can lead to decreased peak bone mass, which has been shown to be a predictor of future osteoporosis and fracture risk.1
Given the large proportion of athletes affected by the Triad, the 2014 Female Athlete Triad Coalition Consensus Statement on Treatment and Return to Play proposed a screening tool.7 The tool takes into account dietary habits, BMI, delayed menarche, oligomenorrhea or amenorrhea, and history of stress reaction/fractures and creates a cumulative risk score.7 The purpose of the tool is to stratify athletes by risk and determine clearance for sport participation.7
In addition to the Triad, iron deficiency and iron deficiency anemia are well-known conditions that further complicate athletic performance and athlete health. Parks et al14 evaluated 2749 NCAA Division I athletes participating in all sports and showed that 30.9% of female athletes had iron deficiency and 2.2% had iron deficiency anemia. Anemia was defined as Hgb <11.6 g·dL for females, and iron deficiency was defined as ferritin <20 ng/mL for both sexes.14
In 2014, the International Olympic Committee published a consensus statement titled “Beyond the Female Athlete Triad: Relative Energy Deficiency in Sport.”12 Relative energy deficiency in sport (RED-S) expands on the Triad and discusses the potential role of iron deficiency. The components of RED-S include menstrual function, bone health, and endocrine, metabolic, hematologic, psychologic, cardiovascular, gastrointestinal, immunologic, and growth and development changes.12 Many of these disturbances are secondary to alterations in the hypothalamic-pituitary-gonadal axis, which leads to dysfunction of thyroid, IGF-1, and estradiol signaling.12 The committee also identified iron deficiency as a direct and indirect contributor to energy deficiency.12 Decreased iron stores can cause decreased appetite, impaired metabolic efficiency, increased energy expenditure, and dysregulation of the growth hormone/IGF-1 axis.12,16 The consensus statement also outlines the effects of 25-hydroxyvitamin D (vitamin D) on risk of bone stress injury (BSI) and healing.12 It emphasizes that <30 ng/mL of serum 25-hydroxyvitamin D is associated with increased incidence of BSI, and increased 25-hydroxyvitamin D levels reduce healing time and decrease time until return to play.12
Although significant research has examined how low energy availability may affect factors of the Triad (menstrual function and BMD), hematologic parameters (iron deficiency), and the endocrine system (thyrotropin, vitamin D, IGF-1), scant literature is available regarding the extent to which female high school distance runners are affected by each of these components. The primary aim of this study was to explore the prevalence of Triad risk factors, iron supplementation, and other potential hormonal fluctuations (thyroid, IGF-1, vitamin D) in female high school distance runners. Our secondary aim was to pilot the Triad screening tool in high school athletes through adaptation of the guidelines set forth by the Female Athlete Triad Coalition Consensus Statement.7 We focused our attention on Triad screening over RED-S because the Triad screening tool has been used and validated in populations of collegiate distance runners.17 Our study piloted this tool specifically in high school–aged distance runners.
Methods
Participant Recruitment
This study was approved by the institutional review board of the study institution. Female high school distance runners were recruited from Northern California, through outreach to high school cross-country and/or track and field teams, running clubs, running stores, and booths at local road races. A total of 21 high school teams, 102 running clubs, 16 running stores, and 92 road races were contacted. Initial contact was made through telephone call or email. In total, 11 of the high school teams, 37 of the clubs, 12 of the running stores, and 21 of the races agreed to help with study recruitment. Through collaboration with these organizations, the best method for advertising the study was determined. Different strategies included educational talks about the female athlete triad and advertisement of the study, distribution of fliers, and posting of advertisements on social media. Additionally, 12 professional female distance runners posted the study flier on their Instagram pages. Inclusion criteria included female sex, age 13-18 years, and current participation in high school cross-country and/or distance track and field. We did not screen for prior medical conditions, bleeding disorders, or thyroid disease. A preliminary power analysis indicated that a sample size of 30 would provide 80% power to detect a moderate (0.5) correlation using the Pearson correlation test with a 2-sided level of significance of .05.
Enrollment occurred between May and November 2018. A total of 38 athletes participated. Before enrollment, proper consent and assent were obtained based on the age of the participant. Participants received a $25 gift card immediately upon completion of the study visit. Once laboratory results were returned to the study team, the results were shared with the participant and guardian upon request.
