Abstract
Background:
Polypharmacy is ubiquitous in patients on hemodialysis (HD), and increases risk of adverse events, medication interactions, nonadherence, and mortality. Appropriately applied deprescribing can potentially minimize polypharmacy risks. Existing guidelines are unsuitable for nephrology clinicians as they lack specific instructions on how to deprescribe and which safety parameters to monitor.
Objective:
To develop and validate deprescribing algorithms for nine medication classes to decrease polypharmacy in patients on HD.
Design:
Questionnaires and materials sent electronically.
Participants:
Nephrology practitioners across Canada (nephrologists, nurse practitioners, renal pharmacists).
Methods:
A literature search was performed to develop the initial algorithms via Lynn’s method for development of content-valid clinical tools. Content and face validity of the algorithms was evaluated over three interview rounds using Lynn’s method for determining content validity. Canadian nephrology clinicians each evaluated three algorithms (15 clinicians per round, 45 clinicians in total) by rating each algorithm component on a four-point Likert scale for relevance; face validity was rated on a five-point scale. After each round, content validity index of each component was calculated and revisions made based on feedback. If content validity was not achieved after three rounds, additional rounds were completed until content validity was achieved.
Results:
After three rounds of validation, six algorithms achieved content validity. After an additional round, the remaining three algorithms achieved content validity. The proportion of clinicians rating each face validity statement as “Agree” or “Strongly Agree” ranged from 84% to 95% (average of all five questions, across three rounds).
Limitations:
Algorithm development was guided by existing deprescribing protocols intended for the general population and the expert opinions of our study team, due to a lack of background literature on HD-specific deprescribing protocols. There is no universally accepted method for the validation of clinical decision-making tools.
Conclusions:
Nine medication-specific deprescribing algorithms for patients on HD were developed and validated by clinician review. Our algorithms are the first medication-specific, patient-centric deprescribing guidelines developed and validated for patients on HD.
Keywords: deprescribing, hemodialysis, polypharmacy, clinical tool development, chronic kidney disease
Abrégé
Contexte:
La polypharmacie est très répandue chez les patients hémodialysés et augmente le risque d’événements indésirables, d’interactions médicamenteuses, d’inobservance au traitement et de mortalité. La déprescription, appliquée de façon appropriée, peut réduire les risques associés à la polypharmacie. Les directives de déprescription existantes ne conviennent cependant pas aux cliniciens en néphrologie puisqu’elles ne renferment aucune indication spécifique sur la manière de procéder ni sur les paramètres de sécurité à surveiller.
Objectif:
Développer et valider des algorithmes de déprescription pour neuf classes de médicaments en vue de réduire la polypharmacie chez les patients hémodialysés.
Conception:
Des questionnaires et des documents envoyés par voie électronique.
Participants:
Des praticiens en néphrologies de partout au Canada (néphrologues, infirmières-praticiennes, pharmaciens spécialisés en néphrologie).
Méthodologie:
Une recherche bibliographique a été effectuée pour développer les algorithmes initiaux avec la méthode de Lynn pour le développement d’outils cliniques à contenu validé. Le contenu et la validité apparente des algorithmes ont été évalués au cours de trois cycles d’interviews par la méthode de Lynn pour déterminer la validité d’un contenu. Les praticiens interviewés (15 par cycle, pour un total de 45) ont chacun évalué trois algorithmes en classant la pertinence de leurs composants sur une échelle de Likert en quatre points, et en classant leur validité apparente sur une échelle en cinq points. Après chaque cycle, l’indice de validité du contenu a été calculé pour chaque composant et des correctifs ont été apportés en fonction de la rétroaction. Si la validité du contenu n’était pas atteinte après trois cycles, des cycles supplémentaires étaient effectués jusqu’à ce que celle-ci soit atteinte.
Résultats:
Six algorithmes ont atteint la validité après trois cycles de validation. Les trois algorithmes restants l’ont atteint après un cycle supplémentaire. La proportion de cliniciens ayant attribué la mention de validité apparente « d’accord » ou « tout à fait d’accord » se situait entre 84 et 95 % (moyenne des cinq questions, sur trois cycles).
Limites:
Le développement des algorithmes repose sur les protocoles de déprescription existants, destinés à la population générale, et sur l’avis des experts de notre équipe d’étude puisque la documentation portant sur des protocoles de déprescription spécifiques aux patients hémodialysés est insuffisante. Il n’existe aucune méthode universellement acceptée pour valider les outils de décision clinique.
Conclusion:
Neuf algorithmes de déprescription spécifiques aux patients hémodialysés ont été développés et validés par révision des cliniciens. Nos algorithmes sont les premiers guides de déprescription développés et validés spécifiquement pour les médicaments des patients hémodialysés.
Enregistrement de l’essai:
Sans objet — il s’agit d’une série de questionnaires.
