Abstract
Background:
Fish oils are the most widely used nonvitamin, nonmineral dietary supplements in the United States. They are not over-the-counter medications and are neither approved nor indicated for treating disease. Patient knowledge and patterns of fish oil use are not well defined.
Objective:
To determine cardiac patients’ knowledge and patterns of fish oil use.
Methods:
One thousand consecutive patients admitted to an in-patient cardiology service (2015-2017) taking fish oil dietary supplements or prescription omega-3 fatty acids were asked to complete an anonymous questionnaire concerning product knowledge and use.
Results:
A total of 711 (71%) patients completed the questionnaire. Primary reasons for use included general health (34%), heart health (28%), arthritis (9%), and lipid disorders (8%). Few patients (14%) were advised to take fish oil products by a health-care provider. Only 2.5% were taking prescription omega-3 fatty acids. Only 26% knew the active ingredient in their fish oil product. Supplements were purchased through a nonpharmacy retail seller by 81% of respondents.
Conclusions:
Most cardiac patients consuming fish oil dietary supplements do so without medical supervision and without knowledge of the active ingredients. As most patients obtain supplements outside of a pharmacy, opportunities to monitor and educate patients remain a major challenge.
Keywords: dietary supplements, docosahexaenoic acid, eicosapentaenoic acid, fish oil, omega-3 fatty acids
Introduction
The long-chain polyunsaturated omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are the primary active ingredients in fish oil dietary supplements and purified prescription omega-3 fatty acids. EPA and DHA are important nutrients that are often cited as having a range of potential beneficial effects on several aspects of health including cardiovascular health, aging, brain health and development, and eye health.1-6 As a means of general health promotion, dietetic and nutrition organizations recommend daily intake of 500 mg of omega-3 fatty acids as a part of a healthy diet for adults to prevent essential fatty acid deficiency.7-9 Perhaps for these reasons, fish oil products have become the most widely used nonvitamin, nonmineral dietary supplements in the United States.10,11 The use of fish oil dietary supplements was found to have increased more than 9-fold between 1999 and 2012 and nearly doubled between 2007 and 2012.10,11
Despite the rising use of fish oil dietary supplements, misconceptions that have important implications for their use persist. Most individuals are unaware that fish oil dietary supplements are not approved or indicated for the treatment of any disease, are not over-the-counter (OTC) drugs, and do not typically contain the same quality or quantity of active ingredients compared to prescription omega-3 fatty acids.12,13 Hence, dietary supplement fish oils are not interchangeable with prescription omega-3 fatty acid products.
Based on the widespread use of fish oil dietary supplements, this study was conducted to assess patient knowledge and patterns of use of these supplements and prescription omega-3 fatty acids to better understand possible misconceptions and the potential role of health-care professionals, including pharmacists, in guiding and educating patients.
Methods
Study Design and Participants
This study was conducted at Creighton University Medical Center, Omaha, Nebraska from January 2015 through March 2017. The study was approved by the University’s institutional review board. One thousand consecutive patients admitted to the cardiology service who were taking fish oil products were asked to complete an anonymous questionnaire about their understanding and use of these products.
Questionnaire and Measures
Respondents provided demographic information including their age, gender, race, height, weight, education, and cardiovascular and other medical history. The survey consisted of 10 questions with multiple-choice answers about fish oil product use aimed at determining patients’ reasons for taking fish oil products and their knowledge of the active ingredients (eg, DHA, EPA), prescriber recommendations, brand of fish oil product, place of purchase, use of prescription omega-3 fatty acids versus fish oil dietary supplements, monthly costs, and pill burden (number of pills and frequency of administration). The survey was self-administered. Survey responses were anonymous and were handwritten.
Statistical Analysis
Descriptive statistics were used to summarize and describe the data.
Results
Respondents
Of the 1000 cardiac patients who were offered this survey, a total of 711 (71%) completed the questionnaire. A summary of respondent demographics is shown in Table 1. Most were older (mean age: 70.3 years), white (68%), female (57%), and had an annual income of less than US$50, 000 per year. More than 75% had not completed college. Key medical conditions per patient histories are shown in Table 2. The most common cardiovascular-related conditions were hypertension and dyslipidemia. Other common diseases included diabetes, chronic obstructive pulmonary disease/asthma, and depression.
