Abstract
Ginkgo biloba leaf extract (GIN) is a popular Chinese herbal medicine. It has a nephroprotective effect against the nephrotoxicity induced by the chemotherapeutic agent methotrexate (MTX). This work was designed to explore the testicular protective role of GIN on MTX-induced testicular injury in a rat model. The experimental protocol lasted for 10 days for the 4 studied groups. First group: received saline (normal control, NC group). The second group was administered GIN (100 mg/kg/day) orally for 10 days (GIN C). Third group: injected with MTX (20 mg/kg ip) only on the fifth day (MTX group). Fourth group: administered GIN for 10 days with MTX injection on the fifth day (GIN+MTX group). MTX induced testicular injury as evident by a marked rise in the malondialdehyde (MDA) content, interleukin-6 (IL-6) and IL-1β protein levels, nuclear factor kappa-B (NF-κB) protein expression, bcl-2 associated × protein (Bax) mRNA expression, p53 mRNA and protein expressions, and miRNA29-a expression along with a marked decline in the serum level of testosterone and superoxide dismutase (SOD) content in testicular tissue in relation to the NC group. Moreover, histopathological testicular damage with a notable decrease in the Johnsen score together with a significant elevation in the testicular injury score was observed in the MTX group in comparison to the NC group. The administration of GIN ameliorated the biochemical changes as well as the testicular histopathological findings and scores. GIN could protect against MTX-induced gonadotoxicity by its antioxidant, anti-inflammatory, antiapoptotic activities plus the regulation of the miRNA-29a testicular expression.
Keywords: methotrexate, testicular injury, Ginkgo biloba extract, NF-κB, Bax, miRNA-29a
Introduction
Cancer is considered one of the principal causes of mortality worldwide and its management comprises the use of several chemotherapeutic agents. Nevertheless chemotherapy has been linked with multiple unfavorable side effects.1 One of the serious problems encountered with the use of chemotherapy in clinical practice is its organ toxicity.2 Different types of malignancies as well as autoimmune diseases have been effectively treated by the folate antagonist agent methotrexate (MTX).3 The MTX mechanism of action was mediated through the inhibition of folate metabolism and nucleic acid synthesis.4 MTX cytotoxicity, as with other chemotherapeutic agents, was reported to affect many organs including liver, kidney, and testis in both clinical and experimental studies.5-8
Methotrexate testicular toxicity is a devastating adverse effect that can cause sterility.9 Although the exact mechanism through which MTX could induce testicular injury is not fully investigated, it was documented that oxidative stress, inflammatory cytokines, and apoptotic cascades have been involved.10,11
MTX contributes to a condition of imbalance between oxidative and antioxidative status in the affected tissue leading to excessive production of reactive oxygen species (ROS) comprising hydrogen peroxide, hydroxyl radical, and superoxide. In addition to the elevated levels of ROS, a decline in the antioxidant defense mechanism was encountered in the form of decreased activity of antioxidant enzymes like catalase and superoxide dismutase (SOD) as well as the non-enzymatic antioxidants like reduced glutathione (GSH).12,13 As the testis was susceptible to MTX toxicity, subsequent oxidative damage with destruction in the membrane integrity and lipid peroxidation occurred.12-14
Moreover, the release of pro-inflammatory cytokines like interleukin-6 (IL-6) and IL-1β along with cell death through apoptotic cascades was also documented in the pathophysiology of MTX-induced testicular injury.10,13,15 Apoptosis is known as a process of programmed cell death and regulated by the family members of bcl-2 which has been documented to be involved in the testicular apoptosis.16-18 Bcl-2 has been reported to inhibit apoptosis and prolong cell survival while bcl-2 associated x protein (Bax) could block the capability of bcl-2 in inhibiting apoptosis and could be considered as an apoptosis regulator. Moreover, p53 is a tumor suppressor gene and can enhance apoptosis through the upregulation of Bax expression.17,18
On the other hand, microRNAs (miRNAs) are small non-coding RNAs with about 22 nucleotides and it was reported that they were implicated in the regulation of diverse physiological processes such as cell proliferation, apoptosis, fibrosis, inflammation and metabolism.19-21 One of these miRNAs is the miRNA-29s family which has 3 members, miRNA-29a, miRNA-29b, miRNA-29c. The miRNA-29a is a vital part of the miRNA-29 family and was reported to have a proapoptotic activity.21,22
Many studies discussed the role of natural antioxidants in ameliorating MTX side effects.6-8,14 One of the traditional Chinese medicines that was utilized in the management and treatment of different diseases is Ginkgo biloba.23 Terpene lactones and flavone glycosides are the ingredients of the Ginkgo biloba standardized extract (GIN).24 The pharmacological action of the GIN was thought to be related to its antioxidant activity which scavenges free radicals.25
Several reports showed an effective protective effect of GIN in different models of neurotoxicity26 and hepatotoxicity,27 in addition to nephrotoxicity.28,29 Moreover, the protective effect of GIN against testicular toxicity induced by cisplatin,25 doxorubicin,30 cadmium,31 and cellphone radiation32 was reported. Nevertheless, the impact of GIN versus MTX-induced gonadotoxicity is still not been identified yet. Hence, the purpose of this work was to explore if GIN could mitigate the MTX-induced testicular toxicity and if so, elucidate its molecular protective mechanism through the determination of oxidative stress, inflammatory, and apoptotic markers in addition to miRNA-29a expression in testicular tissues.
Materials and Methods
Adult male Sprague Dawley rats weighing 250 ± 50 g were utilized in our protocol. Animals were kept in cages and exposed to a cycle of 12 h light/ dark and a temperature of 25°C. Free access to water and chow was permitted. The study protocol approval was obtained from the Ethics Committee of Faculty of Medicine, Mansoura University and was done in conformity with the Guide for Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 2011).