Introduction
Individuals on hemodialysis (HD) have the highest pill burden of all chronically ill patient populations, taking on average 12 ± 5 distinct medications per day.1,2 This is due to other comorbid chronic conditions (eg, hypertension, diabetes, cardiovascular disease) that require long-term medication management.3,4 Furthermore, patients with end-stage kidney disease on HD have physiological changes that alter drug absorption, distribution, metabolism, and elimination.5-7 Therefore, as the number of medications increases, the potential for adverse outcomes increases concurrently.8-12 Patients on HD are thus at increased risk of adverse outcomes and mortality related to polypharmacy.
Deprescribing is the planned and supervised process of discontinuing medications that may be causing harm or are no longer providing benefit.1 Several basic guidelines and generic algorithms for deprescribing exist; however, they have shortcomings which make them unsuitable for addressing polypharmacy in patients on HD.13-29 First, several of these guidelines are not medication specific. They lack practical suggestions on how to discontinue specific medications (eg, tapering vs abrupt discontinuation) and do not recommend specific monitoring parameters when discontinuing a medication. Second, existing tools developed based on safety and efficacy data for particular patient populations (eg, seniors, oncology, dementia) are not necessarily applicable to patients on HD due to their altered pharmacokinetics and unique drug dosing regimens. Finally, it is unclear how patient values and preferences contributed to previous deprescribing approaches and whether existing deprescribing tools have undergone formal validation processes.
An appropriately designed deprescribing intervention has the potential to achieve patient-centered goals and minimize adverse effects associated with polypharmacy.16,28-30 In turn, formal validation of such a clinical tool increases clinician confidence and facilitates adoption of the designed intervention.31 We previously evaluated medication use patterns and associated costs in HD by accessing provincial databases in Manitoba, British Columbia, and Ontario.32 Using results from this study, we conducted a national survey among nephrology health care study team members to pinpoint nine medication classes that are the focus of our current deprescribing efforts: alpha-1 blockers, benzodiazepines and Z-medications (eg, zopiclone), gabapentinoids, loop diuretics, prokinetic agents, proton pump inhibitors, quinine, statins, and urate-lowering agents.32 Patients on HD are rarely included in clinical trials, and so there is uncertainty about the efficacy and safety associated with their use in this population.32 Given the dearth of deprescribing studies within HD populations, we sought to develop validated deprescribing algorithms for clinical practice. The objectives of this study were to (1) develop algorithms for deprescribing nine specific medication classes in patients on HD while accounting for patient perspectives and (2) formally validate deprescribing algorithms with nephrology experts for content and face validity in a structured manner.
Materials and Methods
This study was composed of two phases. The first phase was the development of the deprescribing algorithms. The second phase was the validation of the algorithms. This study was approved by the University Health Network Research Ethics Board (Study ID: 17-5313).
Phase 1: Development of the Algorithms
In May 2014, five literature searches using Ovid MEDLINE (1946-May 2014) and PubMed (1946-May 2014) were conducted to identify any clinical tools or guidelines related to deprescribing alpha-1 blockers, loop diuretics, proton pump inhibitors, quinine, or statins in patients on HD. No existing algorithms were found and therefore five preliminary deprescribing algorithms were developed based on gray literature searches and expert opinions of the study team.33 These algorithms were refined and reviewed through a validation process and ultimately used in a pilot implementation study.33
In April 2018, 18 new literature searches (two different searches for each of the nine medications) were conducted with the guidance of a librarian using Embase (1947-April 2018), Ovid MEDLINE (1946-April 2018), and Ovid E-Pub (1946 to April 2018) to identify information that could improve the five existing deprescribing algorithms and to create algorithms for the four remaining medications (benzodiazepines and Z-medications, gabapentinoids, prokinetic agents, and urate-lowering agents). Searches addressed the following research questions:
Research Question 1: What is the evidence regarding safety and efficacy of this medication in hemodialysis patients?
Research Question 2: What is the success rate for discontinuing this medication (in any patient population) and is it safe to discontinue this medication?
The full list of search terms is listed in Appendix 1.
Randomized controlled trials, prospective and retrospective observational cohort studies, and case series with sample sizes ≥10 were eligible for inclusion, although some nonprimary literature (reviews and meta-analyses) was also considered. Only full-text manuscripts in English were eligible. For searches related to the first research question, the following were excluded: trials in nonchronic HD populations (eg, acute dialysis patients), trials unrelated to safety/efficacy, and editorials.
Using literature search results, nine algorithms were developed or revised (for the original five algorithms) according to Lynn’s three-step method for the development of content-valid clinical tools: (1) identifying domains of the tool, (2) creating an item list for each domain, and (3) forming the instrument.34 The study group (four nephrologists, five renal pharmacists, three patient partners) met via multiple teleconferences to refine the algorithms to consensus agreement.