Study Design
Participants came to the study institution on a single, self-selected day for the series of questionnaires and tests, detailed below.
Body Mass Index
Each participant had a height and weight measurement to calculate BMI.
Questionnaire
Each participant completed a written survey with questions on reported history of disordered eating or eating disorder, menstrual function, history of BSI and other running-related injuries, sleep quantity and quality, overtraining and burnout, and family history of osteoporosis and/or fracture (see the Appendix, available as supplemental material). The questions in this survey were derived from athlete screening during previous research trials and included questions from the Female Athlete Triad Screening Questionnaire, Triad Consensus Panel Screening Questionnaire, Athlete Sleep Screening Questionnaire, and the Recovery Stress Questionnaire for Athletes.7,4,9,20
Laboratory Assessment
Laboratory values that were collected included a complete blood cell count (CBC), ferritin iron, vitamin D, IGF-1, free triiodothyronine (T3), and estradiol.
Dual-Energy X-Ray Absorptiometry
BMD of the lumbar spine and the whole body less the head, which are the two anatomic sites recommended for screening athletes younger than 20 years, was measured using DXA (Hologic Horizon A). We adjusted for growth delay (with height or height age) and maturational delay (with bone age) and used pediatric reference data to report height- and age-adjusted Z scores. Body fat percentage was also obtained from DXA.
Data Analysis
The following steps were taken to evaluate the data obtained.
Cumulative Risk Scoring
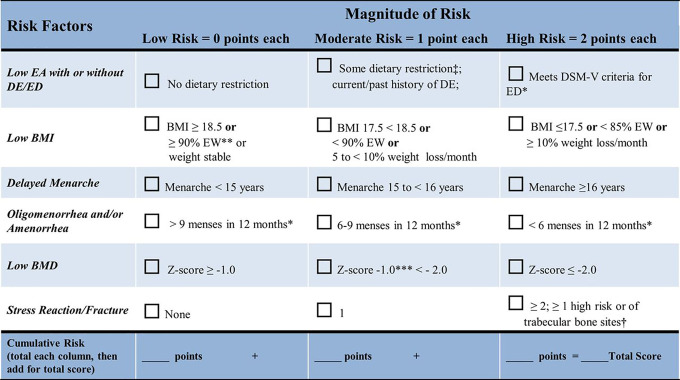
Variables and risk factors of interest were used to calculate a risk score. Each risk factor was quantified as low (0 points), moderate (1 point), or high risk (2 points), using the Female Athlete Triad Coalition Consensus Statement cumulative risk assessment tool (Figure 1).
Figure 1.
Female athlete triad cumulative risk assessment. BMD, bone mineral density; BMI, body mass index; DE, disordered eating; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; EA, energy availability; ED, eating disorder; EW, estimated body weight. *Current or past history. **≥90% estimate weight. ***Weight-bearing sport. ‡Some dietary restriction as evidenced by self-report. †High-risk skeletal sites associated with low BMD and delay in return to play in athletes with one or more components of the triad include stress reaction/fracture of trabecular sites (femoral neck, sacrum, pelvis). (Reprinted with permission from De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289. ©2014 BMJ Publishing Group Ltd.)
Risk factors were defined as follows:
Low energy availability: Low energy availability was scored based on survey questions obtained from the Eating Disorder Examination–Questionnaire.11 Answering all diet-related questions in a negative manner was indicative of “no dietary restriction” and was assigned a score of zero. Answering ≥1 diet-related questions in a positive manner, while not meeting strict Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for an active eating disorder or having an eating disorder in the past, received the categorization of “some dietary restriction/past history of eating disorder” and was assigned a score of 1. Answering diet-related questions in a manner that met DSM-5 criteria for an eating disorder received the categorization of “current DSM-5 eating disorder” and was assigned a score of 2.
Body mass index: Healthy weight, defined by BMI >18.5 kg/m2, was assigned a score of zero. BMI >5th percentile for age but <18.5 kg/m2 was assigned a score of 1, and BMI <5th percentile for age was assigned a score of 2. BMI was adjusted using the Centers for Disease Control and Prevention BMI calculator for age and sex.5
Delayed menarche: The American College of Obstetricians and Gynecologists defines delayed menarche as absence of menarche at 15 years of age.10 Based on this guideline, menarche before age 15 years was assigned a score of zero. Menarche between age 15 and 16 years was assigned a score of 1. Menarche at age >16 years was assigned a score of 2. If the athlete was younger than 15 years, she was given a score of zero.