Study Protocol
This study comprised 4 groups with 8 rats per each group. They were assigned according to the treatment protocol which lasted for 10 days. Group I: normal control group (NC) in which rats received normal saline. Group II: GIN control group (GIN C) in which rats received 100 mg/kg/day Ginkgo biloba extract (GNC, Pittsburgh, USA, Code 194732) by oral gavage.25 Group III: MTX group in which rats were intraperitoneally injected on the fifth day with a single dose 20 mg/kg methotrexate (MTX, 50 mg, TP, Shanxi PUDE Pharmaceutical Co. Ltd., Shanxi, China).14,33 Group IV: GIN + MTX group in which rats received 100 mg/kg Ginkgo biloba extract daily for 10 days by oral gavage25 with MTX injection on day 5.
Blood samples and testicular tissues were collected from all rats in the studied groups at the end of the experimental study. Separated sera were used for testosterone estimation. Testes were removed quickly and one testis was immersed in Bouin’s liquid for 48 hours while the other testis was snap frozen in liquid nitrogen and kept at −80°C for further analytical work.
Biochemical Analyses
Determination of serum testosterone level
Rat testosterone ELISA kit provided by Elabscience Biotechnology Co., Wuhan, China, Cat. No. E-EL-0155 was utilized for the estimation of testosterone serum levels according to the manufacturer’s instructions.
Oxidative stress markers estimation
Oxidative stress markers were determined in testicular tissue homogenate in which the testis was homogenized in PBS and centrifuged for 10 minutes at 4°C. For biochemical analysis, the supernatant was kept at −80°C. Malondialdehyde (MDA) and superoxide dismutase (SOD) kits (Bio-Diagnostic, Cairo, Egypt) were used for the assessment of MDA and SOD levels, respectively in the testicular tissue homogenate.
Inflammatory markers determination
Inflammatory markers IL-6 and IL-1β were assayed in testicular tissues by ELISA according to the manufacturer’s instructions of the rat ELISA kits provided from Cusabio Biotech Co., Wuhan, China, Cat. No. CSB-E04640r, CSB-E08055r, respectively.
Real-time Quantitative Polymerase Chain Reaction (qPCR)
Bax and p53 mRNA expressions determination
A total RNA purification kit (Jena Bioscience, Munich, Germany) was used to isolate the total RNA from the testicular tissues. The cDNA archive kit (Applied Biosystems, Foster City, California, USA) was utilized for converting RNA into its cDNA. The qPCR was carried out by GoTaq PCR master mix (Promega Co., Madison, USA) using a StepOne Real-Time PCR System (Applied Biosystems, Foster City, California, USA). The Bax primers were: forward 5′-CCGGCAGGCCCATACTGAAT-3′; reverse, 5′- CTTGGACAGG GCAGATAGCC-3′; for p53 were forward: 5′-TGGGTCACCTCCACACCTCC-3′, reverse: 5′-GGATGTTGCAGAGTTGTTAG-3′; and for β-actin (housekeeping gene) were forward: 5′-CGTTGACATCCGTAAAGACCTC-3′, reverse: 5′ TAGGAGCCAGGGCAGTAATCT-3′ were used. All values were normalized to the housekeeping gene and relative expression was estimated using the 2–ΔΔCT method.
miRNA-29a expression determination
The miRNeasy extraction kit from Qiagen, Valencia, CA, USA was used for miRNAs extraction. Then to convert the miRNA to the corresponding cDNA, a SuperScript IV TM Reverse Transcription kit provided by Invitrogen, CA, USA was used. The qPCR was performed by utilizing a PowerUpTM SYBRTM Green Master Mix Kit provided by Applied Biosystems on a StepOne Real-Time PCR System (Applied Biosystems, Foster City, California, USA). The primers used were miRNA-29a primer forward 5′-GCGCACTGATTTCTTTTGGTGTTCAG-3′ and reverse: 5′-GCGAGCACAGAATTAATACGAC-3′; and RNU6B primer (housekeeping gene) forward: 5′-CTCGCTTCGGCAGCACATA-3′ and reverse: 5′-CGCTTCACGAATTTGCGTG-3′. All values were normalized to the housekeeping gene and relative expression was calculated using the 2–ΔΔCT method.
Histopathological Examination and Testicular Injury Scoring
Fixed testes in Bouin’s solution were dehydrated and placed in paraffin. The 5µm sections were cut from the testicular tissue and inspected using a light microscope after staining with hematoxylin & eosin (H&E). A spermatogenesis score by the Johnsen score method with grading from 1, no spermatogenesis to 10, full spermatogenesis was used as described previously.34 Moreover, a testicular injury score from 0 which stands for normal histopathology to 3 which stands for severe damage was used for the evaluation of seminiferous epithelial damage, interstitial edema, tubular necrosis, and congestion as previously reported.35
Immunohistochemistry (IHC) Evaluation
The other 5 µm sections of the testicular tissue blocks were deparaffinized and rehydrated. The IHC was accomplished using p53 polyclonal antibody (1:100; Invitrogen, Carlsbad, CA, USA) and NF-κB p65 monoclonal antibody (1:100; ABclonal Biotech Co. Ltd, Woburn, USA) according to the manufacturer’s instructions. The scoring of the IHC staining intensity was done with scoring scale 0, 1, 2, and 3 which stands for negative, weak, moderate, and strong staining, respectively.36
Statistical Analysis
SPSS version 20 and GraphPad Prism version 6, computer software, were used for doing the statistical analysis of this study. One-way analysis of variance (ANOVA) followed by a post hoc Bonferroni test was utilized for the assessment of the differences among groups. However, the statistical analysis of all the histopathological and immunohistochemical scores was done by the Kruskal–Wallis test, followed by Dunn’s test. Data were presented as mean ± SD. A P-value < .05 was statistically significant.
Results
Effect on the Testosterone Level
In Figure 1, the testosterone serum level was markedly decreased after MTX by 53.1% compared with the NC group. Pretreatment with GIN significantly increased the testosterone level by 64% in comparison to the MTX group (P < .001).
Figure 1.