Phase 2: Validation of the Algorithms
Validation was based on a process originally proposed by Lynn.34 The individual components of the instrument to be validated must be independently reviewed and graded by at least five experts to sufficiently control for chance agreement. Although there is little evidence regarding the optimal number of rounds required to build consensus,35,36 we performed a minimum of three rounds of interviews with revisions between each round. Algorithms not achieving expert agreement after three rounds underwent one additional round of revision, reusing five participants from round one.34
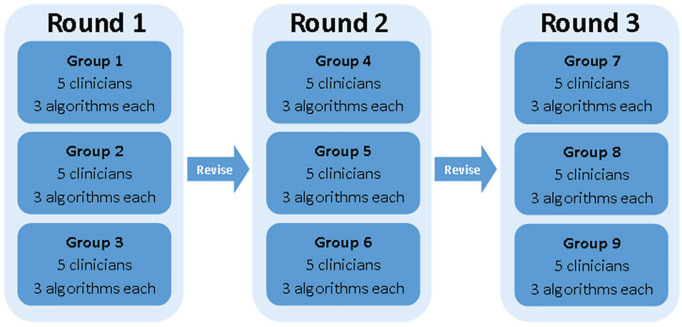
Clinician participants were recruited from major Canadian hospitals and adjacent community care sites based on clinical expertise. Per round of face and content validation, clinician participants independently reviewed three algorithms each (ie, 15 clinicians per round, for a total of 45; Figure 1). Three deprescribing algorithms and their associated two-part questionnaires (part A: content validity; part B: face validity) were disseminated to each participant via email. Questionnaires allowed both qualitative and quantitative responses.37 Participants returned questionnaires to the study team and then discussed ratings and comments in a one-on-one interview in person or via teleconferencing.
Figure 1.
Algorithm validation interview process.
After each round of interviews, revisions were made to the algorithms. Revised algorithms were then presented to the next round of participants. This process was repeated for three rounds of questionnaires and revisions (four if validity was not achieved), after which validation of the algorithm was complete and no further modifications were made.
Questionnaires for validation process
In this study, content and face validity questionnaires were based on Feinstein’s concept of clinical sensibility and the Agency for Healthcare Research and Quality’s Patient Education Materials Assessment Tool for Printable and Audio-Visual Materials.37,38
Part A (content validity)
The algorithms were divided into components and participants ranked the individual components on a four-point Likert scale (one: strongly disagree, two: disagree, three: agree, four: strongly agree; Appendix 2). Participants were asked to provide comments where revision was deemed necessary. Any algorithm components rated as one or two (ie, less relevant) by one or more participant(s) required revision.34
Part B (face validity)
For each algorithm, participants rated a series of statements assessing face validity on a five-point scale, according to their agreement with the statement (from “1: strongly disagree” to “5: strongly agree”; Appendix 2). Any components rated between one and three (ie, strongly disagree to neutral) by one or more participant(s) required revision.
Statistical analysis
Part A (content validity)
Content validity was quantified by calculating a content validity index (CVI) for each section of the flowchart and written guide. A section with a score of three (acceptable, with minor revision) or four (acceptable, as is) was deemed content valid, and sections with a score of one (unacceptable, remove) or two (unacceptable, major revision required) required revision. The CVI of each section was based on the proportion of overall participants ranking the section as valid. The CVI for the entire flowchart or written guide was based on the proportion of sections deemed content valid within each flowchart or written guide. For a panel of five reviewers, 100% must agree (ie, rate three or four) to establish content validity at the P < .05 level.34 Items which did not achieve the required proportion of expert agreement were eliminated or further revised prior to the next round of validation.
Part B (face validity)
Although face validity is a subjective measure, a systemic approach was adopted.39 Face validity was reported as a percentage of study participants rating statements in part B as either four or five (ie, agree or strongly agree). Although there is no standard agreement threshold for face validity, we adopted the consensus threshold of ≥70% that is commonly used when applying the Delphi technique.40-44
Supplemental Materials
Three patient partners (Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease [Can-SOLVE CKD] Patient Council) were included in the study team and provided feedback on the various project aspects as part of the Can-SOLVE CKD Network’s emphasis on patient-oriented research. Our patient partners provided invaluable feedback on the format, feasibility and accessibility of proposed nonpharmacological options, validation approach, and supplemental materials.
The following materials were developed in conjunction with the deprescribing algorithms:
Evidence tables: Data used to inform the creation of our algorithms were summarized in table format for each of the medications.
Monitoring forms: Single-sheet printable forms which can be inserted into the patient’s medical chart to improve ease of monitoring.
Patient information tools: A video and pamphlet were developed for each medication to ensure the patient is appropriately involved in the decision to deprescribe. A manuscript describing the validation process for these tools is underway.
Results
Phase 1: Development of the Algorithms
Literature search
The April 2018 literature search results are summarized in Table 1. A total of 6610 articles were identified. After screening and full-text review, 137 articles were deemed relevant to our study. Information from the relevant articles was used to create and adapt deprescribing algorithms for use in patients on HD.
Table 1.