Oligomenorrhea and/or amenorrhea: >9 menses in 12 months was assigned a score of zero; 6 to 9 menses in 12 months was assigned a score of 1; and <6 menses in 12 months was assigned a score of 2. If the athlete was younger than 15 years, she was given a score of zero.
Bone stress injury: BSI history was obtained from the questionnaire. No prior BSI was assigned a score of zero; 1 BSI was assigned a score of 1; and ≥2 BSIs or a history of 1 high-risk BSI or BSI in a trabecular-rich site (pelvis or femoral neck) was assigned a score of 2.
Bone mineral density: BMD Z score > –1.0 was assigned a score of zero, BMD Z score between –1.0 and –2.0 was assigned a score of 1, and BMD Z score < –2.0 was assigned a score of 2. The lowest Z score between lumbar spine or total body less head was used for risk scoring.
Laboratory Test Results
Results obtained from laboratory draws were used to classify the level of iron deficiency in participants. Athletes were defined as having iron deficiency if ferritin was <35 ng/mL.15 The CBC, vitamin D, IGF-1, free T3, and estradiol were used to evaluate any other blood or hormone irregularities in study participants.
Statistical Analysis
The relationships between ferritin levels and the risk factors for the Triad were analyzed by use of both Spearman and Pearson correlations. BMD was compared with individual risk factors for the Triad using t tests. All other relationships were assessed by use of the Spearman correlation coefficient (r S) and the Mann-Whitney U test. These analyses were completed in RStudio version 1.1.456), with a level of significance of α = .05.
Results
Prevalence of Female Athlete Triad Risk Factors and Iron Supplementation
A total of 38 female high school distance runners were enrolled in the study and completed each component of the study, with no athlete withdrawals. Descriptive data are provided in Table 1.
Table 1.
Descriptive Data of the Study Population
| Mean ± SD | 95% CI | |
|---|---|---|
| Age, y | 16.90 ± 1.00 | 16.60-17.20 |
| Miles run per week | 29.10 ± 15.30 | 24.20-34.00 |
| Body mass index, kg/m2 | 19.80 ± 1.90 | 19.20-20.40 |
| Body mass index, percentile | 36.20 ± 20.80 | 29.60-42.80 |
In this sample of athletes, there was a high prevalence of Triad components, including low BMI, disordered eating, and eating disorders; menstrual irregularities; and decreased BMD. Notably, the average BMI among the sample was 19.8 kg/m2, which equates to a percentile of 36.2 for age-matched participants. Even given the normal BMI age-matched percentile, 76.3% of participants were classified as currently having disordered eating or an eating disorder. Regarding menstrual characteristics, 23.7% of athletes reported delayed menarche, and 45.9% were classified as having oligomenorrhea or amenorrhea. The mean ± SD lumbar spine Z score was –0.6 ± 1 (95% CI, –0.9 to –0.3), with 42.1% of participants falling in the low BMD category (Z score < –1.0). Additionally, prior BSI was reported in 15.8% of participants.
A high prevalence of iron supplementation was noted among the athletes, with 42.1% of participants supplementing iron (Table 2). The mean serum ferritin level in all athletes, including those who supplemented, was 33.6 ± 26.0 ng/mL (95% CI, 26.0-41.2 ng/mL), which was considered iron deficient by our definition.
Table 2.
Study Participants With Risk Factors for the Female Athlete Triad or Those Taking Iron Supplementation
| No. (%) of Participants | |
|---|---|
| Eating disorder/disordered eating | |
| No | 9 (23.7) |
| Yes | 29 (76.3) |
| Age at menarche | |
| <15y | 29 (76.3) |
| 15-16y | 8 (21.1) |
| >16y | 1 (2.6) |
| Oligomenorrhea/amenorrhea | |
| Neither | 20 (54.1) |
| Oligomenorrhea | 7 (18.9) |
| Amenorrhea | 10 (27.0) |
| Stress fracture history | |
| 0 | 32 (84.2) |
| 1 | 5 (13.2) |
| 2 | 1 (2.6) |
| Bone mineral density | |
| Z score > –1.0 | 22 (57.9) |
| Z score ≤ –1.0 | 16 (42.1) |
| Iron supplementation | |
| No | 22 (57.9) |
| Yes | 16 (42.1) |
Body Composition
Body fat percentage and BMD were measured and compared in relation to total Triad risk score, individual risk factors, and hormonal fluctuations. Body fat percentage was significantly inversely correlated with overall Triad risk score (r S = –0.36; P = .028). No association of low BMD was seen with individual Triad risk factors. The association of low BMD with low vitamin D approached statistical significance (r S = 0.28; P = .086), but no significant relationships were seen between BMD and other serum hormonal markers.