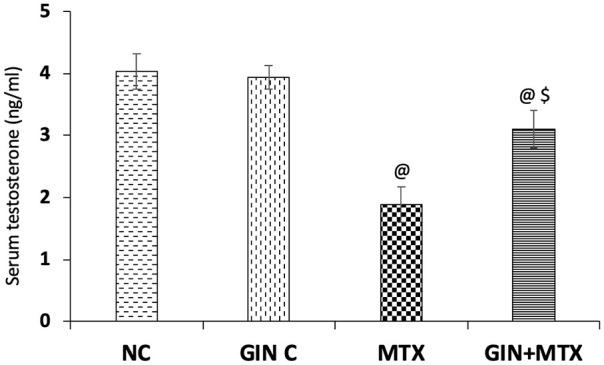
Impact of Ginkgo biloba extract (GIN) on serum testosterone levels in the experimental groups. @P < .001 versus NC group. $P < .001 versus MTX group.
Effect on the Oxidative Stress Markers
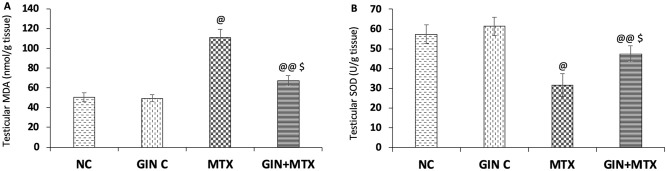
Figure 2 illustrated the effect of GIN on the MDA levels (A) and SOD (B) in the testicular tissue. A notable rise in the testicular MDA level was detected in the MTX group by 1.2-fold compared with the NC; nevertheless, a marked reduction in MDA content by 39.3% was noticed in the GIN + MTX group in comparison to MTX group (P < .001).
Figure 2.
Impact of Ginkgo biloba extract (GIN) on the malondialdehyde (MDA) (A) and superoxide dismutase (SOD) (B) contents in the testicular tissues of the experimental groups. @P < .001, @@P < .05 versus NC group. $P < .001 versus MTX group.
Furthermore, MTX provoked a significant decline in the testicular SOD level by 45.1% in comparison to NC. After GIN treatment, a notable elevation of the SOD testicular level by 50.5% was reported in comparison to the MTX group (P < .001).
Effect on the Inflammatory Markers
Determination of protein levels by ELISA assay
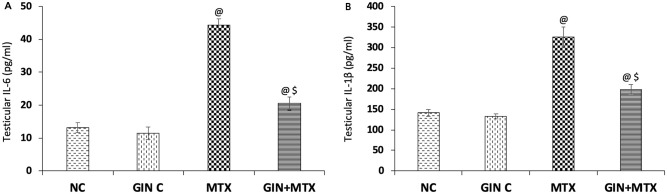
As presented in Figure 3, the injection of MTX induced a marked elevation in the testicular protein levels of IL-6 (A) and IL-1β (B) inflammatory cytokines by 2.4-fold and 1.3-fold, correspondingly in comparison to NC. Moreover, GIN treatment significantly reduced the IL-6 and IL-1β testicular contents by 53.7% and 39.3%, respectively when compared with the MTX group (P < .001).
Figure 3.
Impact of Ginkgo biloba extract (GIN) on the interleukin-6 (IL-6) (A) and IL-1β (B) levels in the testicular tissue of the experimental groups. @P < .001 versus NC group. $P < .001 versus MTX group.
Determination of protein expression by IHC
Concerning the IHC staining of NF-κB which is illustrated in Figure 4, the NC (A) and GIN C (B) groups showed a weak NF-κB testicular expression. In the MTX group, a strong positive NF-κB expression was observed (C), while a moderate expression was seen in the GIN+MTX group (D).
Figure 4.
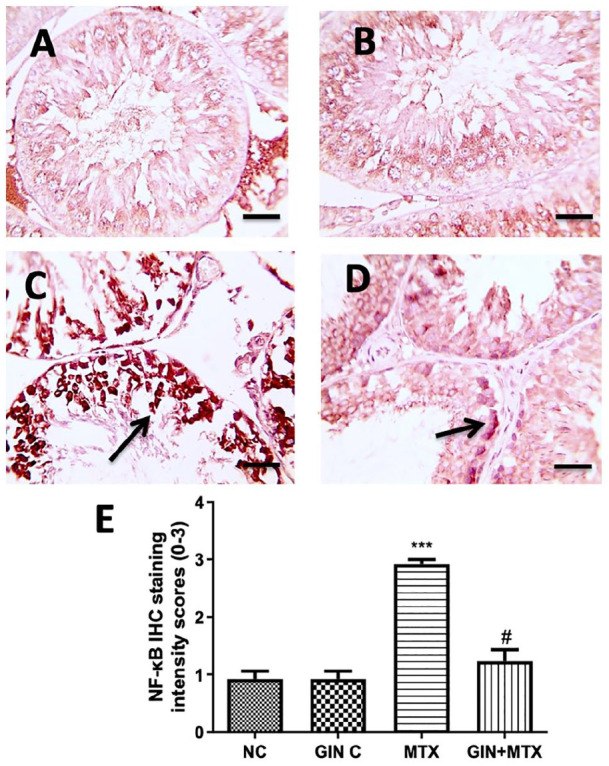
Microscopic pictures of rats’ testes immunostained against inflammatory NF-κB (A–D) and statistical analysis of IHC intensity scores of NF-κB expression (E). Testicular sections showed a weak positive brown expression for NF-κB expression in the NC and GIN C groups (A and B), a strong positive brown staining for NF-κB expressions in the MTX group in the germ cells (C), a moderate positive brown staining for NF-κB expressions in the GIN+MTX treated group (D). IHC counterstained with Mayer’s hematoxylin. X400, bar = 50 µm. ***P < .05 versus NC, #P < .05 versus MTX.
Statistical analysis of the IHC intensity scores in the testicular sections as presented in Figure 4E exhibited a marked increase in the NF-κB expression in the MTX group compared with the NC group, while the GIN+MTX treatment group showed significantly decreased NF-κB expression in respect to the MTX group (P < .05).