Literature Search Results.
| Medication | No. of search results |
No. after initial screening |
No. after full-text screening |
|||
|---|---|---|---|---|---|---|
| Research question 1 | Research question 2 | Research question 1 | Research question 2 | Research question 1 | Research question 2 | |
| Alpha-1 blockers | 276 | 65 | 9 | 1 | 9 | 1 |
| Benzodiazepines and Z-drugs | 382 | 1090 | 7 | 101 | 2 | 17 |
| Gabapentinoids | 1115 | 1142 | 37 | 14 | 22 | 3 |
| Loop diuretics | 562 | 104 | 50 | 9 | 6 | 4 |
| Proton pump inhibitors | 203 | 266 | 48 | 33 | 11 | 9 |
| Prokinetic agents | 43 | 90 | 5 | 1 | 2 | 1 |
| Quinine | 136 | 20 | 16 | 1 | 3 | 0 |
| Statins | 487 | 476 | 126 | 39 | 28 | 14 |
| Urate-lowering agents | 96 | 27 | 11 | 4 | 2 | 3 |
Note. Research question 1: “What is the evidence regarding safety and efficacy of this medication in hemodialysis patients?”; Research question 2: “What is the success rate for discontinuing this medication (in any patient population) and is it safe to discontinue this medication?.”
Nine algorithms were developed according to Lynn’s 3-step method for the development of content-valid clinical tools.34 Six domains were addressed for each algorithm: (1) candidates for deprescribing; (2) baseline monitoring period/parameters; (3) appropriate deprescribing approach; (4) nonpharmacological options to manage symptoms (if applicable); (5) safety and efficacy monitoring period/parameters; and (6) what to do if symptoms continue/worsen. A list of items to be included in each domain was generated for each algorithm based on literature search results and clinical experiences of the group. Domain items were included or discarded through availability of evidence and consensus. For example, in the “nonpharmacological options to manage symptoms” domain, the group included options for which there was published evidence of benefit, and/or have a very low risk for adverse effects. Treatments not meeting these requirements were discarded. To facilitate implementation in clinical practice, a decision-making flowchart for each deprescription was created and supplemented with written guides.
Phase 2: Validation of the Algorithms
Study participants
Between May 2018 and May 2019, 45 clinician participants were recruited from 25 institutions across 10 Canadian provinces for the validation phase. Participant professions included nephrologists, renal pharmacists, and nurse practitioners practicing in dialysis (Table 2).
Table 2.
Participant Characteristics (n = 45).
| Province of practice | Round 1 | Round 2 | Round 3 | Overall |
|---|---|---|---|---|
| Alberta | 1 | 1 | 1 | 3 |
| British Columbia | 2 | 3 | 0 | 5 |
| Manitoba | 2 | 3 | 3 | 8 |
| New Brunswick | 0 | 0 | 2 | 2 |
| Newfoundland and Labrador | 0 | 0 | 1 | 1 |
| Nova Scotia | 4 | 3 | 2 | 9 |
| Ontario | 5 | 2 | 3 | 10 |
| Prince Edward Island | 0 | 1 | 0 | 1 |
| Quebec | 0 | 0 | 2 | 2 |
| Saskatchewan | 1 | 2 | 1 | 4 |
| Profession | ||||
| Nephrologist | 8 | 7 | 9 | 24 |
| Renal pharmacist | 2 | 7 | 5 | 14 |
| Nurse practitioner | 5 | 1 | 1 | 7 |
| Gender | ||||
| Male | 9 | 6 | 6 | 21 |
| Female | 6 | 9 | 9 | 24 |
| Years of practice | ||||
| Median (interquartile range) | 10.5 (4.3-22.5) | 15 (6-23) | 13 (8.8-16.5) | 12.5 (5-20.3) |
| Mean ± SD | 13.8 ± 12.2 | 14.9 ± 10 | 13.2 ± 7.8 | 13.6 ± 10.3 |
Content validation
Overall CVIs per round for the flowcharts and written guides of each deprescribing algorithm are shown in Table 3. Per round CVIs of each flowchart and written guide component are provided in Appendix 3.
Table 3.
Overall Content Validity Indices of Treatment Algorithms, per Round of Content Validation.
| Round 1 overall |
Round 2 overall |
Round 3 overall |
Round 4 overall |
|||||
|---|---|---|---|---|---|---|---|---|
| Flowchart | Written guide | Flowchart | Written guide | Flowchart | Written guide | Flowchart | Written guide | |
| Alpha-1 blockers | 0.8 | 1.0 | 0.7 | 0.8 | 1.0 | 1.0 | N/A | N/A |
| Benzodiazepines | 0.7 | 0.9 | 0.8 | 0.8 | 1.0 | 0.8 | N/A | 1.0 |
| Gabapentinoids | 0.7 | 0.6 | 0.8 | 1.0 | 1.0 | 1.0 | N/A | N/A |
| Loop diuretics | 0.7 | 0.6 | 0.6 | 0.4 | 1.0 | 1.0 | N/A | N/A |
| Proton pump inhibitors | 0.5 | 1.0 | 1.0 | 0.9 | 1.0 | 1.0 | N/A | N/A |
| Prokinetic agents | 0.5 | 1.0 | 0.8 | 1.0 | 1.0 | 1.0 | N/A | N/A |
| Quinine | 0.5 | 0.6 | 1.0 | 0.8 | 1.0 | 1.0 | N/A | N/A |
| Statins | 0.8 | N/A | 1.0 | N/A | 0.8 | N/A | 1.0 | N/A |
| Urate-lowering agents | 0.8 | 0.8 | 0.8 | 0.9 | 1.0 | 0.8 | N/A | 1.0 |
| Overall average for all 9 algorithms | 0.67 | 0.81 | 0.83 | 0.83 | 0.98 | 0.95 | — | — |
Note. N/A—not applicable as item achieved content validity in an earlier round. The statin algorithm does not include a written guide.