Hormone Indicators of Triad Risk
Low free T3 was significantly associated with higher Triad risk score (r S = –0.36; P = .028). Estradiol (r S = –0.30; P = .075), vitamin D (r S = –0.16; P = .325), and IGF-1 (r S = –0.14; P = .416) were not significantly correlated with Triad risk score.
Ferritin and Female Athlete Triad Risk
Serum ferritin levels were not significantly associated with overall Triad risk score (r S = 0.17; P = .304) or individual risk factors. As seen in Table 3, Triad risk scores were not statistically different (P = .142) when participants were categorially classified into “low ferritin” and “normal ferritin,” using the cutoff value of 35 ng/mL15 or when athletes supplementing with iron were compared with nonsupplementing athletes (P = .159).
Table 3.
Female Athlete Triad Risk Scores for High and Low Ferritin Iron Levels and Iron Supplementation
| n | Female Athlete Triad Risk Score, Mean ± SD (95% CI) | P | |
|---|---|---|---|
| Ferritin | .142 | ||
| <35 ng/mL | 25 | 2.8 ± 2.2 (1.9-3.6) | |
| ≥35 ng/mL | 13 | 3.5 ± 1.7 (2.6-4.4) | |
| Iron supplementation | .159 | ||
| No | 22 | 2.7 ± 2.1 (1.8-3.6) | |
| Yes | 16 | 3.5 ± 1.9 (2.6-4.4) |
Discussion
Female Athlete Triad
As hypothesized, a high prevalence of Triad risk factors was seen among the study participants. However, the values obtained in this study (76.3% disordered eating or eating disorder, 23.7% delayed menarche, 45.9% oligomenorrhea/amenorrhea, and 42.1% Z score < –1.0) are higher than seen in the literature. The best existing comparison study, authored by Hoch et al,8 examined all high school athletes for components of the Triad and found that 36% had low energy availability, 54% had menstrual abnormalities, and 16% had low BMD.8 A comparison of disordered eating/eating disorder and low energy availability cannot be made directly because Hoch et al evaluated energy availability through dietary record and calculation of energy deficit rather than by survey. Values in the current study regarding menstrual irregularities confirm the data in Hoch et al’s study. However, the current study had a greater population of athletes classified as having low BMD (Z score < –1.0). This finding could be secondary to the small sample size in both studies, but it could also represent a discrepancy in average BMD between all high school athletes and distance runners. These data may suggest that distance runners are disproportionately affected by low BMD compared with other high school athletes.
The high prevalence of Triad risk factors seen in this study warrants further research into the structure of screening protocols and the process of implementation, specifically in high school–aged runners. Triad screening is routinely conducted at the collegiate level, but these findings support similar or increased Triad risk and need for screening in female high school athletes. The screening tool must account for the inability to accurately assess delayed menarche if the athlete has not yet reached age of normal menarche, an acceptable degree of menstrual irregularity within the first 1 to 2 years after menarche, and lack of consistent research on BMD as an indicator of bone health in the adolescent athlete.
One aim of this study was to determine how to use the Female Athlete Triad Coalition Consensus Statement7 risk score to best predict Triad risk in high school distance runners. Further research is needed to determine the best way to evaluate energy availability. Possibilities include BMI with subjective survey of dietary restriction versus BMI with dietary journal and calculation of energy deficit. Menarche, oligomenorrhea, and amenorrhea cannot be evaluated in athletes who have not reached the normal age of menarche, which comprised a large proportion of the high school athletes surveyed. In this study, athletes who were younger than 15 years received a score of 0 for menarche and oligomenorrhea/amenorrhea, which is a major limitation to both this study and the existing screening tool in this population. Serial evaluations until menarche may be indicated for these athletes. Interestingly, despite the significant number of athletes with decreased BMD on DXA scan, few had corresponding prior BSIs. BSI is used as a proxy for decreased BMD in estimating Triad risk score in the current screening tool, which is based on studies showing that prior BSI is predictive of future BSI.18 However, based on data collected in this study, prior BSI was not a reliable estimate of low BMD in high school distance runners. This phenomenon could be due to lower cumulative stress over time (ie, fewer total miles run) in high school distance runners. The screening tool used in this study, adapted from the Female Athlete Triad Coalition Consensus Statement, may be a starting point for development of a tool specifically for adolescent athletes.