Effect on the Apoptotic Markers
Determination of mRNA expression by qPCR
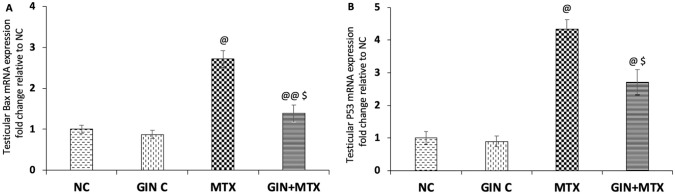
The effect of GIN on the apoptotic marker Bax in the testicular tissue is elucidated in Figure 5A. Injection of MTX led to a marked upregulation of Bax mRNA testicular expression by 1.7-fold when compared with NC. Significant downregulation of testicular Bax mRNA expression by 48.9% was noticed in the GIN +MTX group in comparison to the MTX group (P < .001).
Figure 5.
Impact of Ginkgo biloba extract (GIN) on bcl-2 associated x protein (Bax) (A) and p53 (B) mRNA expressions in testicular tissue of the experimental groups. @P < .001, @@P < .05 versus NC group. $P < .001 versus MTX group.
In addition, the impact of using GIN on testicular p53 mRNA expression is illustrated in Figure 5B. Significant upregulation of p53 mRNA expression by 3.3-fold in comparison to NC was detected. GIN treatment showed a significant downregulation of p53 mRNA expression by 37.6% when compared with the MTX group (P < .001).
Determination of protein expression by IHC
Moreover, p53 protein expression in testicular tissues was determined by IHC as illustrated in Figure 6. Weak p53 expression was observed in the NC (A) and GIN C (B) groups while a strong p53 expression was seen in the MTX group (C). Treatment with GIN showed moderate p53 expression in the GIN+MTX group (D).
Figure 6.
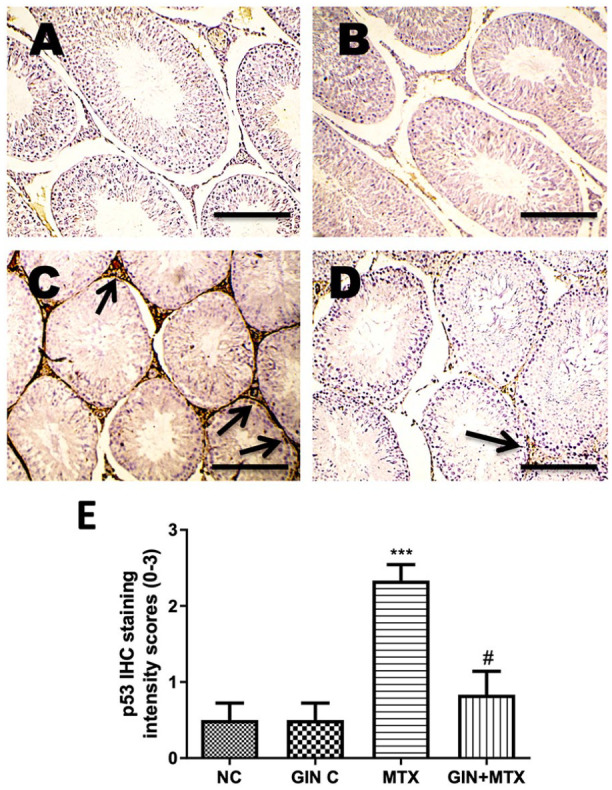
Microscopic pictures of rats’ testes immunostained against apoptotic p53 (A–D) and statistical analysis of IHC intensity scores of p53 expression (E). Testicular sections showed a weak positive brown staining for p53 expression in the NC and GIN C groups (A and B), a strong positive brown staining for p53 expression in the MTX group (C), a moderate positive brown staining for p53 expressions in the GIN+MTX treated group (D). IHC counterstained with Mayer’s hematoxylin. X100, bar = 100 µm. ***P < .05 versus NC, #P < .05 versus MTX.
Statistical analysis of the IHC intensity scores in the testicular sections as illustrated in Figure 6 E showed a marked rise in the p53 expression in the MTX group in comparison to NC. However, the GIN+MTX treatment group showed significantly reduced p53 expression in relation to the MTX group (P < .05).
Effect on miRNA-29a Expression
As illustrated in Figure 7, the MTX treated group exhibited a marked upregulation of miRNA-29a testicular expression by 1.73-fold when compared with NC, whereas GIN administration displayed a significant downregulation of miRNA-29a testicular expression by 44.3% in comparison to the MTX group (P < .001).
Figure 7.
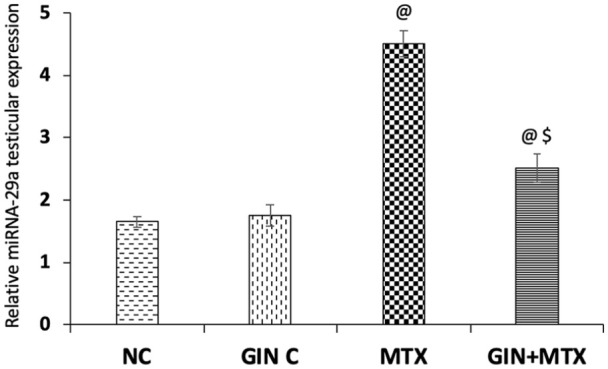
Impact of Ginkgo biloba extract (GIN) on miRNA-29a expression in testicular tissue of the experimental groups. @P < .001 versus NC group, $P < .001 versus MTX group.