Table 1.
Patient Demographics.
| Demographic | N = 711 |
|---|---|
| Age, mean (SD), years | 70.3 (8.9) |
| Weight, mean (SD), pounds | 170.1 (23.8) |
| Height, mean (SD), inches | 68.7 (5.9) |
| BMI ≥30 (kg/m2), n (%) | 135 (19) |
| Women, n (%) | 406 (57) |
| Race, n (%) | |
| White | 483 (68) |
| Hispanic | 117 (16) |
| Black | 81 (11) |
| Asian | 22 (3) |
| Native American | 8 (1) |
| Highest education level, n (%) | |
| High school | 305 (43) |
| Some college | 249 (35) |
| College degree | 83 (12) |
| Professional degree | 38 (5) |
| Graduate degree | 36 (5) |
| Annual income, n (%)a | |
| <US$35K | 294 (41) |
| US$35K to US$49.9K | 249 (35) |
| US$50K to US$74.9K | 85 (12) |
| US$75K to US$99.9K | 64 (9) |
| ≥US$100K | 19 (3) |
Abbreviations: BMI, body mass index; SD, standard deviation.
a Income given in US dollars.
Table 2.
Patient Medical History.
| Medical Condition | n (%), N = 711 |
|---|---|
| Cardiovascular | |
| Hypertension | 560 (79) |
| PCI/CABG | 527 (74) |
| Dyslipidemia | 480 (67) |
| Myocardial infarction | 422 (59) |
| Coronary artery disease | 375 (53) |
| Arrhythmia | 167 (23) |
| Heart failure | 137 (19) |
| Valvular heart disease | 101 (14) |
| Peripheral artery disease | 71 (10) |
| Stroke | 69 (10) |
| Other | |
| COPD/asthma | 242 (34) |
| Diabetes mellitus | 241 (34) |
| Psychiatric/depression | 225 (32) |
| Arthritis | 190 (27) |
| Gastrointestinal disease | 171 (24) |
| Thyroid disease | 119 (17) |
| Renal disease | 90 (13) |
| Liver disease | 21 (3) |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
Reasons for Use
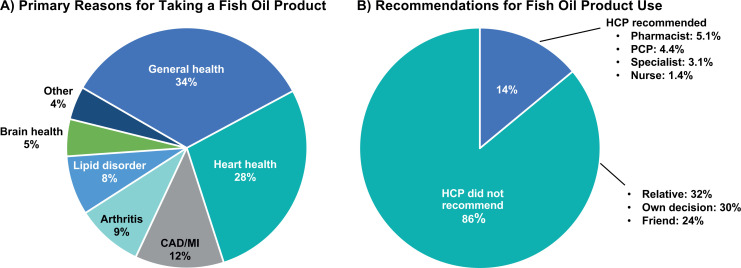
The primary reasons for the use of fish oil dietary supplements cited by the survey respondents were general health (34%, n = 241) and cardiovascular health or diseases including heart health (28%, n = 197), coronary artery disease/myocardial infarction (12%, n = 89), and lipid disorders (8%, n = 54; Figure 1A). Few of those surveyed (14%, n = 99) responded that they were advised by a health-care provider such as a pharmacist or physician to take a fish oil dietary supplement (Figure 1B). Most respondents (86%, n = 612) decided to take fish oil dietary supplements based on recommendation from a friend, a family member, or on their own (Figure 1B).
Figure 1.
Reasons and recommendations for fish oil product use (N = 711). Abbreviations: CAD, coronary artery disease; HCP, health care provider; MI, myocardial infarction; PCP, primary care provider.