Across three rounds of validation, average overall CVIs were 0.67, 0.83, and 0.98 for the flowcharts, and 0.81, 0.83, and 0.95 for the written guides, respectively. Overall CVIs in round one ranged from 0.5 to 0.8 for the flowcharts, and from 0.6 to 1.0 for the written instructions. In round one, three written instructions scored 1.0 and were considered content valid. Overall CVIs in round two ranged from 0.6 to 1.0 for the flowcharts and from 0.8 to 1.0 for the written instructions. Three flowcharts and two written instructions scored 1.0. Overall CVIs in round three for both flowcharts and written instructions ranged from 0.8 to 1.0. A flowchart (statins) and two written guides (benzodiazepines, urate-lowering agents) underwent a fourth validation round before reaching an overall CVI of 1.0.
Face validation
The overall (across all three rounds) levels of agreement with each of the five face validity statements per algorithm are shown in Table 4. Face validity scores for each algorithm are given in Appendix 4.
Table 4.
Agreement With Face Validity of Algorithm (Across All Rounds).
| Q1 (%) | Q2 (%) | Q3 (%) | Q4 (%) | Q5 (%) | Average Q1-Q5 (%) |
|
|---|---|---|---|---|---|---|
| Alpha-1 blockers | 93 | 93 | 93 | 87 | 87 | 91 |
| Benzodiazepines | 87 | 93 | 87 | 87 | 87 | 88 |
| Gabapentinoids | 80 | 93 | 87 | 80 | 80 | 84 |
| Loop diuretics | 100 | 100 | 93 | 87 | 87 | 93 |
| Proton pump inhibitors | 93 | 100 | 87 | 100 | 93 | 95 |
| Prokinetic agents | 100 | 100 | 93 | 80 | 80 | 91 |
| Quinine | 93 | 100 | 100 | 80 | 80 | 91 |
| Statins | 93 | 93 | 87 | 80 | 73 | 85 |
| Urate-lowering agents | 100 | 100 | 87 | 80 | 87 | 91 |
Note. Q1 = the tool is clear and understandable; Q2 = the tool uses appropriate language and wording; Q3 = the tool flows in a logical manner; Q4 = this tool could be used in the hemodialysis unit where I practice; Q5 = I would be confident recommending the use of this tool.
All algorithms achieved >70% overall agreement for the five statements. Agreement ranged from 80% to 100% for statement one (The tool is clear and understandable), 93% to 100% for statement two (The tool uses appropriate language and wording), 87% to 100% for statement three (The tool flows in a logical manner), 80% to 100% for statement four (This tool could be used in the hemodialysis unit where I practice), and 73% to 100% for statement five (I would be confident recommending the use of this tool). Highest scoring algorithms (average across all statements) were proton pump inhibitors (95%) and loop diuretics (93%). Lowest scoring algorithms were benzodiazepines and Z-drugs (88%), statins (85%), and gabapentinoids (84%).
Qualitative feedback
Consistent themes emerged across the validation rounds for all nine algorithms. Several comments recommended reformatting/rewording some components for improved ease of use, conciseness, and clarity. For example, ambiguous sentences were reworded, repetitive items were simplified, and clarity improvements on arrangement of flowcharts. Several clinician participants expressed confusion over the supplemental materials; text was added to the written guides to clarify the intended purpose of the monitoring forms and patient information tools.
The complete list of changes per validation round is given in Appendix 5. Final versions of the nine algorithms are shown in Appendix 6.
Discussion
Our algorithms are the first medication-specific, patient-centric deprescribing guidelines developed and validated for patients on HD. Deprescribing algorithms have been used in other patient populations with success. For instance, existing studies on geriatric populations have demonstrated that the use of specific tools to guide deprescribing effectively reduces polypharmacy.28-30 These interventions have been associated with lower medication costs, fewer long-term care referrals (12% intervention vs 30% control),29 decreased mortality (21% intervention vs 45% control),29 and improved perceptions of overall personal health (88% of patients reported global improvement in health).16
To our knowledge, these algorithms are the first deprescribing tools that have been formally validated for content and face validity by nephrology experts from across Canada. The validation of clinical decision tools and treatment algorithms is becoming a focus in health care due to recent shifts toward quality improvement.45 While there are no standard methods for face and content validation, our results demonstrate that nephrology experts have judged the content of our tools to be accurate, relevant, straightforward, and appropriate for their intended purpose. Our validation processes also revealed facility-specific or practice-specific constraints, such as task ownership (Who will champion the monitoring requirements?) or economic constraints (Nondrug alternative is expensive or not covered by insurance). This resulted in changes to increase the adaptability of the tool across a variety of practice settings and usability between different members of the care team (Appendix 5). Ultimately, this approach helps facilitate the adoption of our algorithms at a wide variety of Canadian centers.