Effect on Histopathology
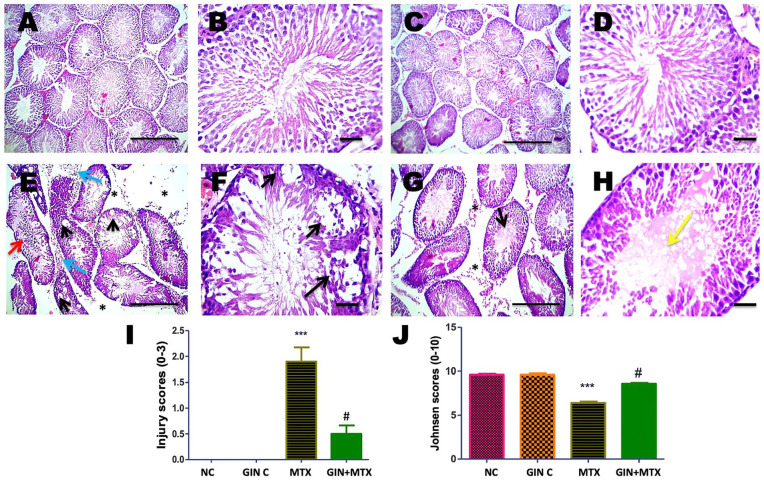
Histopathological investigation of the testicular tissues from all groups is represented in Figure 8. Testis from the NC (A&B) and GIN C (C&D) groups revealed normal histopathology of seminiferous tubules with full spermatogenesis. Moreover, testis from the MTX group showed congestion in the interstitial blood vessels, interstitial edema, leukocytic cells infiltration, marked vacuolization of spermatocyte, necrotic spermatids, and lowered spermatogenesis (E&F). Marked improvement in the histopathological changes was noticed in the treated group GIN+MTX group (G&H). Statistical analysis of the testicular injury scores showed a marked increase in the MTX group in comparison to NC while a significant reduction of the injury score was detected in the GIN+MTX in comparison to MTX, P < .05 (I). Furthermore, a marked decline in the Johnsen score was noticed in the MTX group in comparison to NC. After GIN treatment a notable rise in the Johnsen score was noticed in comparison to the MTX group, P < .05 (J).
Figure 8.
Histopathological pictures of testicular tissues stained with hematoxylin and eosin (H and E) in all groups. Testis showed normal histopathology of seminiferous tubules with full spermatogenesis in NC (A and B) and GIN C (C and D) groups, interstitial edema (asterisks), leukocytic cells infiltration (blue arrows), marked vacuolization of spermatocytes (black arrows), desquamated spermatocytes (red arrow), necrotic spermatids and lowered spermatogenesis in MTX group (E and F). Interstitial edema (asterisks), partially restored spermatogenesis, hyalinization of spermatids is detected in lumen of few seminiferous tubules (yellow arrow) in GIN+MTX treated group (G and H). A, C, E, G X100, bar = 100 µm and B, D, F, H X 400, bar = 50 µm. Statistical analysis of testicular injury (I) and Johnsen (J) scores. ***P < .05 versus NC, #P < .05 versus MTX.
Discussion
The use of chemotherapeutic agents, such as MTX, has been rising with the increased prevalence of cancer worldwide. At the same time, its use is associated with toxic side effects on different body organs including testes.37 In the current work, we evaluated the possible protective effect of GIN when given with MTX in a rat model.
Our study showed that MTX induced a marked decline in the testosterone level in comparison to normal group as described in previous reports.16,37 Our histopathological findings revealed testicular tissue damage after MTX injection with interstitial edema, marked vacuolization of spermatocytes, necrotic spermatids, and lowered spermatogenesis, These findings were consistent with our statistical analysis of the spermatogenesis Johnsen score and testicular injury score, in which MTX showed marked reduction in Johnsen score along with significant elevation of the testicular injury score compared with the normal group. Several studies were consistent with our findings confirming the reproductive toxicity of using MTX.1,37
This study discussed for the first time the GIN protective role versus MTX-induced-reproductive damage. GIN increased significantly the serum level of testosterone and improved the histopathological scores confirming its ameliorating role in the testicular damage induced by MTX. In a similar way, many natural products proved their protective effect versus MTX-induced testicular toxicity.1,14,37 Also, GIN ameliorated the histopathological testicular changes observed after chemotherapeutic agents like cisplatin and doxorubicin.25,30
Oxidative stress is one of the principal pathophysiological mechanisms that is involved in the MTX-induced gonadal toxicity.37 Compared with normal rats, our results reported that MTX induced a notable rise in MDA level with a marked reduction in the SOD level in the testicular tissue and similar findings were reported previously.8,12,37 Injection of MTX led to reactive oxygen species (ROS) overproduction and subsequently to a status of oxidative stress thus this explained the increased levels of MDA, a toxic metabolite released from lipid peroxidation, and the reduction of the antioxidant enzymes like SOD which converted the superoxide into hydrogen peroxide and oxygen.37
These findings contributed to the testicular tissue sensitivity to chemotherapy induced-oxidative injury.30 Therefore, it is acceptable to suggest that antioxidant agents were able to minimize the oxidative stress induced by MTX and could protect the testes from this oxidative damage. Our results documented that the administration of GIN exhibited a marked decrease in the MDA level and a marked elevation in the SOD level in the testicular tissue in comparison with the MTX group, confirming its antioxidant effect. The antioxidant activity of GIN was reported to be related to its ingredients of terpenoids and flavonoids that are able to scavenge the free radicals and minimize the ROS.30,38 GIN was reported to attenuate chemotherapy-induced testicular toxicity through its ability to restore the normal levels of the antioxidant testicular enzymes like SOD and catalase along with reduction of the content of testicular MDA.25,30
Inflammation has a central impact in the pathogenesis of the testicular toxicity induced by MTX.10,13 Inflammatory cytokines are secreted by immune cells and have multiple functions involved in the regulation of the immune and inflammatory responses.13 Furthermore, under normal conditions the transcriptional factor NF-κB is sequestered in the cytoplasm with its associated inhibitory protein IκB, and when activated the IκB dissociates and NF-κB translocates to the nucleus, activating genes of proinflammatory cytokines as IL-6 and IL-1β. NF-κB is thus considered an important regulator of inflammation.13
MTX was reported to induce a marked rise in the inflammatory mediators like IL-6 and IL-1β in testicular tissues10,13,15,39 along with upregulation of the NF-κB mRNA and protein testicular expressions.13,15 In our study, MTX induced a marked testicular inflammation response which was confirmed by the marked elevation in the inflammatory markers of IL-6 and IL-1β testicular levels as well as the marked upregulation of the testicular NF-κB expression, which was in harmony with previous studies.