Patient Knowledge of Active Ingredient
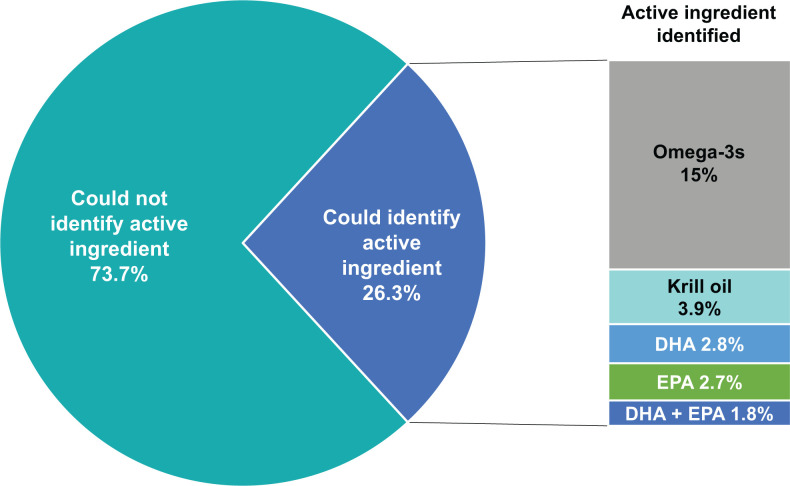
The majority of respondents (74%, n = 524) did not know what the active ingredient was in their fish oil product (Figure 2). Of the 187 (26%) patients who responded that they knew the active ingredient, the active ingredient was identified as omega-3 fatty acids (n = 107), krill oil (n = 28), DHA (n = 20), EPA (n = 19), or DHA plus EPA (n = 13).
Figure 2.
Respondent’s ability to name the active ingredient in their fish oil product. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Patterns of Use and Product Cost
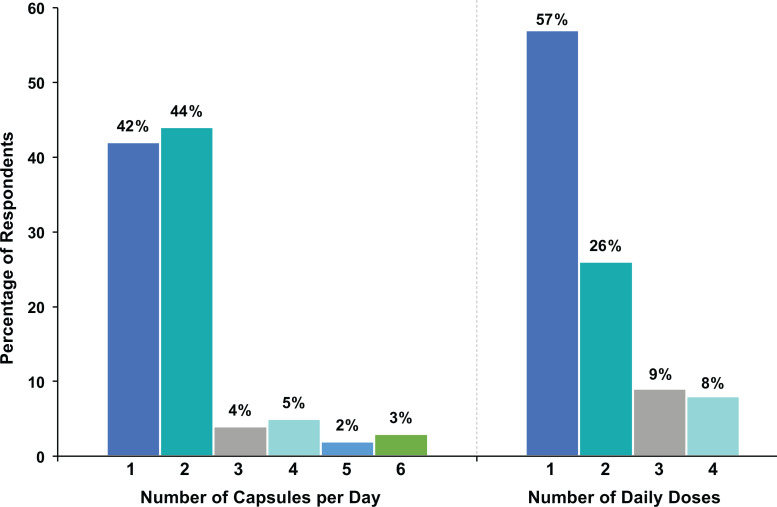
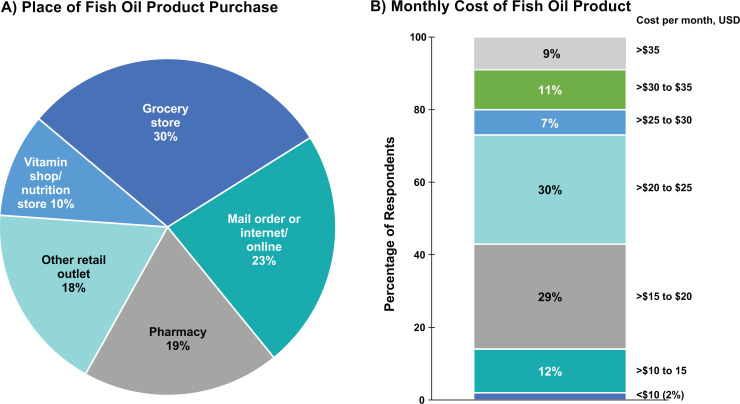
Most patients (86%) responded that they were taking 1 (42%) or 2 (44%) capsules at a frequency of once (57%) or twice (26%) daily (Figure 3). A large majority of respondents (81%) stated that they purchased their fish oil dietary supplement from a nonpharmacy retail seller (Figure 4A). Seventeen different fish oil dietary supplement brands were identified, but 215 respondents did not provide a brand. With regard to cost, 59% of respondents paid US$15 to US$25 per month, whereas 27% paid more than US$25 per month and 14% paid less than US$15 per month for their fish oil product (Figure 4B).