A further strength of our approach was the inclusion of patient partners as members of our study team to ensure that patient perspectives were considered at every step in the project. Recent deprescribing literature has emphasized the “patient-centred deprescribing process.”46 There has been a great focus on understanding the patient perspective toward deprescribing and how it may influence the success or failure of deprescribing initiatives. A recent review summarizes patient-identified enablers (cessation appropriateness, process for cessation, and a general dislike of medications) and barriers (cessation disagreement, lack of process for cessation, and feared consequences of cessation) for deprescribing.46 This review emphasizes the importance of employing a process that includes patient education, support, monitoring, and follow-up—components that we incorporated into our deprescribing protocol. Through involving patient partners from different areas of Canada, we designed the algorithms to act as deprescribing enablers and to assist in overcoming the barriers that affect a diverse Canadian HD population.
The Lynn method is well established for assessing content validity.34,47-49 On average, the overall CVIs for each algorithm steadily increased with every validation round, suggesting an improvement in content validity after each revision. Most medications had recurrent concerns among participants and/or validation rounds based on individual practice experience. In the loop diuretics algorithm, multiple participants felt that a dose of 120-mg/d furosemide was too low for patients on HD. Participants suggested the addition of a higher furosemide dose into the flowchart and including “urine output” as a safety parameter. This was accepted into the final algorithm.
Comments on nonpharmacological options were generally positive, though some concerns arose due to a combination of a lack of literature consensus and differences in practice. Certain participants felt there was insufficient scientific evidence supporting the efficacy of some recommended nonpharmacological options (eg, acupuncture in the benzodiazepines algorithm). Other participants were against certain nonpharmacologic options (eg, cognitive behavioral therapy) as they would be unable to offer them to their patients due to economic constraints. Nonetheless, we decided that options carrying a low risk for adverse effects and showing some positive outcomes in literature should be included. Therefore, all original nonpharmacological options were retained and further options were added based on participant suggestions.
Throughout the face validation process, participants commonly expressed barriers related to algorithm implementation. Many participants practice in units with weekly physician rotations, in understaffed units, or do not have renal pharmacists. In addition, some felt that more emphasis on ensuring communication between members of the patient’s circle of care was needed (eg, family physician, dispensing pharmacy, other specialists, family members). In our 2014 pilot implementation study, communication letters were sent to family physicians to outline the patient’s involvement in a deprescribing trial.33 Future implementation studies will follow this practice. Finally, participants expressed concerns that health care providers may feel uncomfortable deprescribing medications initiated by another provider. Therefore, we intend to perform a preimplementation study to identify real-world barriers and facilitators to algorithm implementation in HD units, and potential strategies to mitigate barriers.
These algorithms were developed to improve outcomes of patients on HD by using an evidence-based approach to decreasing polypharmacy in HD units. However, there is a general lack of literature related to stopping and starting specific medications in HD patients. In designing these algorithms, we relied on literature on deprescribing in the general population and on expert opinions (study team and participants) to guide algorithm creation. Despite this limitation, face validity scores for the tools demonstrated that participants agreed that the tools will be effective in fulfilling their stated purpose.
There is no universally accepted or standard method for the validation of clinical decision-making tools. We selected the Lynn method based on its documented use in the literature, its applicability to this study, and past experience of our study team members with this method.50 There is also little consensus in literature on the threshold for content validity, methods for calculating CVIs, and the number of validation rounds required. Nonetheless, the quality of these tools was strengthened by the national validation using nephrology professionals of various professions and institutions, and the inclusion of patient partners as members of the study team. While we are confident in the content and face validity of our algorithms in a Canadian context, it is possible that health care providers may interpret the content of our algorithms according to location, clinical contexts, or patient and provider values. The impact of these variations will be explored in future implementation research.
Conclusion
We developed and validated nine deprescribing algorithms that incorporate the values and preferences of patients treated with maintenance HD. We conclude that the algorithms possess high content and face validity via high overall CVI scores and high agreement levels with face validity statements from different professions after a rigorous validation process. Future research will focus on evaluating the implementation of these deprescribing tools in multiple HD units across Canada.
Supplemental Material
Supplemental material, Appendix_6_Algorithms,_ALL_-_Final for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease
Supplemental material, CJKHD-20-0099.R1_Translation for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease
Supplemental material, STOPMed-HD_AlgorValid_Appendices_1-5_Final for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the University Health Network Research Ethics Board (Study ID: 17-5313).
Consent for Publication: All authors reviewed and approved the final version of this manuscript.
Availability of Data and Materials: All relevant data and materials are contained in the appendices.