The current study documented that GIN has the ability to minimize the inflammation induced by MTX through a significant decline in the testicular levels of IL-6 and IL-1β and downregulation of NF-κB expression. The anti-inflammatory action of GIN was reported previously in different animal models through suppression of NF-κB activation together with the inflammatory cytokines IL-1β and IL-6.25,40,41
Apoptosis is also involved in the pathophysiological mechanism of MTX-induced gonadotoxicity.11 It was reported that MTX treatment led to defects in DNA synthesis resulting in induction of apoptosis, which is regulated by bcl-2 family proteins involving antiapoptotic proteins such as bcl-2 and proapoptotic protein as Bax as a response to cellular damage.16,42,43 Moreover, Sheikhbahaei et al in 2016 documented that MTX injection induced damage in testicular tissue with degeneration of seminiferous tubules and germ cells apoptosis via pathways of p53, Bax, and bcl-2.11
These features coincided with the findings of this current work which revealed a significant upregulation in Bax mRNA expression as well as p53 mRNA and immunoreactivity protein expressions in the testicular tissue of the MTX group when compared with NC. Additionally, p53 was reported to be upregulated following the DNA damage leading to apoptosis initiation.18 The findings of this study were consistent with other previous reports.11,16,44
Many natural products like resveratrol,8 propolis,1 beta-carotene,12 and thymoquinone11 have been evaluated to protect the testes from testicular injury provoked by MTX chemotherapy and were shown to reduce apoptosis through their antiapoptotic activity by acting on various apoptotic pathways. In a similar way, GIN in our study exhibited an antiapoptotic activity through significant downregulation of testicular Bax mRNA expression and p53 mRNA and protein expressions. Moreover, the antiapoptotic activity of GIN was reported in various animal models confirming its ability to inhibit apoptosis through working on apoptotic pathways including Bax/bcl-2 and p53.30,45,46
The upregulation of miRNA-29a expression was documented in various models involving type II diabetes,20 dextran sodium sulfate-induced ulcerative colitis,47 hyperoxia-induced bronchopulmonary dysplasia,48 and myocardial ischemia/reperfusion22 suggesting its role in inducing cell apoptosis.
Regarding the involvement of miRNA-29 testicular expression, it was reported that the administration of estradiol benzoate as well as doxorubicin, which both induced germ cell apoptosis, was related to the upregulation of the miRNA-29 family expression including miR-29a.49,50 Furthermore, it has been documented that the miRNA-29 family members induce apoptosis in a p53-dependent way.51 This current study was in accordance with those mentioned findings in which the injection of MTX induced upregulation of the testicular miRNA-29a expression in comparison to NC.
A similar mechanistic pathway was documented before in other studies in which the upregulation of miRNA-29a expression was concomitant with oxidative stress activation through increasing MDA along with decreasing SOD levels.22 Moreover, it was documented that upregulation of miR-29a expression increased IL-6 and IL-1β production along with NF-κB activation.52
Many natural products can regulate various miRNAs expression that are involved in many pathophysiological conditions.53,54 Similarly, GIN presented for the first time a marked downregulation in the testicular miRNA29-a expression indicating its ability to reduce the testicular damage induced by MTX. Our finding coincided with what was reported with previous studies which suggested that the suppression of miRNA-29a expression improved medical disorders besides reducing apoptosis.22,47,48
Conclusion
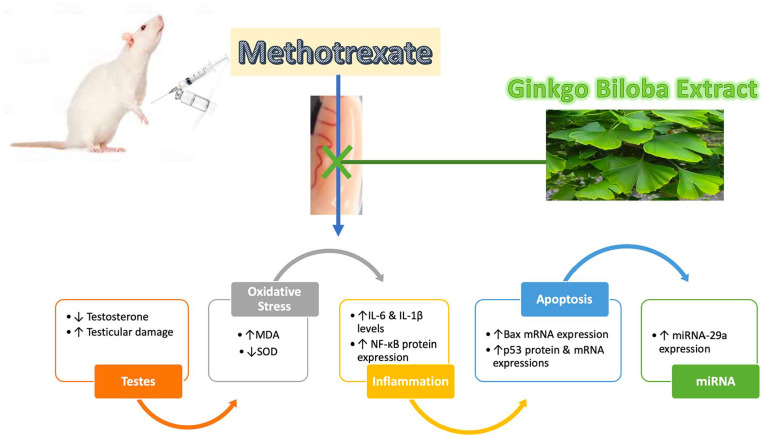
In conclusion, this work showed for the first time a definitive testicular protective effect of GIN against MTX-induced testicular injury. Its molecular protective mechanism involved:(1) minimizing oxidative stress status induced by MTX through declining MDA level besides SOD elevation in the testicular tissue; (2) suppression of the testicular inflammation via reduction of the inflammatory cytokines IL-6 and IL-1β and suppression of NF-κB testicular expression (3) inhibition of testicular apoptosis via downregulation of the apoptotic markers such as Bax mRNA and p53 mRNA and protein testicular expressions; (4) downregulation of the apoptotic factor miRNA-29a testicular expression which was implicated for the first time in the pathogenesis of the MTX-induced gonadal toxicity (Figure 9). Concurrent treatment of GIN with MTX could be utilized to reduce MTX gonadotoxicity; nevertheless, further investigations are required to verify the findings of this study and explore other molecular protective mechanisms of GIN.
Figure 9.
Summarized protective mechanism of Ginkgo biloba extract against testicular injury induced by methotrexate.
Acknowledgments
For the histopathological evaluation, the authors acknowledged Dr. Walaa Fekri.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program. For the histopathological evaluation, the authors acknowledged Dr. Walaa Fekri.