In this study, 37 of 38 athletes chose to receive their screening data. The protocol did not require any follow-up if abnormal results were found, so it is unknown how many of the athletes used the data in consultation with their physicians. However, the large number who decided to obtain the information illustrates that athletes and parents are motivated to obtain data regarding the Triad and believe they may benefit from further screening.
Further research is needed to investigate other factors that may predict Triad risk, including body fat percentage and hormone levels. This study found that low body fat percentage was significantly correlated with overall Triad risk score, which is in line with previous research. For example, a study on body mass–related predictors for the Triad in adolescents illustrated a relationship between low age-adjusted BMI and low ideal body weight with low BMD and menstrual dysfunction.19 These data suggest that body fat percentage is a good proxy for Triad risk in high school distance runners. Additionally, low free T3 was significantly correlated with higher Triad risk score. With further study into the relationship between free T3 and the Triad, serologic evaluation may be a valuable component to Triad risk screening.
Iron Supplementation
A larger than hypothesized group of runners were using iron supplementation, highlighting a need for further investigation into why runners supplement with iron and how many are supplementing without physician guidance. Physicians should inquire about iron supplementation at preparticipation physical examinations so they can provide guidance to the athlete and parents.
Limitations
This study was observational and lacked a control group, making it difficult to account for confounding factors. Additionally, the recruitment process may have selected athletes who were more interested in their own Triad risk by providing free laboratory and DXA scans and returning the results to participants. A gift card was also offered, which may have motivated those who were socioeconomically disadvantaged.
Laboratory testing may have confounding factors, including genetic or environmental factors and active supplementation (iron and others). We did not collect data regarding who was supplementing iron under physician guidance and who was supplementing without physician guidance.
Last, a small sample size left the study underpowered. A sample size of 38 achieves approximately 16% power to detect a Spearman correlation of 0.17 between ferritin and total Triad risk score using a 2-sided hypothesis test with a significance level of .05. Recruitment difficulty was the major limitation to expanding the sample size. In total, 52% of high schools, 36% of running clubs, 75% of running stores, and 23% of road races helped to advertise the study in some way after being contacted. Although we reached out to >231 high schools, clubs, stores, and races, only 38 athletes enrolled in the study. We anticipate that low rates of participation are secondary to the in-person research procedure and requirement to have a parent present at the visit. Additionally, time frame for completion of the study and limited financial support served as barriers to the number of participants who could be enrolled. If this pilot study were to be expanded, a power analysis suggests a sample size of 225 provides 80% power to detect a Spearman correlation coefficient of 0.2 with a 2-sided level of significance of .05.
Conclusion
No study has specifically investigated the prevalence of Triad risk factors among female high school distance runners. This study found a greater prevalence of Triad risk factors compared with prior studies on all female high school sports, which indicates a greater need for screening and prevention among distance runners. Additionally, this research supports existing literature illustrating the interplay of thyroid hormone with the Triad. Last, we found that a large portion of the study population was supplementing with iron.
Further investigation on high school athletes should focus on specific Triad risk factors, modified screening protocols, and the use of different hormonal factors in risk assessment. Athletes should be followed prospectively to determine how the Triad and other hormone fluctuations in adolescence can affect future health, with the goal of preventing future injury and other long-term consequences.
Supplemental Material
Supplemental Material, DS_10.1177_2325967120959725 for Prevalence of Female Athlete Triad Risk Factors and Iron Supplementation Among High School Distance Runners: Results From a Triad Risk Screening Tool by Paige Skorseth, Nicole Segovia, Katherine Hastings and Emily Kraus in Orthopaedic Journal of Sports Medicine
Acknowledgment
The authors thank the Department of Orthopedic Surgery at Stanford University for funding this study.
Footnotes
Final revision submitted March 24, 2020; accepted April 20, 2020.
The authors declared that there are no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Stanford University (protocol No. 45785).