Author Contributions: M.B., M.J.L., and J.F. helped in research idea and study design; M.J.L. and J.F. helped in data acquisition; M.J.L., P.C.K.N., A.D., D.M., B.W., M.T., A.X.G., J.W., M.B., J.M., C.O., A.L., C.M., J.F., C.B., and M.B. helped in data analysis/interpretation; and M.T., A.X.G., C.B., and M.B. helped in supervision/mentorship. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.X.G. is supported by the Dr Adam Linton Chair in Kidney Health Analytics, and a clinician investigator award from the Canadian Institutes of Health Research. The other members of the research team have nothing to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Kidney Foundation of Canada, and the Canadian Institutes of Health Research (CIHR) Strategy for Patient-Oriented Research (SPOR) grant: Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD).
ORCID iDs: Amit X. Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Marisa Battistella  https://orcid.org/0000-0001-9456-4365
https://orcid.org/0000-0001-9456-4365
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827-834. [DOI] [PubMed] [Google Scholar]
- 2. Battistella M, Fleites R, Wong R, Jassal SV. Development, validation, and implementation of a medication adherence survey to seek a better understanding of the hemodialysis patient. Clin Nephrol. 2016;85(1):12-22. [DOI] [PubMed] [Google Scholar]
- 3. Manley HJ, McClaran ML, Overbay DK, et al. Factors associated with medication-related problems in ambulatory hemodialysis patients. Am J Kidney Dis. 2003;41(2):386-393. [DOI] [PubMed] [Google Scholar]
- 4. Chiu Y-W, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ponticelli C, Sala G, Glassock RJ. Drug management in the elderly adult with chronic kidney disease: a review for the primary care physician. Mayo Clin Proc. 2015;90(5):633-645. [DOI] [PubMed] [Google Scholar]
- 6. Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105:437-445. [DOI] [PubMed] [Google Scholar]
- 7. Liles AM. Medication considerations for patients with chronic kidney disease who are not yet on dialysis. Nephrol Nurs J. 2011;38:263-270. [PubMed] [Google Scholar]
- 8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. [DOI] [PubMed] [Google Scholar]
- 9. Fick D, Semla T, Beizer J, et al. American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayes BD, Klein-Schwartz W, Barrueto F. Polypharmacy and the geriatric patient. Clin Geriatr Med. 2007;23:371-390. [DOI] [PubMed] [Google Scholar]
- 11. Huang AR, Mallet L, Rochefort CM, et al. Medication-related falls in the elderly: causative factors and preventive strategies. Drug Aging. 2012;29:359-376. [DOI] [PubMed] [Google Scholar]
- 12. Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmac. 2007;5:345-351. [DOI] [PubMed] [Google Scholar]
- 13. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045-1051. [DOI] [PubMed] [Google Scholar]
- 14. Samsa GP, Hanlon JT, Schmader KE, et al. A summated score for the medication appropriateness index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47(8):891-896. [DOI] [PubMed] [Google Scholar]
- 15. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845-854. [DOI] [PubMed] [Google Scholar]
- 16. Scott IA, Gray LC, Martin JH, Pillans PI, Mitchell CA. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18(4):121-124. [DOI] [PubMed] [Google Scholar]
- 17. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654. [DOI] [PubMed] [Google Scholar]
- 18. Samuel MJ. American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246. [DOI] [PubMed] [Google Scholar]
- 19. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2014;44:213-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindsay J, Dooley M, Martin J, et al. The development and evaluation of an oncological palliative care deprescribing guideline: the “OncPal deprescribing guideline.” Support Care Cancer. 2015;23(1):71-78. [DOI] [PubMed] [Google Scholar]
- 21. Rodríguez-Pérez A, Alfaro-Lara ER, Albiñana-Perez S, et al. Novel tool for deprescribing in chronic patients with multimorbidity: list of evidence-based deprescribing for chronic patients criteria: LESS-CHRON criteria. Geriatr Gerontol Int. 2017;17:2200-2207. [DOI] [PubMed] [Google Scholar]
- 22. Farrell B, Black C, Thompson W, et al. Deprescribing antihyperglycemic agents in older persons: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(11):832-843. [PMC free article] [PubMed] [Google Scholar]
- 23. Abrahamson K, Nazir A, Pressler K. A novel approach to deprescribing in long-term care settings: the SMART campaign. Res Social Adm Pharm. 2017;13(6):1202-1203. [DOI] [PubMed] [Google Scholar]
- 24. Bjerre LM, Farrell B, Hogel M, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(1):17-27. [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes HM, Todd A. Evidence-based deprescribing of statins in patients with advanced illness. JAMA Intern Med. 2015;175(5):701-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersen AW, Shah AS, Simmons SF, et al. Shed-MEDS: pilot of a patient-centered deprescribing framework reduces medications in hospitalized older adults being transferred to inpatient postacute care. Ther Adv Drug Saf. 2018;9(9):523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niehoff KM, Rajeevan N, Charpentier PA, Miller PL, Goldstein MK, Fried TR. Development of the Tool to Reduce Inappropriate Medications (TRIM): a clinical decision support system to improve medication prescribing for older adults. Pharmacotherapy. 2016;36(6):694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane DB Syst Rev. 2014; CD008165. [DOI] [PubMed] [Google Scholar]