ORCID iD: Iman O. Sherif  https://orcid.org/0000-0002-9487-3456
https://orcid.org/0000-0002-9487-3456
References
- 1. Sönmez MF, Çilenk KT, Karabulut D, et al. Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed Pharmacother. 2016;79:44-51. [DOI] [PubMed] [Google Scholar]
- 2. Ramirez LY, Huestis SE, Yap TY, Zyzanski S, Drotar D, Kodish E. Potential chemotherapy side effects: what do oncologists tell parents? Pediatr Blood Cancer. 2009;52(4):497-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan ZA, Tripathi R, Mishra B. Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv. 2012;9(2):151-169. [DOI] [PubMed] [Google Scholar]
- 4. Heidari Khoei H, Fakhri S, Parvardeh S, Shams Mofarahe Z, Baninameh Z, Vardiani M. Astaxanthin prevents the methotrexate-induced reproductive toxicity by targeting oxidative stress in male mice. Toxin Reviews. 2019;38(3):248-254. [Google Scholar]
- 5. Howard SC, McCormick J, Pui C-H, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebrahimi R, Sepand MR, Seyednejad SA, et al. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IĸBα/NFĸB in rats. Daru. 2019:27(2):721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jalili C, Ghanbari A, Roshankhah S, Salahshoor MR. Toxic effects of methotrexate on rat kidney recovered by crocin as a consequence of antioxidant activity and lipid peroxidation prevention. Iran Biomed J. 2020;24(1):39-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuluğ E, Türedi S, Alver A, Türedi S, Kahraman C. Effects of resveratrol on methotrexate-induced testicular damage in rats. ScientificWorldJournal. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armagan A, Uzar E, Uz E, et al. Caffeic acid phenethyl ester modulates methotrexate-induced oxidative stress in testes of rat. Hum Exp Toxicol. 2008;27(7):547-552. [DOI] [PubMed] [Google Scholar]
- 10. Owumi SE, Ochaoga SE, Odunola OA, Farombi EO. Protocatechuic acid inhibits testicular and epididymal toxicity associated with methotrexate in rats. Andrologia. 2019;51(9): e13350. [DOI] [PubMed] [Google Scholar]
- 11. Sheikhbahaei F, Khazaei M, Rabzia A, Mansouri K, Ghanbari A. Protective effects of thymoquinone against methotrexate-induced germ cell apoptosis in male mice. Int J Fertil Steril. 2016;9(4):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. Antiapoptotic and antioxidant effects of β-carotene against methotrexate-induced testicular injury. Fertil Steril. 2009;92(6):2028-2033. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Zhao T, Zhao H, Wang H. Melatonin protects methotrexate-induced testicular injury in rats. Eur Rev Med Pharmacol Sci. 2018;22(21):7517-7525. [DOI] [PubMed] [Google Scholar]
- 14. Belhan S, Çomaklı S, Küçükler S, Gülyüz F, Yıldırım S, Yener Z. Effect of chrysin on methotrexate-induced testicular damage in rats. Andrologia. 2019;51(1):e13145. [DOI] [PubMed] [Google Scholar]
- 15. Belhan S, Özkaraca M, Kandemir FM, et al. Effectiveness of hesperidin on methotrexate-induced testicular toxicity in rats. Kafkas Univ Vet Fak Derg. 2017;23:789-796. [Google Scholar]
- 16. Saad D, Soliman M, Mohamed A, Youssef G. Protective effects of sea cucumber (Holothuria atra) extract on testicular dysfunction induced by immune suppressant drugs in Wistar rats. Andrologia. 2018;50(6):e13017. [DOI] [PubMed] [Google Scholar]
- 17. Taylor MF, Woolveridge I, Metcalfe A, Streuli C, Hickman J, Morris ID. Leydig cell apoptosis in the rat testes after administration of the cytotoxin ethane dimethanesulphonate: role of the Bcl-2 family members. J Endocrinol. 1998;157(2):317-326. [DOI] [PubMed] [Google Scholar]
- 18. Mosadegh M, Hasanzadeh S, Razi M. Nicotine-induced damages in testicular tissue of rats; evidences for bcl-2, p53 and caspase-3 expression. Iran J Basic Med Sci. 2017;20(2):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47. [DOI] [PubMed] [Google Scholar]
- 20. Wu P, Wang Q, Jiang C, et al. MicroRNA‑29a is involved lipid metabolism dysfunction and insulin resistance in C2C12 myotubes by targeting PPARδ. Mol Med Rep. 2018;17(6):8493-8501. [DOI] [PubMed] [Google Scholar]
- 21. Jafarinejad-Farsangi S, Farazmand A, Mahmoudi M, et al. MicroRNA-29a induces apoptosis via increasing the Bax: Bcl-2 ratio in dermal fibroblasts of patients with systemic sclerosis. Autoimmunity. 2015;48(6):369-378. [DOI] [PubMed] [Google Scholar]
- 22. Ding S, Liu D, Wang L, Wang G, Zhu Y. Inhibiting microRNA-29a protects myocardial ischemia-reperfusion injury by targeting SIRT1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther. 2020;372(1):128-135. [DOI] [PubMed] [Google Scholar]
- 23. Cheng D, Liang B, Li Y. Antihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in rats. Biomed Res Int. 2012;2013:162724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dubber M-J, Kanfer I. High-performance liquid chromatographic determination of selected flavonols in Ginkgo biloba solid oral dosage forms. J Pharm Pharm Sci. 2004;7(3):303-309. [PubMed] [Google Scholar]
- 25. Amin A, Abraham C, Hamza AA, et al. A standardized extract of Ginkgo biloba neutralizes cisplatin-mediated reproductive toxicity in rats. J Biomed Biotechnol. 2012;2012:362049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verma S, Ranawat P, Sharma N, Nehru B. Ginkgo biloba attenuates aluminum lactate-induced neurotoxicity in reproductive senescent female rats: behavioral, biochemical, and histopathological study. Environ Sci Pollut Res Int. 2019;26(26):27148-27167. [DOI] [PubMed] [Google Scholar]