Figure 3.
Number of pills per day and frequency of administration.
Figure 4.
Place of fish oil product purchase and cost. Abbreviation: USD, US dollars.
Of the 99 respondents who were advised by a health-care provider to take a fish oil dietary supplement or a prescription omega-3 fatty acid, 27 received a prescription for a Food and Drug Administration (FDA)-approved omega-3 fatty acid drug (3.8% of all respondents). Eighteen of these patients indicated they were filling their prescription (2.5% of all respondents). The prescriptions were for Vascepa® (n = 11) and Lovaza® generic (n = 7).
Discussion
The results of our survey of patients at a university medical center cardiology service found that most cardiac patients were using fish oil dietary supplements without a health-care professional’s recommendation and without medical supervision. Most patients lacked knowledge of the active ingredients in their fish oil products. Only a small minority of patients were using fish oil products because they were advised to do so by their health-care provider. These data suggest that there is an unmet need for health-care professionals to counsel and educate patients regarding fish oil dietary supplements and prescription omega-3 fatty acids. Most patients paid US$15 to US$25 per month and nearly 30% paid more than US$25 per month.
Although most of the respondents in our survey were taking fish oil dietary supplements for general, heart, or brain health, nearly 30% of patients were taking these products for specific diseases such as lipid disorders (8%), arthritis (9%), or coronary artery disease/myocardial infarction (12%). This underscores a common misconception about omega-3 dietary supplement use. Omega-3 dietary supplements are not approved by the FDA as treatments for any disease.4,12,14,15 The only FDA-approved indication for omega-3 prescription products is to treat severe hypertriglyceridemia (triglyceride levels of ≥500 mg/dL). This indication includes a recommended daily dose of 2 to 4 g of prescription quality omega-3 fatty acids.16-18 Fish oil dietary supplements should not be substituted for the FDA-approved prescription omega-3 fatty acids.4,12,14 Only 4% (27 of 711) of cardiac patients in our survey received a prescription from their health-care provider and only 2.5% (18 of 711) were filling their prescription for an FDA-approved omega-3 fatty acid product.
The findings of our survey are supported by other patient surveys regarding use of omega-3 products.19,20 A survey of 496 cardiac patients by Hawkins and colleagues20 reported that 40% of respondents were taking a fish oil product for general health, while 60% were taking the product for a specific disease.20 Only 9% were advised to take a fish oil product by their physician, 10% of patients stated they were taking fish oil for lipid disorders, and only 4% were given a prescription for an FDA-approved omega-3 fatty acid drug.20 Another recent survey by Backes and colleagues19 found that there are gaps in patient knowledge regarding active ingredients and dosing of fish oil dietary supplements, even among well educated and affluent patient populations.19 Their study showed that patients’ actual intake of omega-3 fatty acids was approximately 50% less than what was recommended by their health-care provider. The investigators also noted the existence of confusing product labeling for fish oil dietary supplements that may lead to incorrect dosing. Factors that influenced product selection included substitution of lower cost products and switching from their usual brand, which may also contribute to the dosing differences.