- 29. Lindsay J, Dooley M, Martin J, Fay M, Kearney A, Barras M. Reducing potentially inappropriate medications in palliative cancer patients: evidence to support deprescribing approaches. Support Care Cancer. 2014;22(4):1113-1119. [DOI] [PubMed] [Google Scholar]
- 30. Cross C. Introducing deprescribing into culture of medication. Can Med Assoc J. 2013;185:E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS One. 2016;11(4):e0151066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battistella M, Jandoc R, Ng JY, McArthur E, Garg AX. A province-wide, cross-sectional study of demographics and medication use of patients in hemodialysis units across Ontario. Can J Kidney Health Dis. 2018;5. doi:2054358118760832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611-618. [DOI] [PubMed] [Google Scholar]
- 34. Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382-386. [PubMed] [Google Scholar]
- 35. Goodman CM. The Delphi technique: a critique. J Adv Nurs. 1987;12(6):729-734. [DOI] [PubMed] [Google Scholar]
- 36. Bell CM, Brener SS, Comrie R, et al. Quality measures for medication continuity in long-term care facilities, using a structured panel process. Drug Aging. 2012;29:319-327. [DOI] [PubMed] [Google Scholar]
- 37. Feinstein AR. The theory and evaluation of sensibility. In: Clinimetrics. New Haven, CT: Yale University Press; 1987:141-166. [Google Scholar]
- 38. Shoemaker SJ. The Patient Education Materials Assessment Tool (PEMAT) and user’s guide. Agency for Healthcare Research and Quality; https://www.ahrq.gov/ncepcr/tools/self-mgmt/pemat.html. Published 2014. Accessed November 28, 2019. [Google Scholar]
- 39. Trochim WMK. Research Methods: The Essential Knowledge Base. 2nd ed. Cincinnati, OH: Atomic Dog Publishing; 2000. [Google Scholar]
- 40. Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6(6):e20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banwell HA, Mackintosh S, Thewlis D, Landorf KB. Consensus-based recommendations of Australian podiatrists for the prescription of foot orthoses for symptomatic flexible pes planus in adults. J Foot Ankle Res. 2014;7(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kleynen M, Braun SM, Bleijlevens MH, et al. Using a Delphi technique to seek consensus regarding definitions, descriptions and classification of terms related to implicit and explicit forms of motor learning. PLoS One. 2014;9(6):e100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hicks C. Research Methods for Clinical Therapists: Applied Project Design and Analysis. 5th ed. Edinburgh, Scotland: Churchill Livingstone; 2009. [Google Scholar]
- 44. Vernon W, Parry A, Potter M. Consensus obtained in a Delphi study of shoe wear pattern experiences amongst podiatrists. Journal of Forensic Identification. 2003;53:15-41. [Google Scholar]
- 45. Khalil PN, Kleespies A, Angele MK, et al. The formal requirements of algorithms and their implications in clinical medicine and quality management. Langenbecks Arch Surg. 2011;396(1):31-40. [DOI] [PubMed] [Google Scholar]
- 46. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793-807. [DOI] [PubMed] [Google Scholar]
- 47. Bannigan K, Watson R. Reliability and validity in a nutshell. J Clin Nurs. 2009;18(23):3237-3243. [DOI] [PubMed] [Google Scholar]
- 48. Almanasreh E, Moles R, Chen TF. Evaluation of methods used for estimating content validity. Res Social Adm Pharm. 2019;15(2):214-221. [DOI] [PubMed] [Google Scholar]
- 49. Rutherford-Hemming T. Determining content validity and reporting a content validity index for simulation scenarios. Nurs Educ Perspect. 2015;36:389-393. [DOI] [PubMed] [Google Scholar]
- 50. Ragazzo J, Cesta A, Jassal SV, Chiang N, Battistella M. Development and validation of a uremic pruritus treatment algorithm and patient information toolkit in patients with chronic kidney disease and end stage kidney disease. J Pain Symptom Manage. 2020;59(2):279-292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_6_Algorithms,_ALL_-_Final for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease
Supplemental material, CJKHD-20-0099.R1_Translation for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease
Supplemental material, STOPMed-HD_AlgorValid_Appendices_1-5_Final for Development and Validation of Nine Deprescribing Algorithms for Patients on Hemodialysis to Decrease Polypharmacy by Melissa J. Lefebvre, Patrick C. K. Ng, Arlene Desjarlais, Dennis McCann, Blair Waldvogel, Marcello Tonelli, Amit X. Garg, Jo-Anne Wilson, Monica Beaulieu, Judith Marin, Cali Orsulak, Anita Lloyd, Caitlin McIntyre, Jordanne Feldberg, Clara Bohm and Marisa Battistella in Canadian Journal of Kidney Health and Disease