- 27. El-Maksoud EMA, Lebda MA, Hashem AE, Taha NM, Kamel MA. Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ Sci Pollut Res Int. 2019;26(25):25844-25854. [DOI] [PubMed] [Google Scholar]
- 28. Abd-Ellah MF, Mariee AD. Ginkgo biloba leaf extract (EGb 761) diminishes adriamycin-induced hyperlipidaemic nephrotoxicity in rats: association with nitric oxide production. Biotechnol Appl Biochem. 2007;46(1):35-40. [DOI] [PubMed] [Google Scholar]
- 29. Sherif IO, Al-Shaalan NH, Sabry D. Ginkgo biloba extract alleviates methotrexate-induced renal injury: new impact on PI3K/Akt/mTOR signaling and MALAT1 expression. Biomolecules. 2019;9(11):691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeh YC, Liu TJ, Wang LC, et al. A standardized extract of Ginkgo biloba suppresses doxorubicin-induced oxidative stress and p53-mediated mitochondrial apoptosis in rat testes. Br J Pharmacol. 2009;156(1):48-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Souza Predes F, Monteiro JC, Matta SLP, Garcia MC, Dolder H. Testicular histomorphometry and ultrastructure of rats treated with cadmium and Ginkgo biloba. Biol Trace Elem Res. 2011;140(3):330-341. [DOI] [PubMed] [Google Scholar]
- 32. Gevrek F, Aydin D, Ozsoy S, Aygun H, Bicer C. Inhibition by Egb761 of the effect of cellphone radiation on the male reproductive system. Bratisl Lek Listy. 2017;118(11):676-683. [DOI] [PubMed] [Google Scholar]
- 33. Daggulli M, Dede O, Utangac MM, et al. Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med. 2014;7(12):5511. [PMC free article] [PubMed] [Google Scholar]
- 34. Johnsen SG. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1(1):2-25. [DOI] [PubMed] [Google Scholar]
- 35. Erpek S, Bilgin MD, Dikicioglu E, Karul A. The effects of low frequency electric field in rat testis. Revue Méd Vét. 2007;158(4):206-212. [Google Scholar]
- 36. Kim S-W, Roh J, Park C-S. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50(6):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pınar N, Çakırca G, Özgür T, Kaplan M. The protective effects of alpha lipoic acid on methotrexate induced testis injury in rats. Biomed Pharmacother. 2018;97:1486-1492. [DOI] [PubMed] [Google Scholar]
- 38. Smith J, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol. 2004;64(4):465-472. [DOI] [PubMed] [Google Scholar]
- 39. Makary S, Abdo M, Fekry E. Oxidative stress burden inhibits spermatogenesis in adult male rats: testosterone protective effect. Can J Physiol Pharmacol. 2018;96(4):372-381. [DOI] [PubMed] [Google Scholar]
- 40. Lee C-Y, Yang J-J, Lee S-S, et al. Protective effect of Ginkgo biloba leaves extract, EGb761, on endotoxin-induced acute lung injury via a JNK-and Akt-dependent NFκB pathway. J Agric Food Chem. 2014;62(27):6337-6344. [DOI] [PubMed] [Google Scholar]
- 41. Gargouri B, Carstensen J, Bhatia HS, Huell M, Dietz GP, Fiebich BL. Anti-neuroinflammatory effects of Ginkgo biloba extract EGb761 in LPS-activated primary microglial cells. Phytomedicine. 2018;44:45-55. [DOI] [PubMed] [Google Scholar]
- 42. Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5(2): a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Padmanabhan S, Tripathi D, Vikram A, Ramarao P, Jena G. Cytotoxic and genotoxic effects of methotrexate in germ cells of male Swiss mice. Mutat Res. 2008;655(1-2):59-67. [DOI] [PubMed] [Google Scholar]
- 44. Sukhotnik I, Nativ O, Roitburt A, et al. Methotrexate induces germ cell apoptosis and impairs spermatogenesis in a rat. Pediatr Surg Int. 2013;29(2):179-184. [DOI] [PubMed] [Google Scholar]
- 45. Raafat BM, Saleh A, Shafaa MW, Khedr M, Ghafaar AA. Ginkgo biloba and Angelica archangelica bring back an impartial hepatic apoptotic to anti-apoptotic protein ratio after exposure to technetium 99mTc. Toxicol Ind Health. 2013;29(1):14-22. [DOI] [PubMed] [Google Scholar]
- 46. Wu C, Zhao X, Zhang X, Liu S, Zhao H, Chen Y. Effect of Ginkgo biloba extract on apoptosis of brain tissues in rats with acute cerebral infarction and related gene expression. Genet Mol Res. 2015;14(2):6387-6394. [DOI] [PubMed] [Google Scholar]
- 47. Lv B, Liu Z, Wang S, et al. MiR-29a promotes intestinal epithelial apoptosis in ulcerative colitis by down-regulating Mcl-1. Int J Clin Exp Pathol. 2014;7(12):8542. [PMC free article] [PubMed] [Google Scholar]
- 48. Hu Y, Xie L, Yu J, Fu H, Zhou D, Liu H. Inhibition of microRNA-29a alleviates hyperoxia-induced bronchopulmonary dysplasia in neonatal mice via upregulation of GAB1. Mol Med. 2020;26(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meunier L, Siddeek B, Vega A, et al. Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: role of microRNA miR-29 family in the down-regulation of DNA methyltransferases and Mcl-1. Endocrinology. 2012; 153(4):1936-1947. [DOI] [PubMed] [Google Scholar]
- 50. Akinjo OO, Gant TW, Marczylo EL. Perturbation of epigenetic processes by doxorubicin in the mouse testis. Toxicol Res (Camb). 2016;5(4):1229-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park S-Y, Lee JH, Ha M, Nam J-W, Kim VN. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol. 2009;16(1):23. [DOI] [PubMed] [Google Scholar]
- 52. Tang B, Li X, Ren Y, et al. MicroRNA-29a regulates lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages through the Akt1/NF-κB pathway. Exp Cell Res. 2017;360(2):74-80. [DOI] [PubMed] [Google Scholar]
- 53. Lin Q, Ma L, Liu Z, et al. Targeting microRNAs: a new action mechanism of natural compounds. Oncotarget. 2017;8(9):15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Biersack B. Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Noncoding RNA Res. 2016;1(1):12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]