There are several general but important distinctions between dietary supplements and products classified as OTC or prescription medications that patients and health-care professionals may be unaware of, including differences in regulation, manufacturing, product purity/quality, and product labeling/content.4,12,14,15 It is critical to note that dietary supplements are not categorized as OTC products.12,15 It is also important to understand that dietary supplements are not subject to the same manufacturing standards and quality controls as OTC medications.4,12,15 Manufacturers of dietary supplements are not required to demonstrate the safety or effectiveness of their products prior to marketing.21,22 The Dietary Supplementation Health and Education Act has designated that dietary supplements are assumed to be safe until proven otherwise.23,24 By contrast, OTC medications and prescription drugs are highly regulated by the FDA and must demonstrate safety and efficacy in clinical trials prior to receiving marketing approval. The need for health-care professional education on this subject was underscored in a survey of 200 physicians and 150 US pharmacists which found that less than half (41%) correctly stated that OTC medications are regulated and approved by the US FDA and dietary supplements are not.25
In terms of product purity and manufacturing, several studies have found that fish oil dietary supplements can have inconsistent DHA and EPA levels and impurities such as saturated fat, cholesterol, oxidation products, and other contaminants that may exceed recommended safety standards and may adversely affect health.14,26-30 The safety of oxidation products is not completely understood, although some data suggest that oxidized lipids can contribute to oxidative stress and cause inflammatory effects in animals and humans, potentially increasing the risk of atherosclerosis and thrombosis.31 The prescription products contain highly purified omega-3 fatty acids. The manufacturing process of prescription omega-3 drugs is tightly controlled and regulated.12,14
Patients taking dietary supplement fish oil products should receive education about their relative benefits and risks. As many patients are taking dietary fish oil supplements without supervision from their primary health-care provider, an important opportunity exists for pharmacists to educate patients using these supplements. Patients using dietary supplement fish oil products are adding to their pill burden. Polypharmacy is a documented cause of medication nonadherence. Although we did not evaluate prescription adherence in our survey, it is possible that patients taking dietary supplement fish oil products may be doing so while failing to be adherent to other necessary prescriptions with demonstrated medical indications.
The need for greater awareness of these issues is demonstrated by a recent American College of Cardiology survey of cardiologists and cardiovascular team members, in which only 60% to 65% of respondents reported being extremely or very familiar with fish oil dietary supplements or prescription omega-3 fatty acids.32 Unfortunately, because many individuals may not buy their fish oil products at a pharmacy, pharmacists may have limited opportunities to counsel patients. Despite this potential obstacle, community pharmacists should consider routinely asking their patients about their medication profile, including the use of dietary supplements, update patient profiles, and provide education accordingly.
This study was exploratory in nature and was limited by its single-center design and descriptive statistics, which limit the ability to generalize these findings to larger, more diverse populations. As with other surveys, results may be affected by response bias.
Conclusions
The results of this survey suggest that most cardiac patients who take fish oil products are using dietary supplements without medical supervision. Patients’ general knowledge regarding the active ingredients of fish oil products is poor. Because most fish oil dietary supplements contain less than 1 g of omega-3 fatty acids per capsule, the results of our survey suggest that the daily dose of omega-3 fatty acids that most patients are taking is low. Although low-dose omega-3 supplementation may be beneficial in general promotion of health, there is little to no clinical evidence supporting a clinical benefit for such low doses in the treatment of any disease. Health-care professionals, including pharmacists, can play an important role in educating patients on the appropriate use of fish oil products. However, most patients in our survey obtained fish oil dietary supplements outside of a pharmacy and without medical supervision, suggesting that opportunities to monitor and educate patients concerning appropriate use remain major challenges.
Acknowledgments
Medical writing and editorial assistance was provided by Peloton Advantage, Parsippany, NJ, and funded by Amarin Pharma Inc, Bedminster, NJ.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.E.H. serves on the Amgen speaker’s bureau. R.T. and K.A.P. report no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(suppl 6):1467S–1476S. [DOI] [PubMed] [Google Scholar]