Supplemental Material: Supplemental material for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/2325967120959725959725.
References
- 1. Ackerman KE, Misra M. Bone health and the female athlete triad in adolescent athletes. Phys Sportsmed. 2011;39(1):131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barrack MT, Rauh MJ, Nichols JF. Prevalence of and traits associated with low BMD among female adolescent runners. Med Sci Sports Exerc. 2008;40(12):2015–2021. [DOI] [PubMed] [Google Scholar]
- 3. Beals KA, Hill AK. The prevalence of disordered eating, menstrual dysfunction, and low bone mineral density among US collegiate athletes. Int J Sport Nutr Exerc Metab. 2006;16:1–23. [DOI] [PubMed] [Google Scholar]
- 4. Bender AM, Lawson D, Werthner P, Samuels CH. The clinical validation of the athlete sleep screening questionnaire: an instrument to identify athletes that need further sleep assessment. Sports Med Open. 2018;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. 2020. https://www.cdc.gov/healthyweight/bmi/calculator.html. [Google Scholar]
- 6. Christo K, Prabhakaran R, Lamparello B, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics. 2008;121(6):1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Souza MJ, Nattiv A, Joy E, et al. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289. [DOI] [PubMed] [Google Scholar]
- 8. Hoch AZ, Pajewski NM, Moraski L, et al. Prevalence of the female athlete triad in high school athletes and sedentary students. Clin J Sport Med. 2009;19(5):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kellmann M, Kallus KW. Recovery-Stress Questionnaire for Athletes: User Manual. Human Kinetics; 2001. [Google Scholar]
- 10. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Committee Opinion No. 651. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2015;126:e143–e146. [DOI] [PubMed] [Google Scholar]
- 11. Mond JM, Hay PJ, Rodgers B, Owen C. Eating Disorder Examination Questionnaire (EDE-Q): norms for young adult women. Behav Res Ther. 2006;44(1):53–62. [DOI] [PubMed] [Google Scholar]
- 12. Mountjoy M, Sundgot-Borgen JK, Burke LM, et al. IOC consensus statement on relative energy deficiency in sport (RED-s). 2018 update. Br J Sports Med. 2018;52:687–697. [DOI] [PubMed] [Google Scholar]
- 13. Otis CL, Drinkwater B, Johnson M, et al. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–1882. [DOI] [PubMed] [Google Scholar]
- 14. Parks RB, Hetzel SJ, Brooks MA. Iron deficiency and anemia among collegiate athletes: a retrospective chart review. Med Sci Sports Exerc. 2017;49(8):1711–1715. [DOI] [PubMed] [Google Scholar]
- 15. Pedlar CR, Brugnara C, Bruinvels G, Burden R. Iron balance and iron supplementation for the female athlete: a practical approach. Eur J Sport Sci. 2018;18(2):295–305. [DOI] [PubMed] [Google Scholar]
- 16. Petkus D, Murray-Kolb LE, De Souza MJ. The unexplored crossroads of the female athlete triad and iron deficiency: a narrative review. Sports Med. 2017;47(9):1721–1737. [DOI] [PubMed] [Google Scholar]
- 17. Tenforde AS, Carlson JL, Chang A, et al. Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am J Sports Med. 2017;45(2):302–310. [DOI] [PubMed] [Google Scholar]
- 18. Tenforde AS, Fredericson M, Sayres LC, Cutti P, Sainani KL. Identifying sex-specific risk factors for low bone mineral density in adolescent runners. Am J. Sports Med. 2015;43(6):1494–1504. [DOI] [PubMed] [Google Scholar]
- 19. Thralls KJ, Nichols JF, Barrack MT, Kern M, Rauh MJ. Body mass-related predictors of the female athlete triad among adolescent athletes. Int J Sport Nutr Exerc Metab. 2016;26(1):17–25. [DOI] [PubMed] [Google Scholar]
- 20. Weiss Kelly AK, Hecht S; Council on Sports Medicine and Fitness. The female athlete triad. Pediatrics. 2016;138(2):e20160922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, DS_10.1177_2325967120959725 for Prevalence of Female Athlete Triad Risk Factors and Iron Supplementation Among High School Distance Runners: Results From a Triad Risk Screening Tool by Paige Skorseth, Nicole Segovia, Katherine Hastings and Emily Kraus in Orthopaedic Journal of Sports Medicine