- 2. Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(suppl 1):171S–175S. [DOI] [PubMed] [Google Scholar]
- 3. Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53(12):2525–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manku MS. Best practices: why EPA only? Intern Med News Suppl. 2015. [Google Scholar]
- 5. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. [DOI] [PubMed] [Google Scholar]
- 6. Siriwardhana N, Kalupahana NS, Moustaid-Moussa N. Health benefits of n-3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res. 2012;65:211–222. [DOI] [PubMed] [Google Scholar]
- 7. Kris-Etherton PM, Innis S. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. J Am Diet Assoc. 2007;107(9):1599–1611. [PubMed] [Google Scholar]
- 8. Vannice G, Rasmussen H. Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet. 2014;114(1):136–153. [DOI] [PubMed] [Google Scholar]
- 9. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. [DOI] [PubMed] [Google Scholar]
- 10. Clarke TC, Black LI, Stussman BJ, et al. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Nat Health Stat Rep. 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 11. Kantor ED, Rehm CD, Du M, et al. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316(14):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilleman D, Smer A. Prescription omega-3 fatty acid products and dietary supplements are not interchangeable. Manag Care. 2016;25(1):46–52B. [PubMed] [Google Scholar]
- 13. Mason RP, Hilleman DE. Omega-3 fatty acid fish oil dietary supplements for disease management: are they appropriate for patients? Published 2016. Accessed May 24, 2018 https://www.lipid.org/node/1903
- 14. Fialkow J. Omega-3 fatty acid formulations in cardiovascular disease: dietary supplements are not substitutes for prescription products. Am J Cardiovasc Drugs. 2016;16(4):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gutstein AS, Copple T. Cardiovascular disease and omega-3s: prescription products and fish oil dietary supplements are not the same. J Am Assoc Nurse Practitioners. 2017;29(12):791–801. [DOI] [PubMed] [Google Scholar]
- 16. Lovaza [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2015. [Google Scholar]
- 17. Vascepa [package insert]. Bedminster, NJ: Amarin Pharma Inc; 2017. [Google Scholar]
- 18. Epanova [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2017. [Google Scholar]
- 19. Backes JM, Melton BL, Ruisinger JF, et al. A comparison of patients’ prescribed, self-reported, and actual-intake of supplemental EPA/DHA [poster 161]. Presented at: National Lipid Association Scientific Sessions; May 19-20, 2016; New Orleans, LA. [Google Scholar]
- 20. Hawkins EB, Ling H, Burns TL, et al. Perceptions, knowledge, and patterns of use of “fish oil” products in cardiac patients [abstract 23]. Pharmacotherapy. 2012;32(10):e184. [Google Scholar]
- 21. American Cancer Society. Dietary supplements: what is safe? Published 2014. Accessed May 24, 2018 Available at: http://www.cancer.org/acs/groups/cid/documents/webcontent/002385-pdf.pdf
- 22. Lopez JAG, Ito MK. PLA chapter update: prescription fish oil and Blue Cross of Idaho. LipidSpin. 2010;8(3):32–34. [Google Scholar]
- 23. Food and Drug Administration. Regulatory information: dietary supplement health and education act of 1994. Published 1997. Accessed May 24, 2018 http://health.gov/dietsupp/ch1.htm
- 24. Cohen PA. Hazards of hindsight—monitoring the safety of nutritional supplements. N Engl J Med. 2014;370(14):1277–1280. [DOI] [PubMed] [Google Scholar]
- 25. Higginson R. What’s in your supplement? Even the experts are stumped. Published 2015. Accessed May 24, 2018 http://publicmind.fdu.edu/2015/supplements/final.pdf
- 26. Albert BB, Derraik JG, Cameron-Smith D, et al. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015;5:7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halvorsen BL, Blomhoff R. Determination of lipid oxidation products in vegetable oils and marine omega-3 supplements. Food Nutr Res. 2011;55 doi:10.3402/fnr.v3455i3400.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Truong P, Johnson C, Gabriel D. Variability of cholesterol and saturated fat content in dietary supplements [abstract P72]. Circulation. 2007;115(8):e238. [Google Scholar]
- 29. Mason RP, Sherratt SCR. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun. 2017;483(1):425–429. [DOI] [PubMed] [Google Scholar]
- 30. Jackowski SA, Alvi AZ, Mirajkar A, et al. Oxidation levels of North American over-the-counter n-3 (omega-3) supplements and the influence of supplement formulation and delivery form on evaluating oxidative safety. J Nutr Sci. 2015;4:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turner R, McLean CH, Silvers KM. Are the health benefits of fish oils limited by products of oxidation? Nutr Res Rev. 2006;19(1):53–62. [DOI] [PubMed] [Google Scholar]
- 32. Hypertriglyceridemia: insights on needs of cardiologists, CV team to reduce residual risk. Cardiology Magazine. Published 2018. Accessed May 24, 2018 http://www.acc.org/latest-in-cardiology/articles/2018/04/17/12/42/feature-hypertriglyceridemia-insights-on-needs-of-cardiologists-cv-team-to-reduce-residual-risk.